Abstract
Ramie fibers have poor flame-retardant properties, which limits their application. To improve the flame-retardant properties of ramie fabric (RF), a durable flame-retardant coating was successfully realized on RF by combining covalent bonding and electrostatic adsorption. Si/P/N flame-retardant coatings were constructed on RF using cationic polyethyleneimine (PEI) and anionic sodium hexametaphosphate (PSP) via the layer-by-layer (LBL) assembly approach with the introduction of 3-glycidoxypropyltrimethoxysilane (GPTMS) as an organic cross-linker. Compared with the untreated RF samples, the fabrics treated with the flame-retardant coating PEI/PSP via the LBL method presented reductions of 51.06%, 48.30%, and 40.05% in the fire growth rate, peak heat release rate, and total heat release, respectively, in the cone calorimeter test. In addition, at a weight gain of 31.57%, the fabric self-extinguished in the UL-94 test within 10 s after leaving the ignition source, resulting in a damaged length of 6.13 cm. G-3 retained the limiting oxygen index (LOI) of 26.40% after 6 laundering cycles (LCs). The TG results revealed that the char residue of G-3 at 800 °C reached 30.34 wt%. The surface of the flame-retardant coating of GPTMS-PEI/PSP had good char formation. This study provides a feasible method for realizing durable flame-retardant RFs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural fibers are extensively utilized across various industries owing to their high availability, processability, and exceptional biodegradability (Jiang et al. 2023). Among these materials, ramie fabric (RF), as a natural cellulosic fabric, offers superior breathability and moisture absorption compared with cotton fabric. Owing to the smaller diameter of ramie fibers and their commendable tensile modulus/strength in contrast to cotton fibers, they serve as ideal filler materials in reinforced polymer composites (Ng et al. 2022; Khalili et al. 2020; Lu et al. 2023). Therefore, RFs show great promise for industrial applications because of their high mechanical properties (Wang et al. 2023a). However, akin to other natural fibers, ramie fibers primarily consist of cellulose, lignin, and hemicellulose, resulting in inadequate flame-retardant properties, thus restricting their applicability (Wang 2019; Zhang et al. 2013). Given the significant number of casualties attributed to fires annually, the implementation of flame-retardant treatments on ramie fibers has become imperative.
The traditional methods used to achieve flame retardancy for RFs are grafting, impregnation baking and layer-by-layer (LBL) assembly. Wang et al. (2021) employed polyethyleneimine (PEI) and ammonium polyphosphate (APP) materials to fabricate N/P coatings on RF via the LBL technique, successfully achieving the LOI of 30.6%. Wang et al. (2013) successfully assembled PEI/Cu or Zn ions onto the surface of fabrics via LBL, resulting in excellent flame retardant properties. Wang (2018) subsequently developed an efficient flame-retardant coating using a certain flame-retardant by impregnation and baking methods on RF. Jiang et al. (2023) grafted APP onto the hydroxyl groups of RF to impart flame retardant properties. All of the above methods can achieve better flame-retardant effects.
Among the array of technologies available for applying flame-retardant coatings onto fabric surfaces, the LBL approach stands out as a remarkably effective, enduring, and user-friendly strategy for bolstering the flame-retardant properties of fabrics (Alebeid and Zhao 2017; Pan et al. 2023; Li et al. 2019a). Owing to its emphasis on hydrogen bonding, particularly leveraging electrostatic adsorption of polyelectrolytes for constructing functionalized coatings, the LBL technique is widely applied in diverse fields, such as biology, medicine, and chemistry. The fine control of coating deposition allows researchers to design coatings with targeted functionalities (e.g., enhanced flame retardancy) while maintaining the essential properties of the fabrics (Yang et al. 2023; Zheng et al. 2022). This technology provides an effective way to improve the overall performance of the RF while addressing the flammability issue. Therefore, the functionalization of RF with flame-retardant coating via LBL technology is an appropriate approach.
Sodium hexametaphosphate (PSP) and PEI are considered to be polyelectrolytes with flame-retardant effects. Lazar et al. (2020) used chitosan and PSP to construct intumescent flame-retardant coatings on cotton fabrics via LBL, and 15 bilayers could self-extinguish. Li et al. (2011) used poly(allylamine) and PSP to construct intumescent nanocoatings on fabrics via LBL, and the fabrics were able to self-extinguish in vertical combustion tests. Vest et al. (2023) blended PEI and PSP to form a polyelectrolyte complex, and then used a buffer solution as a cross-linking agent, resulting in excellent flame retardant properties for flammable silk fabrics.
