Abstract
Primary cilia are specialized organelles that extend from the cell surface and concentrate signal transduction components. In the nervous system, primary cilia-associated signals, such as sonic hedgehog (Shh), regulate cell proliferation and neuronal fate. Primary cilia assembly and maintenance require a multi-subunit intraflagellar transport (IFT) protein complex. Defects in primary cilia and IFT proteins are associated to severe pathological phenotypes. In the retina, the study of primary cilia has been mainly restricted to the specialized photoreceptor outer segment. The presence and physiological role of primary cilia in other retinal cells have not been clearly elucidated. Müller cells are the main glia of the retina where they exert distinct functions to maintain homeostasis. In pathological conditions, Müller cells mount a unique regenerative response through the processes of dedifferentiation, proliferation, and differentiation into neuronal lineages. The involvement of IFT proteins or a primary cilium in these processes has not been explored. In this study, we used mature Müller glia primary cultures to reveal the presence of the primary cilia by immunoreactivity to acetylated α-tubulin and γ-tubulin, which localize to the axoneme and ciliar basal body, respectively. We demonstrate that si-RNA-mediated downregulation of IFT20 gene expression, a main component of the IFT machinery, blocks Shh-induced Müller cell proliferation. We present evidence that IFT20 ablation impairs the dedifferentiation capacity of Müller cells induced by Shh and by glutamate. Our demonstration that Müller glia expresses IFT20 and harbors primary cilia, and opens new venues of research on the role of primary cilia in the retina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary cilium is a non-motile microtubule-based organelle, ranging in length from approximately 1–30 μm, which is coupled to the cytoskeleton and extends from the plasma membrane of most mammalian cells (Singla and Reiter 2006). Structurally, primary cilia consist of a ciliary axoneme formed by nine microtubule doublets (9 + 0) that emanate from a modified centriole, the basal body. It is assembled and maintained by intraflagellar transport (IFT), a process mediated by molecular motors and IFT particles that are composed by 15 distinct polypeptides comprising two large complexes (Cole et al. 1998; Rosenbaum and Witman 2002). Although for a long time, primary cilia were considered to be non-functional evolutionary vestiges, in recent years, they have generated enormous interest due to the observation that disruption of ciliary structure or function causes multiple disorders in humans that affect nearly every major body organ including kidney, brain, limb, liver, bone, and retina (Badano et al. 2006; Adams et al. 2009; Novarino et al. 2011; Mahjoub 2013). Specifically, retinal ciliopathies have been associated with most prevalent retinal diseases that include retinitis pigmentosa, macular degeneration, cone-dystrophy, cone-rod dystrophy, Leber congenital amaurosis, and retinal degenerations associated with Usher síndrome (Adams et al. 2009).
Based on the fact that the ciliary compartment concentrates a number of receptors, ion channels, transporters, and downstream signaling proteins, primary cilia are now regarded as sensory cellular antennae that play key roles in development and adult physiology (Singla and Reiter 2006; Berbari et al. 2009). Hence, primary cilia are critical for the efficient activation of the Hedgehog (Hh) signaling pathway (Huangfu et al. 2003; Goetz and Anderson 2010; Ruat et al. 2012) and have important roles in other developmental signaling pathways including Wnt, transforming growth factor beta (TGFβ), and Notch (Gerdes et al. 2007; Corbit et al. 2008; Wallingford and Mitchell 2011; Ezratty et al. 2011). Studies in animal experimental models and in humans have demonstrated that mutations of IFT proteins disturb ciliary assembly, and are associated to neurological disorders such as the Bardet–Biedl and Joubert syndromes (Aldahmesh et al. 2014; Louie and Gleeson 2005); furthermore, that the assembly of a primary cilium plays important roles in brain neurogenesis (Amador-Arjona et al. 2011; Han et al. 2008; Breunig et al. 2008; Ruat et al. 2012, Tong et al. 2014). Thus, it has been demonstrated that Shh signaling, acting through the primary cilia, is required for the formation and establishment of postnatal hippocampal progenitors from radial glia (Breunig et al. 2008; Han et al. 2008) and the expansion of cerebellar progenitors through development (Spassky et al. 2008). The presence of a single cilium was also described as an ultrastructural characteristic of precursor cells in the subventricular zone of the adult mouse (Doetsch et al. 1999; Tong et al. 2014).
