Abstract
The intrauterinal development in mammals represents a very sensitive period of life in relation to many environmental factors, including ionizing radiation (IR). The developing nervous system is particularly vulnerable to IR, and the consequences of exposure are of importance because of its potential health risks. The aim of our work was to assess whether prenatal irradiation of rats on the 17th day of embryonic development with a dose of 1 Gy would affect the formation of new cells and the number of mature neurons in the hippocampus and the selected forms of behaviour in the postnatal period. Male progeny of irradiated and control females was tested at ages of 3 weeks, 2 and 3 months. The number of mitotically active cells in the gyrus dentatus (GD) of the hippocampus was significantly reduced in irradiated rats aged 3 weeks. In irradiated rats aged 2 months, a significant reduction of mature neurons in CA1 area and in GD of the hippocampus was observed. The IR negatively influenced the spatial memory in Morris water maze, significantly decreased the exploratory behaviour and increased the anxiety-like behaviour in elevated plus-maze in rats aged 2 months. No significant differences were observed in animals aged 3 months compared with controls of the same age. A significant correlation between the number of mature neurons in the hilus and of the cognitive performances was found. Our results show that a low dose of radiation applied during the sensitive phase of brain development can influence the level of neurogenesis in the subgranular zone of GD and cause an impairment of the postnatal development of mental functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, it was assumed that formation of new neurons from neuronal stem cells (neurogenesis) is restricted to prenatal and early postnatal development and that the adult mammalian brain is unable to facilitate this process. Now, the adult neurogenesis is a generally accepted conception of neurobiology, based on large body of experimental evidences (Altman 1963; Gould et al. 1992; Cameron et al. 1993).
At least at two places of the brain, the subventricular zone and the subgranular zone (SGZ) of the gyrus dentatus (GD) of the hippocampus, the adult neurogenesis has been proved unambiguously (reviewed by Abrous et al. 2005; Zhao et al. 2008). It has been shown that in these brain structures in mammals, including humans, new neurons are produced by proliferation and differentiation of neuronal stem cells during the whole life (Taupin 2006; Gould 2007; Eriksson et al. 1998). Since this phenomenon was accepted, it became important to understand what triggers and what inhibits neurogenesis, how it is regulated, what its function is and how it is affected by various environmental factors.
It is known that ionizing radiation (IR) is able to selectively damage or kill dividing cells and therefore is often used as a methodical tool to stop cell proliferation (Hellström et al. 2009). Because of high mitotic activity during embryonic development, this phase of ontogenesis is especially sensitive towards IR.
Pregnant women are at risk of exposure to IR resulting from necessary medical procedures, workplace exposure, diagnostic or therapeutic interventions or accidental cases in nuclear power plants. The effects of radiation are directly related to the level of exposure and the stage of foetal development (Williams and Fletcher 2010). Besides the fact that in utero exposure to IR can have teratogenic, carcinogenic or mutagenic effects, several studies have suggested that it can induce cognitive deficits in adult animals (Raber et al. 2004; Madsen et al. 2003; Snyder et al. 2005).Their results indicated that these cognitive impairments could be associated with radiation-induced inhibition of hippocampal neurogenesis (HNG).
The underlying mechanisms responsible for radiation-induced cognitive impairment remain, however, elusive. The possible mechanism includes alterations in the neurogenic cell populations in GD (Rola et al. 2004; Winocur et al. 2006; Monje and Palmer 2003; Saxe et al. 2006), loss of mature neurons in GD (Raber et al. 2004; Fan et al. 2007), alterations in NMDA receptor subunits (Shi et al. 2006), genetic risk factors (Villasana et al. 2006) and lower expression of the immediate-early gene Arc (activity-regulated cytoskeleton-associated protein) (Rosi et al. 2008).
These cognitive dysfunctions often manifest as deficits in hippocampal-dependent learning and memory, including spatial information processing (Abayomi 1996; Crossen et al. 1994; Roman and Sperduto 1995; Surma-aho et al. 2001). The MWM task has often been used in the validation of rodent models for neurocognitive disorders and the evaluation of possible neurocognitive treatments. Lesions in distinct brain regions like hippocampus, striatum, basal forebrain, cerebellum and cerebral cortex were shown to impair MWM performance (D’Hooge and De Deyn 2001; Brandeis et al. 1989). Data showing a positive correlation between dentate neurogenesis and behavioural performance (Kempermann 2002; van Praag et al. 1999) indicate that newly born neurons from SGZ are functionally integrated into the hippocampal circuitry (Ramirez-Amaya et al. 2006; Zhao et al. 2006). However, the mechanisms of the integration of new neurons into the existing neuronal network and their exact role in brain functions need to be clarified.