Traditional halogen-based flame retardants release a substantial quantity of toxic gases and exhibit significant corrosive properties after combustion because hydrogen halides pose severe environmental hazards. Consequently, they are being phased out (An et al. 2022; Ge et al. 2020; Vanderveen and Deboer 2012). Therefore, nitrogen-phosphorus-based intumescent flame retardants are increasingly supplanted with traditional flame retardants because of their increased safety (Yang et al. 2021). PEI, which is renowned for its robust adsorptive capacity, is predominantly utilized as a solvent in sewage treatment processes (Lu et al. 2022). Notably, its high nitrogen content makes it an ideal candidate for increasing the nitrogen content in flame-retardant coatings. In addition, PSP is an environmentally friendly flame retardant with high phosphorus content and no halogen, which is less polluting to the environment and is considered a green flame retardant (Chen et al. 2020). Finally, the silicon provided by 3-glycidyloxypropyltrimethoxysilane (GPTMS) was combined to construct an efficient Si/P/N ternary synergistic flame-retardant system. On the basis of the properties of the above materials, we constructed an environmentally friendly flame-retardant coating on RF.
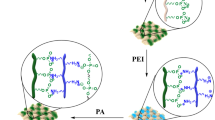
In this study, first, we used GPTMS for surface treatment to react with hydroxyl linkages. Immediately after that, the amine group in PEI was able to react with the epoxy group of GPTMS via a ring-opening reaction to form a covalent C‒N bond (Aghayan et al. 2021). The fabric was subsequently repeatedly immersed in a solution containing PEI and PSP by LBL to obtain the desired mass. In addition, the flame-retardant properties of these coatings were evaluated via a series of tests, including LOI tests, UL-94 vertical burning tests and cone calorimeter tests. To elucidate the inherent mechanism of the flame-retardant coatings, we also conducted a targeted analysis of the char residue of RF after combustion. In addition, gas-phase flame-retardant mechanisms for RF and G-3 were analyzed via TG-FTIR. This study provides some insights into the preparation of durable and environmentally friendly flame-retardant RFs.
Experimental
Materials and chemicals
RF was purchased from a ramie textile factory in Sichuan Province, China. PSP solid, PEI and GPTMS solutions were purchased from Shanghai Macklin Co., Ltd., and Yien Chemical Technology Co., Ltd. Additionally, 65% hydrochloric acid and anhydrous NaOH were obtained from Aladdin Reagent Co., Ltd., Shanghai, China. In addition, all the deionized water was obtained from the laboratory.
LBL assembly coating GPTMS-PEI/PSP on RF
The dried RF is immersed in a 5 wt% GPTMS solution at 60 °C for 3 h. The samples are designated G-RF and need to be washed with this solution for 5 min at the end of the coating assembly.
The fabrics were soaked in 3 wt% PEI solution for 5 min (pH adjusted to 8‒9 with 1 mol/L HCl) and squeezed at 1 min intervals. Then, the fabric was washed with deionized water for 1 min to remove excess weak electrolytes from the surface and dried in an oven at 65 °C for 45 min. Finally, the fabric was immersed in a 6 wt% PSP solution for 5 min, with the same post-treatment process as above. The above steps were repeated until the weight of the fabrics increased by 9.38%, 20.41% and 31.57% with respect to the original samples, and the resulting samples were named G-1, G-2 and G-3, respectively. The experimental flowchart of the flame-retardant coatings is shown in Fig. 1. The weight gain (wt%) of RF was calculated according to the following formula:
where W1 is the initial weight of the RF and W2 is the weight of the RF flame-retardant treated.
Characterization
The structural composition and functional groups of RF before and after flame-retardant coating were analyzed via a NEXUS 470 Fourier transform infrared spectrometer (Nicolet Instrument Corporation, Madison, WI, USA). Spectral data were collected in the range of 4000–400 cm−1 at a resolution of 4 cm−1, with 32 scans acquired for each spectrum. SEM analysis was performed via a scanning electron microscope (SEM, Regulus 8100, Hitachi, Japan) to observe the sample and its residue morphology after combustion, with sample surfaces treated with gold sputter coating and examined at an accelerating voltage of 5 kV. EDS analysis was conducted via energy dispersive X-ray spectroscopy (EDS, Octane Elect Plus, EDAX Inc.) on samples sputtered with a metal layer to enhance conductivity, and the results were examined at an accelerating voltage of 5 kV. XPS analysis of the char residues was performed via an ESCALAB Xi+ X-ray photoelectron spectrometer (Thermo Fisher Scientific Inc., China).