In the adult mammalian retina, a specialized population of radial glia, the Müller cells, retains the capacity to deddiferentiate from a differentiated to a progenitor-like state, re-enters the cell cycle, and acquires neurogenic potential upon retinal injury, therefore acting as dormant stem cells of the retina (Das et al. 2006; Ooto et al. 2004; Reh and Fischer 2001). Extrinsic and intrinsic factors cooperate to regulate the dedifferentiation capacity and neurogenic potential acquisition of postnatal Müller cells (Fischer et al. 2002; Osakada et al. 2007; Das et al. 2006; Wan et al. 2007, 2012; Reyes-Aguirre et al. 2013; Wang et al. 2013). It has been demonstrated, for example, that the secreted growth factors, such as heparin-binding epidermal growth factor and ciliary neurotrophic factor, insulin-like growth factor, cytokines, modulators of the Wnt transduction pathway, or microRNAs such as let-7 modulate the activation or suppression of signaling pathways involved in the stimulation of Müller glia reprogramming or Müller cell differentiation (for a recent review see Goldman 2014).
Among them, Shh has proven to promote stem-cell potential of rat in immature Müller glia both in vivo and in vitro, enhancing the regenerative response of these cells (Wan et al. 2007).
Recent immunofluorescence analysis aimed at the localization of intraflagellar transport proteins (Sedmak and Wolfrum 2010) and the primary cilia in the whole thickness of the mouse retina, other than the extensively described photoreceptor outer segment (Wheway et al. 2014). These reports demonstrated the presence of five IFT proteins (IFT-20, 88, 140, 57, and 52) in the photoreceptor cilia and in subcompartments of bipolar, horizontal, and ganglion cells; and a longitudinal staining pattern of the ciliary marker acetylated α-tubulin suggestive of the presence of primary cilia in Müller cells (Kim et al. 2013). However, neither the expression of IFT proteins nor the formation of the primary cilia or its participation in Shh promotion of stem-cell potential of Müller cells has been conclusively elucidated.
Here, we demonstrate that the mature Müller glia-enriched primary cultures express IFT20 and expose well-delineated structures that are revealed by immunoreactivity to the axonemal acetylated α-tubulin (ac-tub) and to γ-tubulin, which localizes to the basal bodies from where the primary cilium emerges. We also demonstrate that si-RNA-mediated downregulation of IFT20 gene expression blocks Shh-induced Müller cell proliferation and the induction of expression of the progenitor gene marker, nestin in these cells. Furthermore, we present evidence that IFT20 ablation also affects Müller cell dedifferentiation when the process is initiated not by Shh but by a different triggering factor, i.e., glutamate.
Materials and Methods
Animals
Experiments were carried out with 12-day-old Long-Evans rats that were maintained on a 12–12 light–dark cycle, had access to standard rat chow and water ad libitum, and were lethally anesthetized with an IP injection of sodium pentobarbital and decapitated. All experiments were conducted in accordance with the Association for Research in vision and Ophthalmology (ARVO) Statement for the Use of Animals for Ophthalmic and Vision Research, and the guidelines of the internal animal care committee (CICUAL-CINVESTAV, Protocol number 356-06).
Cell Culture
Enucleated eyes from 12-day-old Long-Evans rats were placed in Dulbecco’s Minimal Essential Medium (DMEM; Gibco BRL, Gaithersburg, MD) containing 10 % fetal bovine serum (FBS) and 1:1,000 penicillin–streptomycin and stored overnight in the dark at room temperature. The eyes were incubated the next day for 30 min at 37 °C in DMEM containing 0.1 % trypsin and 70 IU/ml collagenase (Sigma Chemical Co., St. Louis, MO), transferred to 10 % FBS-DMEM, and cultured for 7–10 days until confluent. The retina was dissected away from the rest of the tissue and dissociated with a Pasteur pipette. Cells from two retinas were seeded onto a 75 cm2 flasks until confluent and cultured in OptiMEM (Gibco BRL, Gaithersburg, MD) containing 4 % FBS. The medium was changed 24 h after seeding to eliminate suspended cells. This technique is routinely used as a source of purified Müller cell cultures that are devoid of neuronal contamination (Ramirez and Lamas 2009). Cells were maintained as controls or exposed to the following compounds: Sonic Hedgehog (Sigma Chemical) 5 nM for proliferation studies and 20 nM for induction of nestin expression; Cyclopamine (Sigma Chemical) 10 μM or glutamate 50 µM.
MTT Cell Proliferation Assay
Cells were seeded in a 96-well culture plates at a density of 4 × 103 cells/well. Triplicate wells were used for each experimental condition. Ten μl of 5 mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Invitrogen) were added to each culture well. The reaction mixture was incubated for 4 h at 37 °C. After addition of 50 μL of dimethyl sulfoxide (Sigma), the absorbance at 540 nm was measured with a NanoDrop 2000 Spectrophotometer.
RNA Interference
The siRNA transfection procedure for IFT20 knockdown has already been described (Yoshimura et al. 2011). The IFT20 siRNA duplex oligoribonucleotides (Invitrogen) had the following sequence: 5-GAAACUUGCUGAAAUCCAUAGCGAA-3. Cells transfected with a non-coding siRNA sequence obtained from Invitrogen were used as mock controls. Fifty nM of oligoribonucleotides were transfected into Müller glia using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions, and the experiments with transfected cells were conducted 48 h after transfection.
