Abstract
Caudatin, one of the species of C-21 steroidal glycosides mainly isolated from the root of Cynanchum bungei Decne, exhibits potent anticancer activities. However, the mechanism remains poorly defined. In the present study, the growth inhibitory effect and mechanism of caudatin on human glioma cells were evaluated in vitro. The results revealed that caudatin time- and dose-dependently inhibited U251 and U87 cells growth. Flow cytometry analysis indicated that caudatin-induced growth inhibition against U251 and U87 cells was mainly achieved by the induction of G0/G1 and S-phase cell cycle arrest through triggering DNA damage, as convinced by the up-regulation of p53, p21, and histone phosphorylation, as well as the down-regulation of cyclin D1. Moreover, caudatin treatment also triggered the activation of ERK and inactivation of AKT pathway. LY294002 (an AKT inhibitor) addition enhanced caudation-induced AKT inhibition, indicating that caudatin inhibited U251 cells growth in an AKT-dependent manner. Taken together, these results indicate that caudatin may act as a novel cytostatic reagent against human glioma cells through the induction of DNA damage-mediated cell cycle arrest with the involvement of modulating MAPK and AKT pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant glioma represents one of the most common and aggressive primary brain tumors (Su et al. 2006; Chen et al. 2008). Glioma possess high infiltration and invasion capacity into adjacent tissues through migrating along blood vessels, displacing the junction among glial cells and blood vessels, invading, compressing, and destructing the brain parenchyma (Du et al. 2009; Iarosz et al. 2015; Rhee et al. 2009). The survival rate for glioma is extremely low and the mean survival is only 16 months (Ku et al. 2011; Niu et al. 2011). Due to the high invasiveness, high incidence, and resistance toward standard chemotherapy, the cure rate is very low, and new therapeutic strategies are urgently needed (Bourkoula et al. 2014; Liu et al. 2013).
Natural products exhibit potential application in cancer chemotherapy due to their novel pharmacological activities and less toxicity. Cynanchum auriculatum, mainly distributed in China and the other Asia countries, was used as a medicine to nourish the blood and prolong the life in the ancient times (Peng et al. 2008). It has been shown that C. auriculatum displayed various pharmacological functions, including immunoenhancement, hepatoprotection, anti-aging, anticancer, and even some nervous system and mental disorders, such as epilepsy, depression, and Meniere’s syndrome (Johnson and O’Neill, 2012; Lwin et al. 2013). C-21 steroidal glycoside, a member of Asclepiadaceae families, is thought to be the major active component of C. auriculatum. It has been demonstrated that C-21 steroidal glycosides showed multiple pharmacological activities, such as anti-proliferation/invasion of tumor cells, immunoregulation, high efficiency of removing hydroxyl radicals and oxygen free radicals, and protection of liver and nerve cells (Luo et al. 2013). Caudatin, extracted and purified from the root of Cynanchum bungei Decne, is a compound of C-21 steroidal glycosides species (chemical structure Fig. 1) (Ma et al. 2011). It is reported that caudatin could induce apoptosis in several cell lines. Besides its prohibitive effect on cancer cell proliferation and invasion, the roles of inhibiting the secretion of HBsAg and HBV DNA replication were also reported (Wang et al. 2012). All the properties make caudatin have powerful chemoprevention and chemotherapy potential against tumor as well as virus infection. However, the underlying mechanisms are not well understood, especially in human brain tumors. Therefore, in the present study, the anticancer activities and mechanism of caudatin on human glioma cells were clarified, and the results revealed that caudatin effectively inhibited human glioma cells growth by triggering DNA-mediated cell cycle arrest with the involvement of modulation of MAPK and AKT pathways.
Materials and Methods
Chemicals
Caudatin, propidium iodide (PI), MTT, and other reagents were all purchased from Sigma. BCA assay kit was bought from CWBIO (Cat CW0014). DMEM and fetal bovine serum (FBS) were bought from Gibco. Parenzyme and penicillin–streptomycin were purchased from Solarbio. All of the solvents related were of high-performance liquid chromatography (HPLC) grade. The water mentioned in this study was provided by a Milli-Q water purification system from Millipore. All antibodies used in this study were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Culture
U251 cells (ATCC, USA) were incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % FBS and penicillin (100 U/ml) and streptomycin (50 U/ml) at 37 °C under a humidified atmosphere with 5 % CO2.