The flame retardancy of the samples was assessed via the LOI in accordance with the standard oxygen index test GB/T5454-1997 (oxygen index apparatus RSJ-5, Hesheng Analysis Instrument Co., Ltd., China). The vertical flammability test was performed with sample dimensions of 300 mm × 80 mm × 0.6 mm, following the guidelines of GB/T 2406–93 (HS-RSJ-5 apparatus, Hesheng Analysis Instrument Co., Ltd., China). The cone calorimetry test (iCone Classic, FTT, UK) for the RF was conducted via a FTT cone calorimeter, which applied a heat flux of 35 kW/m2, in compliance with the ISO 5660 standard.
The degree of graphitization of the char residues was analyzed via Raman spectroscopy with a DXR2xi spectrometer (Thermo Fisher Scientific, USA). The thermal stability of the fabrics was examined via a DTG-60H (Nicolet Instrument Corporation, Madison, WI, USA) thermal analyzer under a nitrogen atmosphere at a heating rate of 10 °C/min, over a temperature range from 30 °C to 800 °C. Each sample had a mass of 5‒10 mg. The degradation of the samples resulting from the thermal decomposition of the fabrics was analyzed via Fourier transform infrared spectroscopy in conjunction with thermogravimetric analysis (TG-FTIR) (Nicolet Instrument Corporation, Madison, WI, USA). In addition, the fabrics were soaked in water at 45 °C for 40 min with 0.3 wt% detergent to test the washing resistance of the flame-retardant coatings.
Results and discussion
FTIR characterization and XPS spectroscopy of the coated RF
LBL and covalently bonded GPTMS-PEI-PSP were used to construct flame-retardant coatings on RF. The formation of the GPTMS-PEI/PSP hybridized network structure was investigated via FTIR and XPS spectra.
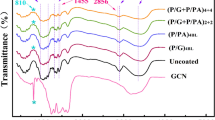
FTIR spectroscopy was used to determine the molecular characteristics of the G-RF and GPTMS-PEI/PSP coatings on RF. As illustrated in Fig. 2, the bands at 3460, 2820, 1585 and 1164 cm−1 in the RF spectrum are characteristic bands of ramie fibers (Cheng et al. 2019; Chen et al. 2023; Liao et al. 2021). In the G-RF spectra, the shift of the –OH peak suggests that the hydroxyl groups in the fabric may have undergone a reaction (Hu et al. 2023). In addition, the spectra of G-RF showed different characteristic peaks. Among these peaks, the peak at 2906 cm−1 is characteristic of the –CH2– of GPTMS, the peak at 1058 cm−1 corresponds to the Si–O stretching vibration of GPTMS, and the peak at 1112 cm−1 corresponds to the Si–O–C bond (Li et al. 2022). Covalent bonds formed between GPTMS and RF through hydrolysis and condensation reactions. In addition, the characteristic peak at 901 cm−1 belongs to the unique epoxy group of GPTMS, which further proves that GPTMS reacted with the fabric (Hu et al. 2023). However, the peak intensity of the epoxy group at 901 cm−1 disappears completely in the spectra of GPTMS-PEI/PSP, suggesting a ring-opening reaction between the epoxy group in GPTMS and the amino group in PEI (Fang et al. 2023; Chen et al. 2023). In addition, the spectra of GPTMS-PEI/PSP showed new peaks at 1641, 1315, 1282 and 1029 cm−1, which are attributed to the C–H and C–N stretching vibrations of PEI (Qi et al. 2022b) and the P=O and P–O vibrations of PSP, respectively (Yang et al. 2016a; Li et al. 2019b; Palen et al. 2022). The above results indicate that the GPTMS-PEI/PSP flame-retardant coatings were successfully constructed on the surfaces of the fabrics via LBL (Jenny et al. 2012; Shi et al. 2022).