Apoptosis Assay
Quantitative analysis of live, early/late apoptotic, and dead cells was performed using a Muse™ Annexin V & Dead Cell Kit (MCH100105, Millipore, Hayward, CA, USA) and a Muse Cell Analyzer (Millipore), as described in the manufacturer’s instructions. Briefly, Müller cells were detached from culture plates with trypsin 0.25 % (Gibco). A combination of 100 μL of cell suspension and 100 μL of Muse™ Annexin V & Dead Cell Reagent was incubated for 20 min at room temperature and analyzed in a Muse Cell Analyzer.
RT-PCR and Real-Time Quantitative RT-PCR
Total RNA was isolated from cell cultures or total retina using Trizol (Invitrogen). Complementary DNA (cDNA) was synthesized using Oligo dT and Superscript-II reverse transcriptase (Invitrogen). To evaluate the expression of IFT20 in Müller glia, cDNA samples from Müller cell cultures were applied to Real-Time PCR using TaqMan Gene Expression Assays (Applied Biosystems). PCR was performed at 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Primer pairs were β-actin (Rn00667869_m1), IFT20 (Rn01467420_m1), Smo (5′-GCCAGGAGCTCTCCTTCAG-3′ and 5′ CCGGCAACAGGTCCATCAT-3′), and Gli (5′-TGAGCATTATGGACAAGTGCAAGTA-3′ and 5′-CAACCCGGTGGAGTCAGA-3′). When required, samples were resolved in agarose gel electrophoresis. Data analyses were performed according to the comparative Ct method; values of cDNA expression of the target genes were normalized relative to the expression of β-actin analyzed from the same sample on the same plate and reported as Relative mRNA Expression ± SEM.
Immunocytochemistry
The cells were cultured on Poly-l-Lysine (Sigma Chemical Co.) coated slides fixed with 4 % paraformaldehyde for 10 min, washed three times in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2PO4; 1.47 mM KH2PO4), and blocked in 10 % BSA and 10 % goat normal serum in PBS containing 0.3 % Tween-20. The cells were stained with primary antibodies for glutamine synthase (1:200; Abcam, ab16802), acetylated tubulin (1:1,000; Sigma T6793), gamma tubulin (1:500; Abcam ab11321), and Nestin (1:200; Millipore MAB353), which were diluted in the blocking solution before they were added. The slides were allowed to sit overnight at 4 °C. After extensive washing, the double staining was developed using a 1: 500 dilution of Alexa 568 anti-mouse antibody and Alexa 488 anti-rabbit antibody (Molecular Probes, Invitrogen, Carlsbad, CA). Finally, the cell nuclei were counterstained with 0.1 mg/ml 4′, 6-diamidino-2-phenylindole (DAPI) for 10 min. The slides were mounted with Vectashield (Vector Laboratories, Burlingame, CA), and they were viewed on an epifluorescence microscope Zeiss Axiovert 40 CFL, with a digital camera Carl Zeiss Axiocam MRm and using AxioVision Rel.4.8. software.
Scanning Electron Microscopy
Müller cells were cultured on poly-l-lysine-coated slides in 24-well tissue culture plates, washed 3 times with PBS, fixed in 4 % glutaraldehyde for 2 h, rinsed in deionized water, and dehydrated in ascending grades of alcohol (10–100 %, 30 min per step). After dehydration, the cells were subjected to critical point drying, were sputter coated with gold, and then examined by scanning electron microscopy (Hitachi su1510).
Statistical Analysis
The data are expressed as the mean ± SEM. The significance of the differences between means was assessed using a one-way and two-way ANOVA, followed by a Tukey’s test for post hoc comparisons where necessary.
Results
Expression of Primary Cilia Markers and the Intraflagellar Transport Protein IFT20 in Postnatal Rat Müller Cells Primary Cultures
The presence of primary cilia was evaluated in differentiated postnatal Müller primary cell cultures characterized morphologically by a flattened large somata with extended cytoplasmic protrusions and the expression of the Müller specific cell marker glutamine synthetase (GS) in over 96 % of the cells (Fig. 1a, b). Morphological analysis by scanning electron microscopy at higher magnification revealed the presence of distinct projections, approximately 5 μm in length, which were distinguishable from other smaller projections (Fig. 1c).