MTT Assay
U251 cells (104 cells/well) seeded in 96-well plate were treated with caudatin (0, 25, 50, 100 μM) for 24, 48, and 72 h. After incubation, cell viability was detected by MTT assay. Briefly, MTT could be transformed by succinate dehydrogenase, an important enzyme in cellular metabolism, to a purple formazan crystal in living cells. Therefore, when the cells were treated with caudatin, MTT was added into the wells with 20 µl/well (5 mg/ml) and incubated for another 5 h at 37 °C. Then the medium was replaced by 150 µl/well DMSO. Crystals were dissolved by DMSO and then the intensity of the solvent was measured by microplate reader (Molecular Devices, USA) at 570 nm. The result represents the growth condition of the U251 cells after drug intervention. Cell viability was expressed as the percentage compared to control (as 100 %).
Cell Apoptosis and Cycle Distribution
Cell apoptosis and cell cycle were detected by flow cytometry. Briefly, cells seeded in cell culture dishes (6 cm) were treated with caudatin (0–400 μM) for 48 h. Then the cells were harvested by centrifugation, washed with PBS, fixed with 70 % ethanol at −20 °C overnight, and stained with PI for 2 h in dark. The apoptotic cells show hypodiploid DNA contents (Sub-G1 peak), and therefore the Sub-G1 peak was usually employed to quantify the apoptotic cells. The cell cycle distribution, G0/G1, S, and G2/M, was analyzed by Modfit LT 4.0 software.
Western Blotting Analysis
Cells were cultured in dishes (10 cm) and treated with caudatin. After treatment, cells were washed by PBS and lysed in lysis buffer in the icy environment. The total cellular protein was extracted and the concentration of the protein was quantified by BCA kit according to the manufacturer’s instructions. The total protein was added with samples buffer, boiled for 10 min, and stored at −80 °C environment for subsequent SDS–polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Briefly, total protein (40 μg/lane) was loaded and separated in 5 % (80 V, 40 min) and 10 % SDS-PAGE (110 V, 70 min), respectively. Then the protein was transferred into polyvinylidene difluoride membrane (PVDF) under 110 V for 90 min. After that, the membranes were blocked with 5 % BSA at room temperature for 2 h. Then, the membranes were incubated with primary antibodies (1:1000) overnight at 4 °C and second antibodies (1:2000) for 2 h at room temperature, respectively. At last, the target proteins were scanned with enhanced chemiluminescence reagent (ECL) under an Imaging System (Chemi Doc MP, Bio-Rad). β-actin plays as a positive control.
Results
Caudatin Inhibits Human Glioma Cells Proliferation
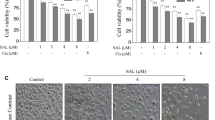
Firstly, MTT assay was employed to examine the anti-proliferation activity of caudatin against U251 and U87 cells. As shown in Fig. 2, treatment of cells with caudatin significantly induced cell growth inhibition against U251 and U87 cells in a time- and dose-dependent manner. For instance, cells exposed to 25, 50, and 100 µM of caudatin for 48 h markedly inhibited the U251 cell viability to 78.7, 67.6, and 51.3 %, respectively. And U251 cells treated with 50 µM caudatin for 24, 48, and 72 h showed an obvious decline in cell viability to 80.2, 67.6, and 41.5 %, respectively. This result suggests that caudatin may act as a potential cytostatic agent in hunting human glioma cells growth. Moreover, U251 cells exhibited more sensibility to caudatin than that of U87 cells. Therefore, U251 cells were selected for mechanism study.