The aim of our experiments was to assess whether whole-brain irradiation with a very low dose of gamma rays during the sensitive period of the embryonic development of the brain will influence the level of HNG and the number of mature neurons in the hippocampus of adult rats. We wished to see also whether the possible suppression of HNG or decrease of the number of mature neurons will correlate with changes in hippocampal-dependent types of behaviour.
Materials and Methods
Animal Model and Experimental Design
Albino Wistar rats were used in the experiments. Rats were maintained on LD 12:12 light regimen (12:12 h light/dark cycles with light on at 7 a.m.). They were fed with a standard laboratory diet and tap water ad libitum. Six-month-old females (n = 10) were allowed to mate with males (n = 10) of the same age. The male progeny of irradiated (n = 29) and of sham-irradiated mothers (n = 17) was housed under standard conditions and used in further experiments. The experiments were performed in accordance with European Community’s legislation. The experiments were approved by the State Veterinary and Alimentary Administration of the Slovak Republic.
Irradiation Procedure
Six pregnant females were exposed to a dose of 1 Gy of gamma rays on the 17th day of gravidity. Irradiation of animals was carried out in the irradiation facility Chisostat (Chirana, Prague, Czech Republic) equipped with a source of gamma rays (60-Co). Four pregnant females were sham-irradiated on the same day of gravidity and represented the control group. The animals were placed in special cages to limit vertical movement during irradiation to provide the same distance between the animals and the radiation source. The controls were manipulated in the same way, with the exception of irradiation.
Behavioural Analyses
Two-month-old (number of control animals, nC = 6; number of irradiated animals, nIR = 8) and 3-month-old rats (nC = 5, nIR = 8) were tested for learning and memory in MWM and for the level of anxiety and exploratory behaviour in elevated plus maze test (EPM) and in open field (OF). The behaviour of animals during testing was video-recorded and evaluated using the computerized video-tracking system Smart Junior (Panlab, Barcelona, Spain).
The OF sized 100 × 100 cm was used, bounded by white wooden walls of 60 cm height. This apparatus was used to measure the locomotor activity (given as the distance walked, number of crossings of the centre and the average speed of walking), the exploratory activity (number of rearings) and the comfort behaviour (given as the number of “face washing” acts and duration of washing). At the start of the test, the rat was placed into the centre of the field. Each trial lasted 8 min.
The EPM with two open and two closed arms of the length of 80 cm and a central square sized 15 × 15 cm was used. The arms of the maze were in a height of 75 cm above the ground. The level of anxiety (time spent in the open arms and the number of defecation boluses), the exploratory activity (number of rearings), the comfort behaviour (number of “face washing” acts) and the locomotor activity (number of crossings of the centre) were recorded in this test. While starting the test, the animal was placed into the central square of the maze. Each trial lasted 5 min.
Water maze training apparatus was prepared according to Morris (1984). The MWM apparatus consisted of a plastic circular pool (100 cm diameter and 50 cm high) filled with water up to 25 cm. The water was made opaque by the addition of powdered milk. A submerged escape platform (15 × 15 cm) was placed 2 cm under the water surface in the southeast quadrant of the maze. A variety of extramaze visual cues were visible from within the maze. The experimenter and an assistant remained at fixed locations approximately 0.5 m away from the outside edge of the tank on each trial. A day before the start of the experiment, the animals were habituated to the maze (without platform) for 2 min. All rats started from the same start position, namely from the opposite the quadrant where the escape platform was placed during acquisition. The time needed to find the hidden platform was measured for each animal. The rats were tested 3 times in 3-hour intervals on the 1st day of the experiment (testing of learning and short-term memory). A repeated test was performed on the 8th experimental day (testing of long-term memory).
BrdU Administration, Perfusion and Fixation
The level of HNG in irradiated and control rats was assessed 7 days after finishing the behavioural tests. All age categories were investigated (3 weeks: nC = 6, nIR = 9; 2 months: nC = 6, nIR = 10; 3 months: nC = 5, nIR = 10). To label the proliferating cells in the hippocampus, the animals received i.p. injections of 5-bromo-2-deoxyuridine (BrdU, Sigma) in a daily dose (100 mg/kg) for three consecutive days. Two hours after the last injection, the rats were anesthetized (chloral hydrate, 400 mg/kg, i.p.) and perfused transcardially (through the left heart ventricle) with 0.9 % saline followed by 4 % fresh paraformaldehyde solution. The brains were removed, postfixed for 24 h in 4 % paraformaldehyde and transferred to 30 % sucrose solution for cryopreservation. Consecutively, 33-μm-thick coronal sections of brain were cut on cryostat at the level of bregma −3.3 ± 0.2 mm. Seven slices containing the hippocampus (every sixth section) were used for further analysis.