Morphological and immunofluorescence analysis of rat postnatal mature Müller glia. a Scanning electron microscopy analysis of a PN12 rat Müller glia primary culture. Scale bar 100 μm. b Representative photomicrograph of PN12 rat Müller glia primary culture labeled with GS (red) and counterstained with DAPI (blue). Scale bar 50 μm. c Representative high magnification scanning electron microscopy analysis of a Müller cell showing a distinct projection, approximately 5 μm in length (arrow). Scale bar 5 μm (Color figure online)
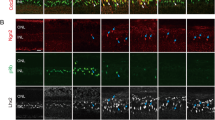
Primary cilia were detected in approximately 10 % of the cells by immunoreactivity to acetylated α-tubulin (ac-tub) that allowed identifying well-delineated structures (Fig. 2a, d), and γ-tubulin (γ-tub), which localizes to the basal bodies from where the primary cilium emerges (Fig. 2c). Double immunolabeling of ac-tub with GS further corroborated that the ac-tub immunopositive structures extended from Müller cells (Fig. 2d).
PN12 rat Müller cells express primary cilia markers and the intraflagellar transport protein IFT20. a–c Representative photomicrographs of PN12 rat Müller glia primary cultures labeled with ac-tub (white or red), γ-tub (green) and counterstained with DAPI (blue). Scale bar 20 μm. Ac-tub immunopositive structures were observed in approximately 10 % of the cells (32 cells from a total of 300 cells counted) d Representative photomicrograph of PN12 rat Müller cell labeled with GS (red) and ac-tub (green), counterstained with DAPI (blue). e RT-PCR analysis of IFT20 expression in the PN12 rat retina (R) and PN12 Müller primary cultures (M). Actin mRNA expression was used as control. RT-PCR reactions were resolved by agarose gel electrophoresis (Color figure online)
Next, we used PN12 Müller primary cultures to determine by RT-PCR the expression of IFT20; a component of the IFT machinery that plays an essential role in the maintenance of the cilia structure (Follit et al. 2006; Jonassen et al. 2008; Yoshimura et al. 2011). Total retina mRNA extracts were used as positive controls. Our results demonstrate that PN12 Müller cell primary cultures express IFT20 (Fig. 2e).
Downregulation of IFT20 Gene Expression Blocks Shh-Induced Mature Müller Cell Proliferation in Culture
Late-stage retinal progenitors gradually transition to mature Müller glia from PN0 to PN12 in the rodent retina (Nelson et al. 2011), and it has been previously demonstrated that Shh promotes proliferation from cultures of Müller cells obtained from immature PN7 rat retinas (Wan et al. 2007). To evaluate whether mature PN12 cell cultures retain the capacity to proliferate in response to Shh, we measured cell proliferation on confluent cultures grown in the absence (control) or presence of 5 nM Shh and the Shh pathway inhibitor, cyclopamine (10 μM). Our results showed that 5 nM Shh is enough to induce a significant increase cell proliferation compared to control cultures, which is prevented by exposure to 10 μM cyclopamine (Fig. 3a).
Downregulation of IFT20 gene expression impairs Shh-induced Müller cell proliferation in culture. a MTT cell proliferation assay of Müller primary cultures grown in the absence (control) or presence of 5 nM Shh, 10 μM Ciclopamine (Cpm), or a combination of Shh and Cpm. The graph represents the mean ± SEM absorbance readings of at least three independent experiments. **p < 0.01, *p < 0.05 compared to control; one-way ANOVA followed by Tuke’s test for post hoc. b Real-time RT-PCR quantification of IFT20, Smo and Gli1 expression in control, and scramble siRNA and IFT20 siRNA-transfected cells 24 h after transfection. Actin mRNA expression was used as an internal control to calculate the relative mRNA expression of IFT20, Smo, and Gli to GAPDH. Data represent the media ± SEM of at least three different experiments. ***p < 0.001, *p < 0.05 compared to control; one-way ANOVA and Tukey’s test for post hoc. c MTT cell proliferation assay of control, scramble siRNA, and IFT20 siRNA-transfected cells. **p < 0.01, *p < 0.05 compared to control; one-way ANOVA and Tukey’s test for post hoc. d Muse Annexin V/Dead Cell Assay in control, scramble siRNA, and IFT20 siRNA-transfected cells. The left panel shows a representative cytofluorimetric dot plot for annexin V detection that allows the identification of four cell populations: live (bottom right), early apoptotic (bottom left), dead (upper left), and late apoptotic (upper right). The graph shows the quantification of surviving cells at days 1, 2, and 3 after transfection of at least three independent experiments. No statistical differences between the groups were found
Efficient activation of Shh signaling pathway depends on IFT proteins that ensure the proper function of primary cilia (Huangfu et al. 2003; Goetz and Anderson 2010; Ruat et al. 2012). To evaluate whether disassembly of the primary cilium could affect Shh-mediated induction of Müller cell proliferation, we decided to inhibit ciliogenesis using a previously described siRNA approach (Yoshimura et al. 2011). Postnatal rat Müller cell primary cultures were independently transfected with an IFT20 siRNA or a control scrambled siRNA (Scr) sequence. Real-time PCR analysis, 24 h after transfection, showed a knockdown of IFT20 expression of approximately 90 % in IFT20-siRNA-transfected cells, while expression was not affected after scrambled siRNA transfection (Fig. 3b). We also examined whether previously reported components of the Shh signaling system (Smo and Gli1) (Murone et al. 1999) were expressed in PN12 Müller primary cultures and whether expression of these proteins was affected by IFT20 downregulation in siRNA-transfected cells. Quantitative RT-PCR demonstrated that, in IFT20 siRNA-transfected cells, expression of Smo and Gli1 is reduced 32 and 48 %, respectively, compared to the level of expression of these proteins in control cells (Fig. 3b).