Caudatin inhibits U251 cells growth. Cytotoxicity of caudatin to U251 (a) and U87 cells (b). Cells seeded in 96-well plate (10,000 cells/well) were treated with 0, 25, 50, or 100 µM caudatin for 24, 48, and 72 h. Cell viability was detected by MTT assay. All data were expressed as the mean ± SD of the three independent experiments. Bars with “*” and “**” represent statistical difference at P < 0.05 and P < 0.01 levels, respectively
Caudatin Triggers Cell Cycle Arrest in Human Glioma Cells
To explore the modes of cell death induced by caudatin, flow cytometry was used to quantify caudatin-induced cell apoptosis and cell cycle distribution. As shown in Fig. 3a, exposure of cells to indicated concentrations of caudatin resulted in significant accumulation of U251 cells in G0/G1 phase, as convinced by the increase of G0/G1 peak, accompanied by the decrease of S and G2/M phases. The statistical analysis of cell cycle distribution exactly confirmed this result (Fig. 3b). However, caudatin inhibited U87 cells growth also by the induction of cell cycle arrest (Fig. S1), but at S-phase arrest, not G0/G1 phase. No significant cell apoptosis in U87 and U251 was observed in 48 h of treatment compared to that of the control group. To evaluate whether cell apoptosis was involved in caudatin-induced cell growth inhibition, the treatment time of caudatin was prolonged to 72 h, and the result indicated that, besides the G0/G1 phase arrest, caudatin also induced obvious cell apoptosis in U251 cells, as convinced by the increase of Sub-G1 peak (Fig. S3).
Caudatin induces G0/G1 phase arrest in U251 cells. a Cell cycle distribution. Cells after treatment with caudatin were stained by PI solution, and the cell cycle distribution (G0/G1, S, and G2/M) was analyzed by flow cytometry. b Statistical analysis of cell cycle distribution. c Effect of caudatin on cyclin D1. Total protein was extracted from caudatin-treated cells, and the protein expression was detected by western blotting. Bars with “*” and “**” represent statistical difference at P < 0.05 and P < 0.01 levels, respectively
To evaluate the molecular mechanism involved in the cell growth inhibition induced by caudatin, two regulators of cell cycle, cyclin D1 and cyclin B1, were detected by western blotting method. As shown in Fig. 3c, caudatin significantly decreased the cyclin D1 expression, but had no effect on cyclin B1 expression. Taken together, these results indicate that caudatin inhibited human glioma cells proliferation by the induction of cell cycle arrest.
Caudatin Triggers DNA Damage
Induction of DNA damage by anticancer drugs is an effective way in combating human cancers. Hence, several DNA damage markers were examined. As shown in Fig. 4, caudatin treatment apparently increased the phosphorylated levels of p53, p21, and histone. p53 can be activated in response to DNA damage by affecting several phosphorylated sites, and active p53 can activate p21 and eventually induce apoptosis or/and cell cycle arrest. Therefore, we speculate that the addition of caudatin into U251 cells caused DNA damage and eventually induced G0/G1 cell cycle arrest.
Caudatin triggers DNA damage. Cells after treatment were collected and lysed, and total protein was used to detect protein expression by western blotting method. The optical density was detected by Quantity-One software to quantify the protein expression. The expression rate (target protein/β-actin) was lined under the bands
Caudatin Causes Dysfunction of MAPK and AKT Signaling pathways
Both MAPK (including ERK, p38, and JNK) and AKT pathways play important roles in regulating cell proliferation, cell survival, and cell apoptotic signaling. Hence, we examined whether phosphorylated status of p38, JNK, ERK, and AKT was involved in caudatin-induced cell growth inhibition. The results found that caudatin treatment dramatically increased ERK expression and decreased AKT expression (Fig. 5), indicating that MAPK and AKT pathways both contributed to caudatin-induced cell growth inhibition. Furthermore, caudatin-induced U87 cells growth inhibition also involved the decline of p-AKT expression (Fig. S2). Moreover, AKT inhibitor (LY294002) pretreatment significantly enhanced caudatin-induced inhibition against AKT expression in U251 cells (Fig. 5c), revealing that caudatin suppressed U251 cells growth in an AKT-dependent manner. No significant changes in expression of p38 and JNK were observed. Besides cell cycle arrest, decreased AKT expression also contributed to caudatin-induced apoptosis (Fig. S4). Taken together, these results above revealed that caudatin inhibited human glioma cells growth mainly by DNA damage-mediated cell cycle arrest with the involvement of modulating MAPK and AKT pathways.