Labelling of Proliferating Cells with BrdU
The BrdU-labelled cells were visualised immunohistochemically. The sections were incubated in 2 M HCl for 30 min at 59 °C and rinsed in 0.1 M boric buffer, at pH 8.5 for 30 min. Endogenous peroxidase reactivity was inhibited by 20 % methanol containing 0.3 % hydrogen peroxide for 30 min. Nonspecific protein activity was blocked in PBS containing 10 % normal goat serum (NGS, Vector Laboratories, Burlingame, CA) and 0.3 % Triton-X 100, for 30 min at room temperature. Then the sections were incubated with monoclonal rat primary antibody against BrdU (1:400, AbD Serotec, UK) in 5 % NGS and 0.3 % Triton-X 100 in PBS at 4 °C overnight followed by 2 h in goat anti-rat IgG biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) in 5 % NGS and 0.3 % Triton-X 100 at room temperature. Sections were incubated with avidin–biotin peroxidase system (ABC, Vector Laboratories, Burlingame, CA) for 1 h at room temperature. Antibody binding was detected by incubation with 3′,3′-diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA). Sections were mounted onto adhesive microscope slides (HistoBond®), dehydrated and coverslipped using Vectamount (Vector Laboratories, Burlingame, CA).
Labelling of Mature Neurons Using NeuN
All age categories were investigated (3 weeks: nC = 6, nIR = 9; 2 months: nC = 6, nIR = 5; 3 months: nC = 5, nIR = 10). The visualisation of mature neurons was established by NeuN-staining. Immunohistochemistry was performed on the prepared coronal free-floating sections. Briefly, the sections were incubated overnight at 4 °C with NeuN antibody (Merck Millipore, Czech Republic, 1:500) in 0.1 mol/l PBS (pH 7.4) with 0.2 % Triton. After washing with 0.1 mol/l PBS (pH 7.4) with 0.2 % Triton, secondary anti-mouse IgG antibody (Vector laboratories, Burlingame, USA, 1:200) was applied for 90 min at room temperature. After further washing, ABC Elite (Vector Laboratories, Burlingame, USA) was applied for 90 min, then the slides were rinsed with PBS followed by Tris Buffer (pH 7.6) and reacted with DAB (0.1 mol/l Tris, 0.04 % DAB, 0.033 % H2O2); the reaction was stopped with phosphate buffer. The slides were dehydrated, cleared and coverslipped for analysis.
Cell Counts and Statistical Analysis
Both BrdU- and NeuN-labelled cells were counted microscopically using the computer software Image Tool (UTHSCSA, San Antonio, USA). Neuronal cell count was performed by a person who was unaware of the treatment conditions. Analysis was performed by cell number quantification in every sixth section (165 µm separation distance, 5–8 sections per animal). BrdU-positive cells were counted (at 100× magnification) in photomicrographs in the SGZ and hilus of GD (Fig. 1). NeuN-positive pyramidal cells were counted in the middle of the linear zone of the CA1 region (at 200× magnification), in the hilus (at 100× magnification) and in the granule cell layer (GCL) (at 200× magnification). NeuN-positive label in CA1 region and in GCL is expressed as the number of neurons per 400 μm. For the hilus region, where the labelled neurons were seen apart from each other, the neuron numbers were counted (Fig. 2). Cells were counted only if structures of appropriate size and shape were demonstrated clearly. Questionable structures were examined under 400× magnification and were not counted if identification remained uncertain.
BrdU-positive cells (arrows) in the subgranular zone (SGZ) of the gyrus dentatus in the hippocampus of a control (C) and b irradiated (IR) animals. Magnification: ×100; c number of BrdU-positive cells in SGZ and hilus (H) of the hippocampus of IR and control C rats aged 3 weeks (3w), 2 months (2 m) and 3 months (3 m). Results are expressed as mean ± SEM. ***P < 0.001
NeuN-positive cells in a, b CA1-area and a’, b’, c’ hilus (H) and granule cell layer (GCL) of gyrus dentatus in the hippocampus of a, a’ control (C) and b, b’ irradiated (IR) animals. Magnification: 100×; c number of NeuN-positive cells in the CA1-area, H and GCL of the hippocampus of IR and C rats aged 3 weeks (3w), 2 months (2 m) and 3 months (3 m). Results are expressed as mean ± SEM. ***P < 0.001; *P < 0.05
The experimental data were analysed using two-way ANOVA and the unpaired t test. Differences were considered significant at P < 0.05.