Cell proliferation analysis showed that siRNA-mediated downregulation of IFT20 gene expression blocked Shh-induced Müller cell proliferation in culture, while transfection of scrambled siRNA had no significant effect on cell proliferation (Fig. 3c). To evaluate whether the IFT20 siRNA-mediated decrease on cell proliferation was associated to increased cell death, we evaluated the apoptosis profile of control and transfected cultures through the analysis of Annexin V expression (Fig. 3d). Quantification of the percentage of viable cells indicated that none of the transfections caused a significant increase on the number of the apoptotic annexin V-immunopositive cells (Fig. 3d).
Downregulation of IFT20 Gene Expression Reduces Shh-Induced Expression of the Progenitor Marker Nestin in Müller Cell Cultures
In addition to inducing Müller cell proliferation, Shh promotes PN7 Müller cell dedifferentiation and the expression of progenitor cell markers in culture (Wan et al. 2007). To determine whether mature PN12 Müller cells were also responsive to Shh-mediated induction of dedifferentiation, we evaluated the expression of the progenitor cell marker nestin, by immunofluorescence with an specific antibody, in PN12 cultured cells for three days in the absence and presence of 20 μM Shh (Fig. 4a). We found that approximately 20 % of Shh-treated cells were immunopositive for nestin expression. When nestin expression was evaluated in IFT20 siRNA-transfected cells, we found that downregulation of IFT20 gene expression reduces Shh-induced nestin expression and only 4 % of the cells resulted immunoreactive (Fig. 4a). Mock or scramble siRNA transfection had no effect on nestin expression (not shown).
Downregulation of IFT20 gene expression blocks Shh-induced and glutamate-induced expression of the progenitor marker nestin. a Shh induces nestin expression in rat Müller glia primary cultures and IFT20 ablation impairs Shh-induced nestin expression. Left panel, representative photomicrograph of PN12 rat Müller glia primary culture labeled with nestin (red) and counterstained with DAPI (blue). Scale bar 20 μm. Right panel, quantification of the number of nestin immunolabeled cells in rat Müller glia primary cultures and IFT20 siRNA-transfected cells cultured for three days in absence (Control) or the presence of 20 nM Shh. Data represent percentage of immunopositive cells of 300 cells counted on non-overlapping fields in three different experiments on ×40 photomicrographs. **p < 0.01, *p < 0.05 compared to control; one-way ANOVA and Tukey’s test for post hoc. b IFT20 ablation partially impairs glutamate-induced nestin expression in PN12 rat Müller cells. Quantification of the number of nestin immunolabeled cells in rat Müller glia primary cultures and in mock, scramble (Scr) and IFT20 siRNA-transfected, and cyclopamine-exposed (Cpm) cells, cultured for four hour in the absence (Control) or the presence of 50 μM glutamate. Data represent percentage of immunopositive cells of 300 cells counted on non-overlapping fields in three different experiments on ×40 photomicrographs. ***p < 0.001; # p < 0.01 compared to control, & p < 0.05 compared to control; two-way ANOVA and Tukey’s test for post hoc (Color figure online)
IFT20 Downregulation Also Affects the Glutamate-Induced Müller Dedifferentiation Pathway
We have previously demonstrated that glutamate, the main excitatory neurotransmitter of the retina, can trigger a rapid dedifferentiation signal in primary cultures of rat Müller glial cells, characterized by changes in cell morphology and the induction of nestin expression, (Reyes-Aguirre et al. 2013), but the participation of IFT20 or the necessity for the correct assembly of a primary cilium in this process had not been evaluated. To this end, IFT-20 siRNA, scramble siRNA and mock-transfected cells were cultured in the absence or presence of 50 μM glutamate. To evaluate the participation of the Shh signal transduction pathways, a group of cells was also exposed to the Shh pathway inhibitor, cyclopamine. Nestin expression in these cultures was assessed by immunofluorescence and the percentage of immunoreactive cells was quantified (Fig. 4b). Our results show that, as expected, glutamate exposure for 4 h induces significant increases in the percentage of nestin expressing cells in mock and scramble siRNA-transfected cultures (from 6.3 to 28 %, and from 4.5 to 28 %, respectively). IFT20 downregulation and cyclopamine exposure partially impair glutamate-induced nestin expression. The percentage of nestin immunopositive cells is reduced by approximately 50 % in glutamate-exposed IFT20 siRNA-transfected cells compared to glutamate-exposed mock or Scr-transfected cells (from 28.3 to 14.1 %, respectively). Ciclopamine exposure induces a similar reduction on the number of nestin expressing cells. Noteworthy, our results also demonstrate that even after IFT20 ablation or in the presence of cyclopamine, glutamate exposure induces a significant increase in the number of nestin expressing cells.