Effects of caudatin on MAPK and AKT pathways. Effects of caudatin on MAPK (a) and AKT pathways (b). c Effect of AKT inhibitor (LY294002) on AKT expression in caudatin-treated U251 cells. Cells were pre-treated with LY294002 for 2 h before the caudatin treatment. The protein expression was detected by western blotting and was quantified by Quantity-One software
Discussion
Caudatin as a natural product exhibits diverse pharmacological activities (Fei et al. 2012a, b; Li et al. 2013; Ma et al. 2011; Peng et al. 2008). Induction of cell apoptosis or/and cell cycle arrest by natural products are the important ways in hunting cancer cell growth. The cell death process is highly precisely regulated by several signaling pathways which is critical during the development and regulation of cellular homeostasis as well as in the pathogenesis of a variety of diseases including many brain tumors (Charriaut-Marlangue et al. 1998; Czarnota et al. 1999; Jesionek-Kupnicka et al. 1997).
Cell cycle arrest is one of the momentous mechanisms through which many pharmaceutical products exert antitumor effects (Xiong et al. 2014; Xu and Kim, 2014). Many cytotoxic medicaments and DNA damaging drugs inhibit cell growth by the induction of cell cycle arrest at the G0/G1, S, or G2/M phase (Jin et al. 2014; Liu et al. 2014). As detected by flow cytometry, we observed that the growth inhibition effect of caudatin is highly related to cell cycle arrest of U251 cell lines in the G0/G1 phase. G0/G1 transition phase is regulated by the controlled expression and activity of various cytokines, including cyclins, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CKIs) (Brooks 2005; Warenius et al. 2008). Cyclin and CDK can from a complex to regulate the cell cycle (Chen et al. 2005). Aberrant elevation of cyclins and down-regulation of CKIs have been proven to be poor prognostic features in many cancers (Scott et al. 2004). Numerous studies have demonstrated that p21 played a crucial role in regulating G1/S transition (Chen et al. 2005). Its overexpression and association with CDKs are implicated in the induction of blockade at a specific stage of the cell cycle. Transcriptional regulation of the p21 gene is controlled by the tumor suppressor protein p53, acting on the p53 responsive element in the distal region of the p21 promoter in response to intracellular signals such as DNA damage (Curro et al. 2014; Liu et al. 2006). Cyclin D1, a regulator of cellular proliferation, is itself regulated by ERK1/2. In the present study, caudatin treatment dose dependently caused a distinct down-regulation in cyclin D1 (a G0/G1 phase-regulating protein), whereas the levels of G2/M phase-regulating cyclin B1 were not changed. Therefore, caudatin-mediated cell cycle arrest at G0/G1 phase might be through the inhibition of the formation of CDK/cyclin complexes by the down-regulation of cyclin D1, along with p21 activation in a p53-dependent event in U251 cells.
Progression of cell cycle is governed by the sequential assembly and activation of holoenzyme complexes (Yang et al. 2007). Plenty of evidence indicates that the MAPK and AKT pathways are important mediators of the processes of cell growth and death in response to stressful stimuli, such as UV irradiation, heat, hydrogen peroxide, and DNA damage (Squires et al. 2003; Wang et al. 2010; Ho et al. 2012). Moreover, ERK and JNK have been implicated in p53 phosphorylation that in turn participates in the regulation of p53 activity (Su 2014). In order to testify the apoptotic signaling pathways induced by caudatin, we detect the phosphorylation level of ERK, JNK, and p38 in the caudatin-treated cells. The result suggested that ERK was activated and AKT was inactivated after caudatin treatment in a dose-dependent manner, suggesting that ERK and AKT, but not p38 and JNK, contributed to caudatin-induced G0/G1 phase arrest in U251 cells. Furthermore, the activation of p53 and p21 suggested that caudatin-induced G0/G1 phase arrest may be mediated by MAPKs through p53-dependent pathways.
In conclusion, our results demonstrated that caudatin inhibited the U251 cell proliferation in a dose-dependent manner by inducing G0/G1 arrest through the induction of DNA damage, which was mediated by markedly decreased expression of cyclin D1, as well as the obviously increased expression of p21 and p53 with the involvement of regulating MAPK and AKT pathways. The findings explored that caudatin may act as a beneficial antitumor agent with potential application in chemoprevention and chemotherapy of human glioma.