Results
Irradiation of developing rat embryos on the 17th day of embryogenesis caused a high decrease (P < 0.001) of neurogenesis in GD of the hippocampus, assessed by BrdU-labelling in animals aged 3 weeks, in comparison with non-irradiated controls, but no significant differences were observed in animals aged 2 or 3 months (Fig. 1).
The number of mature neurons in CA1 region and in GD of the hippocampus, assessed by NeuN-staining, was highly decreased (CA1: P < 0.001; hilus: P < 0.001; GCL: P < 0.05) in irradiated rats aged 2 months, but not at age of 3 weeks or 3 months, compared with controls of the same age (Fig. 2).
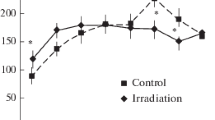
In two age categories (2 and 3 months), we performed behavioural testing to determine whether reductions in the indicators of neurogenesis and in number of mature neurons were associated with cognitive impairments. We first looked for radiation-induced alterations in exploratory activity in a novel environment, locomotor activity and anxiety levels and measures that could conceivably influence performance in later tests of hippocampal-dependent learning and memory. Data from OF (Table 1) indicated that there were no radiation-induced alterations. In contrast, the exploratory behaviour was significantly lower and the level of anxiety measured was higher in EPM in irradiated animals aged 2 months than in controls. These differences were not present in animals aged 3 months (Fig. 3).
We used MWM to determine whether the observed cellular changes were associated with alterations in hippocampal-dependent spatial memory. The ability of learning and the short-term memory in MWM were not affected by irradiation. Shortening of escape latency was recorded with each iteration of the test. The long-term memory was significantly impaired (P < 0.05) in irradiated animals aged 2 months. Animals needed significantly longer time to locate the hidden platform compared with age-matched controls (Fig. 4a). No significant differences were found in animals aged 3 months (Fig. 4b).
Time needed to find the hidden platform in Morris´s water maze in irradiated (IR) and control (C) rats aged a 2 (2 m) and b 3 (3 m) months tested on the 1st experimental day (1D) during 3 sessions (1T, 2T, 3T) in 3-h intervals and on the 8th experimental day. Data given as arithmetic mean ± SEM. *P < 0.05
We found a significant linear regression with high correlation of data between the number of NeuN-stained neurons in hilus and the cognitive performances of individual animals in MWM at the 3rd trial on day 1 (Fig. 5a) and on day 8 (Fig. 5b).
The relationship between the number of NeuN-positive cells in the hilus of the hippocampus and a the short-term memory performance in irradiated rats (2 mIR) aged 2 months in 3rd session on the 1st experimental day (1D3T) given as the time needed to find the hidden platform in MWM. The linear regression coefficient b = −0.5799, the correlation coefficient r = −0.8572, P < 0.05; b the long-term memory performance in 2 mIR on the 8th experimental day (8D), given as the time needed to find the hidden platform in MWM. The linear regression coefficient b = −2.3614, the correlation coefficient r = −0.88679, P < 0.01
Discussion
The effects of IR on the foetal development in mammals has been studied extensively (e.g. by Hamdorf et al. (1990); Bruni et al. 1993; Sienkiewicz et al. (1999); Kitamura et al.(2001)) in animal experiments, as well as in humans exposed to diagnostic or therapeutic radiation (Donnelly et al. 2011). Bromet et al. (2011) and Shaw et al. (2011) reviewed the effects of IR on atomic bomb attack survivors and on the victims of nuclear power plant accidents. These studies document that relatively low doses of IR applied during intrauterine development cause changes in structure and functions of the brain which persisted into adulthood.
Sensitivity to radiation (dose–response) is markedly similar among all mammalian species when equivalent developmental periods are compared (Schull et al. 1990). The foetus is most susceptible to radiation during organogenesis in the period from 5th up to 13th day of gravidity in mice (Russell and Russell 1954), from 8th to 15th day in rats (Brent and Gorson 1972; Shaw et al. 2011; Rice and Barone 2000), which corresponds to 2nd to 7th week after conception in humans (Williams and Fletcher 2010) as well as in the early foetal period from 13th to 20th day in mice (Russell and Russell 1954), from 15th to 20th day in rats (Brent and Gorson 1972) and from 8th to 15th week after conception in humans (Williams and Fletcher 2010).
The foetal period (13th–18th day of gestation) in small rodents represents an active phase of brain development involving proliferation, migration and differentiation of cells in cerebral cortex and associated structures, which corresponds to a period starting with 4th week after conception in humans (Tau and Peterson 2010). Exposure at this period of development to moderate doses of gamma radiation can induce permanent deficits in the brain histology, which can adversely affect the learning and memory in adult age (Hossain et al. 2005).