Discussion
In this study, we demonstrate that postnatal mature Müller glia may harbor a primary cilium and that the presence of this organelle may be relevant for the proliferation and dedifferentiation of these cells. In the retina, primary cilia have attracted considerable attention due to the presence of a modified and highly specialized primary cilium in photoreceptor cells that play a crucial role in the selective transport of proteins and other molecules to and from the outer segments (Sedmak and Wolfrum 2010). Immunoreactivity to ciliary markers and distribution of IFT proteins have also been described on the outer plexiform layer (OPL) and ganglion cell layer (GCL) of the rodent retina, suggesting that retinal neuronal processes might share the features of cilia (Kim et al. 2013). Based on a characteristic longitudinal staining pattern of the ciliary marker, acetylated α-tubulin previous reports suggested the presence of primary cilia in Müller glia in the mouse retina in vivo (Kim et al. 2013). Here, we used Müller glia-enriched primary cultures to conclusively identify the presence of primary cilia, by immunofluorescence with antibodies that specifically recognize ac-tub and γ-tub, two critical microtubule proteins that are enriched in the axonemes and basal bodies of primary cilia, respectively (Alieva et al. 1999; Aldaz et al. 2005). Ac-tub immunopositive “hair-like” structures were intensively immunolabeled, distinguishable from other cytoplasmic microtubules recognized by the antibody, and associated to γ-tub co-labeling. Consistent with the notion that assembly of cilia is closely associated to cell cycle progression (Seeley and Nachury 2010) and given that Müller cell cultures were exponentially growing in the presence of serum and not synchronized; we only observed ac-tub immunopositive structures in approximately 10 % of the cells, but ciliated cells were present in all the cultures evaluated.
In addition to their dedifferentiation and neuronal differentiation capacity, it has been proposed that Müller cells, obtained from the immature retina, might instruct a pool of resident retinal progenitor cells to acquire the characteristics of neural stem cells (Simón et al. 2012). The fact that the cultures used in our study were obtained after full maturation of the rodent retina (Nelson et al. 2011) excludes the possible contribution of late-stage retinal progenitors.
An antecedent of this work is the demonstration that the Shh signaling pathway promotes proliferation and stem cell potential of postnatal day 7 (PN7) Müller glia in culture and in vivo (Wan et al. 2007). The components of the Shh signaling pathway are concentrated within the ciliary compartment and proper cilia assembly is crucial for efficient pathway activation (Corbit et al. 2005; Ocbina and Anderson 2008; Haycraft et al., 2005). Here, we determined that siRNA-mediated downregulation of IFT20 resulted in decreased expression of the Shh signaling pathway components Smo and Gli1, and caused the inhibition of Shh-mediated increase of cell proliferation and progenitor cell marker nestin expression in mature (PN12) IFT20-siRNA-transfected Müller cells. The effect of IFT20 downregulation on nestin expression is partial and a percentage (4 %) of the cells is still able to induce nestin expression. The explanation could be related to a methodological issue, as siRNA-mediated ablation of IFT-20 expression is limited.
Although the regenerative potential of rodent Müller cells is limited, dedifferentiation and acquisition of stem cell characteristics by Müller glia-derived retinal progenitors can be induced by a variety of signal transduction pathways that include those triggered by basic fibroblast growth factor (bFGF), prostaglandin E2 (PGE2) (Wang et al. 2013); Wnt (Liu et al. 2013), oxidative stress (Abrahan et al. 2009), and glutamate (Takeda et al. 2008; Reyes-Aguirre et al. 2013), in addition to Shh. The molecular mechanisms underlying the induction of Müller cell dedifferentiation upon these triggering signals remain elusive and, therefore, the question remains whether different signal transduction pathways may converge or follow distinct non-overlapping routes to finally drive Müller cell reprogramming. We here demonstrate that IFT20 ablation not only decreases Shh-mediated Müller cell dedifferentiation, but also the expression of the progenitor marker nestin induced by glutamate. These results would indicate that a correct assembly of the primary cilia or IFT20 expression is required for proper induction of Müller cell dedifferentiation by glutamate, suggesting that both signal transduction pathways converge. However, despite IFT20 downregulation, glutamate exposure is still capable of inducing a significant increase in nestin expression in glutamate-treated compared to non-treated-transfected cells. On the whole, these results suggest that the expression of nestin may be induced through independent signal transduction pathways initiated by diverse dedifferentiation signals. The molecular mechanisms underlying a possible crosstalk between Shh and glutamate, as dedifferentiation triggering signals, remain un-deciphered.