References
Bourkoula E, Mangoni D, Ius T, Pucer A, Isola M, Musiello D, Marzinotto S, Toffoletto B, Sorrentino M, Palma A, Caponnetto F, Gregoraci G, Vindigni M, Pizzolitto S, Falconieri G, De Maglio G, Pecile V, Ruaro ME, Gri G, Parisse P, Casalis L, Scoles G, Skrap M, Beltrami CA, Beltrami AP, Cesselli D (2014) Glioma-associated stem cells: a novel class of tumor-supporting cells able to predict prognosis of human low-grade gliomas. Stem Cells 32:1239–1253
Brooks G (2005) Cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors: detection methods and activity measurements. Methods Mol Biol 296:291–298
Charriaut-Marlangue C, Remolleau S, Aggoun-Zouaoui D, Ben-Ari Y (1998) Apoptosis and programmed cell death: a role in cerebral ischemia. Biomed Pharmacother 52:264–269
Chen YW, Huang SC, Lin-Shiau SY, Lin JK (2005) Bowman-Birk inhibitor abates proteasome function and suppresses the proliferation of MCF7 breast cancer cells through accumulation of MAP kinase phosphatase-1. Carcinogenesis 26:1296–1306
Curro M, Gugliandolo A, Gangemi C, Risitano R, Ientile R, Caccamo D (2014) Toxic effects of mildly elevated homocysteine concentrations in neuronal-like cells. Neurochem Res 39:1485–1495
Czarnota GJ, Kolios MC, Abraham J, Portnoy M, Ottensmeyer FP, Hunt JW, Sherar MD (1999) Ultrasound imaging of apoptosis: high-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo. Br J Cancer 81:520–527
Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, Cao X, Ling EA, Hao A (2009) Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia 57:724–733
Fei HR, Chen HL, Xiao T, Chen G, Wang FZ (2012a) Caudatin induces cell cycle arrest and caspase-dependent apoptosis in HepG2 cell. Mol Biol Rep 39:131–138
Fei HR, Cui LY, Zhang ZR, Zhao Y, Wang FZ (2012b) Caudatin inhibits carcinomic human alveolar basal epithelial cell growth and angiogenesis through modulating GSK3β/β-catenin pathway. J Cell Biochem 113:3403–3410
Ho PJ, Chou CK, Yeh SF (2012) Role of JNK and p38 MAPK in Taiwanin A-induced cell death. Life Sci 91:1358–1365
Iarosz KC, Borges FS, Batista AM, Baptista MS, Siqueira RA, Viana RL, Lopes SR (2015) Mathematical model of brain tumour with glia-neuron interactions and chemotherapy treatment. J Theor Biol 368:113–121
Jesionek-Kupnicka D, Buczynski J, Kordek R, Sobow T, Kloszewska I, Papierz W, Liberski PP (1997) Programmed cell death (apoptosis) in Alzheimer’s disease and Creutzfeldt-Jakob disease. Folia Neuropathol 35:233–235
Jin J, Lin G, Huang H, Xu D, Yu H, Ma X, Zhu L, Ma D, Jiang H (2014) Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. Int J Biol Sci 10:285–295
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359–364
Ku BM, Lee YK, Ryu J, Jeong JY, Choi J, Eun KM, Shin HY, Kim DG, Hwang EM, Yoo JC, Park JY, Roh GS, Kim HJ, Cho GJ, Choi WS, Paek SH, Kang SS (2011) CHI3L1 (YKL-40) is expressed in human gliomas and regulates the invasion, growth and survival of glioma cells. International journal of cancer. Int J Cancer 128:1316–1326
Li X, Zhang X, Liu X, Tan Z, Yang C, Ding X, Hu X, Zhou J, Xiang S, Zhou C, Zhang J (2013) Caudatin induces cell apoptosis in gastric cancer cells through modulation of Wnt/beta-catenin signaling. Oncol Rep 30:677–684
Liu GY, Liao YF, Chang WH, Liu CC, Hsieh MC, Hsu PC, Tsay GJ, Hung HC (2006) Overexpression of peptidylarginine deiminase IV features in apoptosis of haematopoietic cells. Apoptosis 11:183–196
Liu H, Schmitz JC, Wei J, Cao S, Beumer JH, Strychor S, Cheng L, Liu M, Wang C, Wu N, Zhao X, Zhang Y, Liao J, Chu E, Lin X (2014) Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncol Res 21:247–259
Luo Y, Sun Z, Li Y, Liu L, Cai X, Li Z (2013) Caudatin inhibits human hepatoma cell growth and metastasis through modulation of the Wnt/beta-catenin pathway. Oncol Rep 30:2923–2928