In our experiments, irradiation of the developing brain with a very low dose of gamma rays on the 17th day of embryogenesis caused a marked decrease in the number of mitotically active cells in SGZ of GD in early phases of life. We noticed a significant decrease of BrdU-positive cells in SGZ and in the hilus in rats aged 3 weeks, in comparison with non-irradiated controls. No significant differences were observed in animals aged 2 or 3 months. Tomášová et al. (2012) found no statistically significant differences in the level of HNG in GD in 3-month-old irradiated (1 Gy of gamma rays on the 16th day of gravidity) and control rats too. The authors considered this finding as a result of regeneration processes of the brain tissue after irradiation with a very low dose of IR. This conclusion is supported by results of Isono et al. (2012). They irradiated neural stem cells with X-rays with a dose of 1 Gy and have seen that the cells stopped proliferation only temporarily. In contrast, the stem cells ceased proliferation following irradiation with a dose of over 5 Gy. Fan et al. (2007) also demonstrated that radiation doses of 5–10 Gy could cause persistent reduction in precursor cell proliferation in GD by more than 90 %.
We observed significant changes in the number of BrdU-positive cells between the age groups of control animals. Our results confirm that the mitotic activity of cells in GD decreases with age. Significant reduction in proliferative activity with age was also observed by Ben Abdallah et al. (2008).
In young adult rats, aged 2 months, we found a significant decrease in the number of mature neurons (NeuN-positive cells) in CA1 region, in hilus and in GCL of the hippocampus. Since new neurons leaving the SGZ migrate into the adjacent GCL (Jin et al. 2001), this finding is consistent with reduced neurogenesis in rats aged 3 weeks. We also suppose that this reduction could be due to the irradiation during the sensitive period of intrauterine development, when intensive differentiation of neuronal cells runs.
Radiation doses as low as 0.5–1.5 Gy applied on the 14th–17th days of intrauterine development have been found to significantly reduce the number of neurons in various parts of the hippocampus (Eliott and Grunberg 2005; Gross 2000; Minamisawa and Hirokaga 1996). In the experiments of Hossain et al. (2005), the exposure of mice to 1 Gy at 14th or 17th day of gestation resulted in significant decrease in the number of neurons in the CA3 and CA4 regions of hippocampus, while a dose of 1.5 Gy significantly affected the CA1 region too. Miki et al. (1999) also noticed the histological changes in the CA3 region after prenatal irradiation of rats, but they did not register any changes in GD and CA1 region. We also did not observe differences in the number of mature neurons in animals aged 3 months.
In our work, the decrease in the number of mature neurons in irradiated rats aged 2 months corresponded with changes in innate behaviour and with decreased performance in spatial memory test.
We tested innate behaviour, that does not require any laboratory training, in OF and EPM. The data from OF revealed no radiation-induced alterations. Similarly, Jensh et al. (1986, 1987) did not observe behavioural changes in this test in rats irradiated with doses of 0.1, 0.2 and 0.6 Gy on the 9th and 17th days of pregnancy. In contradiction to these findings, Tomášová et al. (2012) recorded an increase in locomotory and exploratory activities in prenatally (16th day of gravidity) irradiated (1 Gy) rats in OF. Sienkiewicz et al. (1992, 1999) also observed increased locomotor activity after exposure to a 1 Gy irradiation dose on the 15th day of gravidity. Exposure on this day produced no deficit in performance on the spatial task, but caused a highly significant deficit in the visually cued task. Thus, increased locomotor activity could be the way how animals compensated impaired visual ability. Exposure on day 18 produced a highly significant deficit in performance on the spatial task and an improvement in the cued task. Exposure on day 13 produced no significant deficits on either task. These differential effects on performance appear to be consistent with radiation-induced insult to different memory systems within the developing brain. Conversely, Hossain and Devi (2000) noticed dose-dependent suppression of activities in OF. Baskar and Devi (2000) found out that prenatally irradiated mice showed a dose-dependent suppression of locomotory and exploratory activities in OF. These discrepancies may be due to inter-strain genetic differences, different gestational age at irradiation and different age at testing. Gestational age at the time of exposure to IR seems to be the most important factor in determining the nature of the insult to the developing brain (Schull and Otake 1999).
In contrast, the exploratory behaviour and locomotor activity were significantly lower and the level of anxiety was higher measured in EPM test in irradiated animals aged 2 months than in controls. Tomášová et al. (2012) also registered an increase of anxiety in irradiated rats, expressed by significantly shortening the time spent in the open arms of the maze. Low level of exploration and increased anxiety were the reaction of animals to the new, potentially dangerous environment also in studies of Ramos and Mormede (1998). Measurement of anxiety in EPM can be influenced by motor activity in as much that a rat that shows reduced levels of activity will also show reduced exploration. It is likely that the decreased exploration of the open arms in 2 months aged animals was due to radiation-induced changes in the level of motoric activity.