To our knowledge, this is the first evaluation of the consequences of IFT20 ablation and, presumable the disruption of the primary cilia, in these cells. However, the expression of IFT20 in Müller cells might have additional physiological implications other than those related to the presence of a primary cilium, as there is increasing evidence that IFT proteins might be involved in functions other than ciliogenesis (Baldari and Rosenbaum 2010). Thus, in addition to the basal centriole and the cilia, IFT20 has been localized to the Golgi complex (Follit et al. 2006, 2008) and associated to the exocytosis process even in non-ciliated cells (Jekely and Arendt 2006; Finetti et al. 2009). Interestingly, the remarkable contribution of Müller glia to retinal homeostasis, structure, and function (Reichenbach and Bringmann 2013) includes the exocytotic release of neuronal activity modulators, such as glutamate (Slezak et al. 2012).
Our findings highlight novel characteristics of Müller glia: the expression of IFT-20 and the presence of primary cilia. Because Müller cells and Müller-derived retinal progenitors hold promise for the treatment of retinal diseases, and given the strong connection between primary cilia-associated defects and various clinical phenotypes (Adams et al. 2009; Fry et al. 2014; Valente et al. 2014), and this knowledge may open new venues of research in the regenerative medicine field.
References
Abrahan CE, Insua MF, Politi LE, German OL, Rotstein NP (2009) Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro. J Neurosci Res 87(4):964–977
Adams NA, Awadein A, Toma HS (2009) The retinal ciliopathies. Ophthalmic Genet 28:113–125
Aldahmesh MA, Li Y, Alhashem A, Anazi S, Alkuraya H, Hashem M, Awaji AA, Sogaty S, Alkharashi A, Alzahrani S, Al Hazzaa SA, Xiong Y, Kong S, Sun Z, Alkuraya FS (2014) IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet-Biedl syndrome. Hum Mol Genet 23(12):3307–3315
Aldaz H, Rice LM, Stearns T, Agard DA (2005) Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 435:523–527
Alieva IB, Gorgidze LA, Komarova YA, Chernobelskaya OA, Vorobjev IA (1999) Experimental model for studying the primary cilia in tissue culture cells. Membr Cell Biol 12(6):895–905
Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV (2011) Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci 31(27):9933–9944
Badano JL, Mitsuma N, Beales PL, Katsanis N (2006) The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7:125–148
Baldari CT, Rosenbaum J (2010) Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol 22:75–80
Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19(13):R526–R535
Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T (2008) Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA 105:13127–13132
Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL (1998) Chlamydomonas Kinesin-II–dependent Intraflagellar Transport (IFT): IFT Particles Contain Proteins Required for Ciliary Assembly in Caenorhabditis elegans Sensory Neurons. J Cell Biol 141(4):993–1008
Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF (2005) Vertebrate Smoothened functions at the primary cilium. Nature 437:1018–1021
Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF (2008) Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10:70–76
Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I (2006) Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol 299(1):283–302
Doetsch F, García-Verdugo JM, Alvarez-Buylla A (1999) Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci 96:11619–11624
Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E (2011) A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145(7):112911–112941
Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT (2009) Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol 11(11):1332–1339
Fischer AJ, McGuire CR, Dierks BD, Reh TA (2002) Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci 22:9387–9398
Follit JA, Tuft RA, Fogarty KE, Pazour GJ (2006) The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17:3781–3792
Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ (2008) The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet 4(12):e1000315
Fry AM, Leaper MJ, Bayliss R (2014) The primary cilium: guardian of organ development and homeostasis. Organogenesis 10(1):62–68
Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S et al (2007) Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 39:1350–1360
Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11:331–344
Goldman D (2014) Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci 15(7):431–442
Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A (2008) Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11:277–284
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK (2005) Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1:e53
Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87
Jekely G, Arendt D (2006) Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. BioEssays 28:191–198
Jonassen JA, San Agustin J, Follit JA, Pazour GJ (2008) Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183:377–384