Lwin Z, MacFadden D, Al-Zahrani A, Atenafu E, Miller BA, Sahgal A, Menard C, Laperriere N, Mason WP (2013) Glioblastoma management in the temozolomide era: have we improved outcome? J Neurooncol 115:303–310
Ma XX, Wang D, Zhang YJ, Yang CR (2011) Identification of new qingyangshengenin and caudatin glycosides from the roots of Cynanchum otophyllum. Steroids 76:1003–1009
Niu CS, Li DX, Liu YH, Fu XM, Tang SF, Li J (2011) Expression of NANOG in human gliomas and its relationship with undifferentiated glioma cells. Oncol Rep 26:593–601
Peng Y, Li Y, Wang D, Liu X, Zhang J, Qian S, Duan J (2008) Determination of caudatin-2,6-dideoxy-3-O-methy-beta-d-cymaropyranoside in rat plasma using liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 22:575–580
Rhee W, Ray S, Yokoo H, Hoane ME, Lee CC, Mikheev AM, Horner PJ, Rostomily RC (2009) Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia 57:510–523
Scott SA, Kimura T, Dong WF, Ichinohasama R, Bergen S, Kerviche A, Sheridan D, DeCoteau JF (2004) Methylation status of cyclin-dependent kinase inhibitor genes within the transforming growth factor beta pathway in human T-cell lymphoblastic lymphoma/leukemia. Leuk Res 28:1293–1301
Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, Fox LH, Dickens M, Prigent SA, Manson MM (2003) Relevance of mitogen activated protein kinase (MAPK) and phosphatidylinositol-3-kinase/protein kinase B (PI3 K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol 65:361–376
Su CC (2014) Tanshinone IIA inhibits gastric carcinoma AGS cells through increasing p-p38, p-JNK and p53 but reducing p-ERK, CDC2 and cyclin B1 expression. Anticancer Res 34:7097–7110
Su G, Meyer K, Nandini CD, Qiao D, Salamat S, Friedl A (2006) Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. Am J Pathol 168:2014–2026
Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong DW, Yu ZL (2010) Oridonin induces G2/M cell cycle arrest and apoptosis through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep 24:647–651
Wang LJ, Geng CA, Ma YB, Huang XY, Luo J, Chen H, Guo RH, Zhang XM, Chen JJ (2012) Synthesis, structure-activity relationships and biological evaluation of caudatin derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem 20:2877–2888
Warenius HM, Seabra L, Kyritsi L, White R, Dormer R, Anandappa S, Thomas C, Howarth A (2008) Theranostic proteomic profiling of cyclins, cyclin dependent kinases and Ras in human cancer cell lines is dependent on p53 mutational status. Int J Oncol 32:895–907
Xiong F, Jiang M, Huang Z, Chen M, Chen K, Zhou J, Yin L, Tang Y, Wang M, Ye L, Zhan Z, Duan J, Fu H, Zhang X (2014) A novel herbal formula induces cell cycle arrest and apoptosis in association with suppressing the PI3 K/AKT pathway in human lung cancer A549 cells. Integr Cancer Ther 13:152–160
Xu MY, Kim YS (2014) Antitumor activity of glycyrol via induction of cell cycle arrest, apoptosis and defective autophagy. Food Chem Toxicol 74:311–319
Yang SH, Chien CM, Lu CM, Chen YL, Chang LS, Lin SR (2007) Involvement of c-Jun N-terminal kinase in G2/M arrest and FasL-mediated apoptosis induced by a novel indoloquinoline derivative, IQDMA, in K562 cells. Leuk Res 31:1413–1420
Acknowledgments
The study was supported by the National Natural Science Foundation of China No.81471212, 81271275, 81070947, and 30770759 to B.-L. Sun and Natural Science Foundation of Shandong No. ZR2012HZ006 to B.-L. Sun.
Conflict of interest
The authors declare that there is no conflict of interest for all the authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao-yan Fu and Shuai Zhang are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, Xy., Zhang, S., Wang, K. et al. Caudatin Inhibits Human Glioma Cells Growth Through Triggering DNA Damage-Mediated Cell Cycle Arrest. Cell Mol Neurobiol 35, 953–959 (2015). https://doi.org/10.1007/s10571-015-0190-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0190-x