Since cells involved in neurogenesis are extremely sensitive to low and moderate doses of IR (Tada et al. 2000; Monje et al. 2002; Mizumatsu et al. 2003), reduced production of new neurons in GD might play a role in radiation-induced cognitive impairments.
There are evidences that adult neurogenesis has a role in the learning process (Shors et al. 2001, 2002; Madsen et al. 2003; Raber et al. 2004) and that new neurons produced in GD of adult rats are required for long-term memory in a spatial version of the water maze( Snyder et al. 2005).
In our experiment, the reduction of the number of mature neurons in 2-month aged irradiated animals was recorded. In this experimental group, also an impaired long-term memory, manifested as a significant prolongation of time needed to locate the hidden platform in MWM, was observed. There is a possibility that these records are associated with decreased exploratory behaviour and increased anxiety observed in these animals. One of the possible explanations of impairment function of GD and CA1 region may be a reduction of neuronal progenitor cells by IR and its consequent impact on the neuronal population of granular and pyramidal neurons.
Baskar and Devi (1996, 2000) showed that very low doses (0.25–0.50 Gy) of gamma rays applied during sensitive phases of intrauterine development (11.5, 12.5, 14.5 or 17.5 day of gravidity) cause learning disorders and memory dysfunctions in mice and that the intensity of damage increases with the dose of radiation and was higher during the late organogenesis (11.5 and 12.5 day of gravidity).
In the study of Tomášová et al. (2012), the prenatal irradiation negatively influenced the short-term spatial memory of rats in MWM, although the long-term memory was not impaired. Sienkiewicz et al. (1999) also observed the spatial memory deficits when irradiated mice with 1 Gy of gamma rays on the 18th day of intrauterine development. Neurogenesis in the hippocampus of rodents, especially in GD, runs intensively on this day of gravidity (Rodier 1980). Takai et al. (2004) and Zhang et al. (2007) recorded the significant memory impairment in MWM in rats aged 2 months irradiated on the 15th day of embryonic development with 1.5 Gy. Jensh et al. (1986, 1987) observed no significant alterations in MWM-behaviour of rats aged 2 months irradiated on the 9th and 17th days of gestation with doses in a range of 0.1–0.6 Gy. These doses of radiation appeared to have been probably too low to damage mitotically active cells and mature neurons. However, when they used the dose of 2 Gy on the 17th day of gestation, morphological and behavioural changes were recorded (Jensh et al. 1995).
In addition, we found a significant direct relationship between the number of NeuN-positive cells in the hilus of hippocampus and the spatial memory in MWM. Yan et al. (2007) also detected a significant correlation between these parameters. Hilus belongs to one of the layers of GD, which is believed to be involved in learning and memory. Loss of hilus neurons in old people has been linked to the decline in relational memory (West 1993). Thus, our present data, along with the findings of other investigators (Sienkiewicz et al. 1999; Rola et al. 2004; Monje 2008), suggest that radiation-induced spatial memory impairment may be due to the hippocampal neuronal injury or neurodegeneration caused by irradiation.
In parallel, both the neuronal changes in hippocampus and changes in behavioural parameters disappeared with age. We recorded the increment in the total number of neurons in irradiated rats aged 3 months compared with the rats aged 2 months. We can hypothesize that if prenatal irradiation caused the loss of neural precursor cells, this could be compensated by production of new neurons in the process of HNG in GD or regeneration process in CA1 region later during the life. These processes may also be interconnected. It has been shown that neurogenesis may respond to various pathological stimuli. Damaged brain regions, whether neurogenic or not, may be able to initiate regeneration processes (Thompson et al. 2008). Pathological changes within the hippocampal trisynaptic circuit can stimulate neurogenesis after acute injury of the hippocampus (Kuhn et al. 1997). Chronic icv infusions of bFGF and EGF greatly enhanced the potential of endogenous progenitors to proliferate in response to injury and the cells regenerated into CA1 pyramidal neurons. The regenerated neurons were integrated into the neural circuitry as they received afferences, projected onto their normal target, the subiculum, and reversed the deficits in cognitive functions (Nakatomi et al. 2002). This constitutes the most impressive example of network reconstruction leading to behavioural recovery that takes advantage of adult endogenous neurogenesis. In summary, enhancement of neurogenesis in the DG and other brain regions may compensate for disconnected circuits that occur after injury and contribute to the improvement of functional outcome (Abrous et al. 2005). Analyses made at different ages could show therefore different stages of these processes in the brain tissue.
Our results show that a dose of radiation as low as 1 Gy received during the sensitive phase of brain development can cause an impairment of the postnatal development of mental functions.