Kim YK, Kim JH, Yu YS, Ko HW, Kim JH (2013) Localization of primary cilia in mouse retina. Acta Histochem 115(8):789–794
Liu B, Hunter DJ, Rooker S, Chan A, Paulus YM, Leucht P, Nusse Y, Nomoto H, Helms JA (2013) Wnt signaling promotes Müller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci 54(1):444–453
Louie CM, Gleeson JG (2005) Genetic basis of Joubert syndrome and related disorders of cerebellar development. Hum Mol Genet 14:R235–R242
Mahjoub MR (2013) The importance of a single primary cilium. Organogenesis 9(2):61–69
Murone M, Rosenthal A, de Sauvage FJ (1999) Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol 9:76–84
Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA (2011) Genome-wide analysis of Müller glial differentiation reveals a requirement for notch signaling in postmitotic cells to maintain the glial fate. PLoS ONE 6(8):e22817
Novarino G, Akizu N, Gleeson JG (2011) Modeling human disease in humans: the ciliopathies. Cell 147:70–79
Ocbina PJ, Anderson KV (2008) Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn 237:2030–2038
Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M (2004) Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA 101:13654–13659
Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M (2007) Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci 27:4210–4219
Ramirez M, Lamas M (2009) NMDA receptor mediates proliferation and CREB phosphorylation in postnatal Müller glia-derived retinal progenitors. Mol Vis 15:713–721
Reh TA, Fischer AJ (2001) Stem cells in the vertebrate retina. Brain Behav Evol 58:296–305
Reichenbach A, Bringmann A (2013) New functions of Müller cells. Glia 61:651–678
Reyes-Aguirre LI, Ferraro S, Quintero H, Sánchez-Serrano SL, Gómez-Montalvo A, Lamas M (2013) Glutamate-induced epigenetic and morphological changes allow rat Müller cell dedifferentiation but not further acquisition of a photoreceptor phenotype. Neuroscience 254:347–360
Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3(11):813–825
Ruat M, Roudaut H, Ferent J, Traiffort E (2012) Hedgehog trafficking, cilia and brain functions. Differentiation 83(2):S97–S104
Sedmak T, Wolfrum U (2010) Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J Cell Biol 189(1):171–186
Seeley ES, Nachury MV (2010) The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci 123(4):511–518
Simón MV, De Genaro P, Abrahan CE, de los Santos B, Rotstein NP, Politi LE (2012) Müller glial cells induce stem cell properties in retinal progenitors in vitro and promote their further differentiation into photoreceptors. J Neurosci Res 90(2):407–421
Singla V, Reiter JF (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313(5787):629–633
Slezak M, Grosche A, Niemiec A, Tanimoto N, Pannicke T, Münch TA, Crocker B, Isope P, Härtig W, Beck SC, Huber G, Ferracci G, Perraut M, Reber M, Miehe M, Demais V, Lévêque C, Metzger D, Szklarczyk K, Przewlocki R, Seeliger MW, Sage-Ciocca D, Hirrlinger J, Reichenbach A, Reibel S, Pfrieger FW (2012) Relevance of exocytotic glutamate release from retinal glia. Neuron 74(3):504–516
Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A (2008) Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol 317:246–259
Takeda M, Takamiya A, Jiao JW, Cho KS, Trevino SG, Matsuda T, Chen DF (2008) Alpha-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Invest Ophthalmol Vis Sci 49(3):1142–1150
Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A (2014) Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci USA 111(34):12438–12443
Valente EM, Rosti RO, Gibbs E, Gleeson JG (2014) Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 10(1):27–36
Wallingford JB, Mitchell B (2011) Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 25:201–213
Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM (2007) Sonic hedgehog promotes stem-cell potential of Müller glia in the mammalian retina. Biochem Biophys Res Commun 363:347–354
Wan J, Ramachandran R, Goldman D (2012) HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell 22(2):334–347
Wang L, Lang LL, Wang Y, Shi S, Liu L (2013) Prostaglandin E(2) enhances proliferation, dedifferentiation and stem-like properties of rat retinal Müller glial cells in vitro. Ophthalmic Res 49(2):100–107
Wheway G, Parry DA, Johnson CA (2014) The role of primary cilia in the development and disease of the retina. Organogenesis 10(1):69–85
Yoshimura K, Kawate T, Takeda S (2011) Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia 59(2):333–344
Acknowledgments
The authors wish to express their gratitude to A. Huerta (Cinvestav) and to all the members of Dr. Lamas’ laboratory for the discussions on these data. This work was supported by a grant from Conacyt (160614) to M. Lamas. M. Ramirez is the recipient of a Catedra Conacyt para Jovenes Investigadores (Conacyt).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferraro, S., Gomez-Montalvo, A.I., Olmos, R. et al. Primary Cilia in Rat Mature Müller Glia: Downregulation of IFT20 Expression Reduces Sonic Hedgehog-Mediated Proliferation and Dedifferentiation Potential of Müller Glia Primary Cultures. Cell Mol Neurobiol 35, 533–542 (2015). https://doi.org/10.1007/s10571-014-0149-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-014-0149-3