References
Abayomi OK (1996) Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 35:659–663
Abrous DN, Koehl M, Le Moal M (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569
Altman J (1963) Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec 145:573–591
Baskar R, Devi PU (1996) Long-term effects of prenatal exposure to low level of gamma radiation in neurophysiology of mouse. Indian J Exp Biol 34:887–890
Baskar R, Devi PU (2000) Influence of gestational age to low level gamma irradiation on postnatal behavior in mice. Neurotoxicol Teratol 22:593–602
Ben Abdallah NM-B, Slomianka L, Vyssotski AL, Lipp H-P (2008) Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging 31(1):151–161
Brandeis R, Brandys Y, Yehuda S (1989) The Use of the Morris Water Maze in the Study of Memory and Learning. Int J Neurosci 48:29–69
Brent RL, Gorson RO (1972) Radiation exposure in pregnancy. Curr Probl Radiol 2:1–48
Bromet EJ, Havenaar JM, Guey LT (2011) A 25 year retrospective review of the psychological consequences of the Chernobyl accident. Am J Clin Oncol 23(4):297–305
Bruni JE, Persaud TVN, Huang W, Froese G (1993) Postnatal development of the rat CNS following in utero exposure to a low dose of ionizing radiation. Exp Toxicol Pathol 45(4):223–231
Cameron HA, Woolley CS, McEwen BS, Gould E (1993) Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Eur J Neurosci 56:337–344
Crossen JR, Garwood D, Glatstein E, Neuwelt EA (1994) Neurobehavioral sequel of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol 12:627–642
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 36:60–90
Donnelly EH, Smith JM, Farfán EB, Ozcan I (2011) Prenatal radiation exposure: background material for counseling pregnant patients following exposure to radiation. Disaster Med Pub Health Preparedness 5(1):62–68
Eliott BM, Grunberg NE (2005) Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res 165:187–196
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317
Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J (2007) Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci 25:38–46
Gould E (2007) How widespread is adult neurogenesis in mammals? Nat Rev Neurosci 8:481–488
Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS (1992) Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci 12:3642–3650
Gross CG (2000) Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci 1:67–73
Hamdorf G, Shahar A, Cervós-Navarro J, Scheffler A, Sparenberg A, Skoberla A (1990) Morphological changes in cultures of hippocampus following prenatal irradiation in the rat. J Neurosci Res 26(3):327–333
Hellström NAK, Björk-Eriksson T, Blomgren K, Kuhn G (2009) Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells 27:634–641
Hossain M, Devi PU (2000) Effect of irradiation at the early fetal stage on adult brain function in the mouse: locomotor activity. Int J Radiat Biol 76(10):1397–1402
Hossain M, Chetana M, Devi PU (2005) Late effect of prenatal irradiation on the hippocampal histology and brain weight in adult mice. Int J Dev Neurosci 23:307–313
Isono M, Otsu M, Konishi T, Matsubarad K, Tanabea T, Nakayamae T, Inouea N (2012) Proliferation and differentiation of neural stem cells irradiated with X-rays in logarithmic growth phase. J Neurosci Res 73:263–268
Jensh RP, Brent RL, Vogel WH (1986) Studies concerning the effects of low level prenatal x-irradiation on postnatal growth and adult behaviour in the wistar rat. Int J Radiat Biol Relat Stud Phys Chem Med 50(6):1069–1081
Jensh RP, Brent RL, Vogel WH (1987) Studies of the effect of 0,4 and 0,6 Gy prenatal X-irradiation on postnatal and adult behavior in the Wistar rat. Teratology 35:53–61
Jensh RP, Eisenman LM, Brent RL (1995) Postnatal neurophysiologic effects of prenatal x-irradiation. Int J Radiat Biol 67(2):217–227
Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA (2001) Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. PNAS 98:4710–4715
Kempermann G (2002) Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 22:635–638
Kitamura M, Itoh K, Matsumoto A, Hayashi Y, Sasaki R, Imai Y, Itoh H (2001) Prenatal ionizing radiation-induced apoptosis of the developing murine brain with special references to the expression of some proteins. Kobe J Med Sci 47:59–76
Kuhn HG, Winkler J, Kempermann G et al (1997) Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci 17:5820–5829
Madsen TM, Kristjansen PEG, Bolwig TG, Wortwein G (2003) Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience 119:635–642
Miki T, Sawada K, Sun XZ, Hisano S, Takeuchi Y, Fukui Y (1999) Abnormal distribution of hippocampal mossy fibres in rats exposed to X-radiation in utero. Dev Brain Res 112:275–280
Minamisawa T, Hirokaga K (1996) Long-term changes on open-field activity of male mice irradiated with low levels of gamma rays at late stage of foetal development. J Radiat Res 37:117–124
Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR (2003) Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res 63:4021–4027
Monje M (2008) Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev 14:238–242
Monje ML, Mizumatsu S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955–962
Monje ML, Palmer T (2003) Radiation injury and neurogenesis. Curr Opin Neurol 16:129–134
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Nakatomi H, Kuriu T, Okabe S, Yamamoto SI, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110:429–441
Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR (2004) Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162:39–47
Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA (2006) Integration of new neurons into functional neural networks. J Neurosci 26:12237–12241
Ramos A, Mormede P (1998) Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 22(1):33–57
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Persp 108:511–533
Rodier PM (1980) Chronology of neuron development: animal studies and their clinical implications. Dev Med Child Neurol 22:525–545
Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike J (2004) Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188:316–330
Roman DD, Sperduto PW (1995) Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys 31:983–998
Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR (2008) Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein). Cancer Res 68:9763–9770
Russell LB, Russell WL (1954) An analysis of the changing radiation response of the developing mouse embryo. Cell Physiol 43:103–149
Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci 103:17501–17506
Schull WJ, Otake M (1999) Cognitive function and prenatal exposure to ionizing radiation. Teratology 59:222–226
Schull WJ, Norton S, Jensh RP (1990) Ionizing radiation and the developing brain. Neurotoxicol Teratol 12(3):249–260
Shaw P, Duncan A, Vouyouka A, Ozsvath K (2011) Radiation exposure and pregnancy. J Vasc Surg 53(1):28S–34S
Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D’Agostino R, Brunso-Bechtold JK (2006) Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res 166:892–899
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 414:372–376
Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E (2002) Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12:578–584
Sienkiewicz ZJ, Saunders RD, Butland BK (1992) Prenatal irradiation and spatial memory in mice: investigation of critical period. Int J Radiat Biol 62:211–219
Sienkiewicz ZJ, Haylock RG, Saunders RD (1999) Differential learning impairments produced by prenatal exposure to ionizing radiation in mice. Int J Radiat Biol 75(1):121–127
Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852
Surma-aho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, Paetau A, Kallio M, Pyykkonen J, Jaaskelainen J (2001) Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology 56:1285–1290
Tada E, Parent JM, Lowenstein DH, Fike JR (2000) X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 99:33–41
Takai N, Sun X-Z, Ando K, Mishima K, Takahashi S (2004) Ectopic neurons in the hippocampus may be a cause of learning disability after prenatal exposure to X-rays in rats. J Radiat Res 45:563–569
Tau GZ, Peterson BS (2010) Normal development of brain circuits. Neuropsychopharmacol 35(1):147–168
Taupin P (2006) Adult neurogenesis in mammals. Curr Opin Mol Ther 8(4):345–351
Thompson A, Boekhoorn K, Van Dam AM, Lucassen PJ (2008) Changes in adult neurogenesis in neurodegenerative diseases: cause or consequence? Genes Brain Behav 7(1):28–42
Tomášová L, Šmajda B, Ševc J (2012) Effects of prenatal irradiation on behaviour and hippocampal neurogenesis in adult rats. Acta Physiol Hung 99(2):126–132
Van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci 96:13427–13431
Villasana L, Acevedo S, Poage C, Raber J (2006) Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res 166:883–891
West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging 14(4):287–293
Williams PM, Fletcher S (2010) Health effects of prenatal radiation exposure. Am Fam Physician 82(5):488–493
Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S (2006) Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16:296–304
Yan B, Bi X, He J, Zhang Y, Thakur S, Xu H, Gendron A, Kong J, Li X-M (2007) Quetiapine attenuates spatial memory impairment and hippocampal neurodegeneration induced by bilateral common carotid artery occlusion in mice. Life Sci 81:353–361
Zhang R, Sun X-Z, Cui Ch, Sakata-Haga H, Sawada K, Ye Ch, Fukui Y (2007) Spatial learning and expression of neural cells adhesion molecule L1 in rats X-irradiation prenatally. J Med Invest 54:322–330
Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications af adult neurogenesis. Cell 132:645–660
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Ingrid Obšitošová and Eva Petrovičová. The project was supported by research Grant VEGA No. 1/0292/12 and Institutional Grant of P.J. Šafárik University in Košice VVGS PF No. 2012/27.
Conflict of interest
Natália Kokošová, Lenka Tomášová, Terézia Kisková and Beňadik Šmajda declares that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kokošová, N., Tomášová, L., Kisková, T. et al. Neuronal Analysis and Behaviour in Prenatally Gamma-Irradiated Rats. Cell Mol Neurobiol 35, 45–55 (2015). https://doi.org/10.1007/s10571-014-0144-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-014-0144-8