To further demonstrate whether GPTMS, PEI and PSP were successfully modified onto the fabrics, the XPS spectra of RF and G-3 were examined. As shown in Fig. 3, the characteristic peaks of C 1s and O 1s at 285.2 and 532.1 eV were detected in RF and G-3, respectively (Jiang et al. 2019). In contrast to RF, G-3 showed new peaks at 400.1, 191.4, 134.2, 153.3 and 102.3 eV belonging to N 1s, P 2s, P 2p, Si 2s and Si 2p, respectively. Specifically, the N 1s spectrum of G-3 shows two peaks at 398.9 and 400.9 eV, which are attributed to the C–N and N–H of PEI (Alongi et al. 2012). Similarly, the P 2p spectrum shows two peaks at 133.6 and 134.7 eV, which are attributed to the C–O–P and P=O of PSP, respectively (Alongi et al. 2012). Finally, the Si 2p spectra showed a peak at 102.1 eV, which is attributed to the Si–O of GPTMS (Miao et al. 2021). The combination of the XPS and FTIR results demonstrated that the GPTMS-PEI/PSP system was successfully attached to the fabric surface.
Surface morphology and elemental analysis of RF
As depicted in Fig. 4, the untreated RF fibers exhibited relatively smooth characteristics, featuring a tidy and consistent arrangement of fibers. However, following treatment, substantial alterations occurred on the surface of the ramie fibers. A conspicuous coating developed on the surface of the treated fibers, resulting in surface roughening and the discernible presence of solid particles. These transformations stem from the attachment of flame retardants to the surface of the fabric.
In addition, as shown in Fig. 5, the elemental composition of G-3 was also analyzed via EDS, which revealed that the P content on the fabric surface was 4.07 wt%, the N content was 6.32 wt%, and the Si content was 0.45 wt%. All the elements can be uniformly distributed on the fabric surface, which indicates that the dispersion of the flame-retardant coating is relatively good and that no aggregation phenomenon occurs.
LOI and UL-94 test
Table 1 lists the LOI test results for each coating. The LOI for RF was only 19.5%, indicating a high degree of fire hazard. However, the flame retardancy of the fabric improved as the weight of the LBL flame retardant coating increased. Specifically, with a 9.38% increase in fabric weight, the LOI increased to 24.20%. Furthermore, as the weight of the fabric increased by 31.57%, the LOI significantly improved to 30.20%, demonstrating a notable increase in flame retardancy.
As shown in Fig. 6 and Table 1, in the UL-94 test, the untreated RFs burned out in a short period of time, leaving no residual char. G-1 and G-2 leave a more complete char layer than RF does. After 20 s of ignition, the flame length of G-2 was significantly shorter than that of G-1, indicating a decrease in the heat release rate. When the mass of the sample was increased to 31.57%, the flame spread in the G-3 sample was significantly inhibited, and the sample self-extinguished within 10 s after leaving the ignition source, resulting in a damage length of 6.13 cm. In addition, the water washing resistance of G-3 was tested. When G-3 was subjected to 1 laundering cycle, the LOI value was 29.30% and the damaged length for the UL-94 test was 14.84 cm. When G-3 was subjected to 2 laundering cycles (LCs), the LOI value was 28.60% and it was still self-extinguishing in the UL-94 test, but the damaged length increased to 20.23 cm. When G-3 was subjected to 6 LCs, the LOI value of 26.40% was maintained, which may be attributed to the enhanced water washing resistance of the flame-retardant coating through covalent C–N cross-linking.
Cone calorimetry test
To further assess the flame retardancy of PEI/PSP, the samples were subjected to cone calorimeter tests, as depicted in Fig. 7. The test parameters included the heat release rate (HRR), total heat release (THR), CO2 production rate (CO2PR), and fire growth rate (FGR). Some key parameters, including the time to ignition (TTI), average CO yield (AV-COY) and percentage of char residue, are also recorded in Table 2.
Figure 7 and Table 2 show that the TTI and burning time of G-3 are shorter than those of the RFs. This phenomenon arises from the premature decomposition of GPTMS-PEI/PSP on the RF surface under heat exposure. The pHRR and THR of G-3 decreased from 204.64 kW/m2 and 14.83 MJ/m2 in the untreated RFs to 106.36 kW/m2 and 8.89 MJ/m2, corresponding to reductions of 48.30% and 40.05%, respectively. Importantly, the FGR is an important parameter for estimating a material’s safety in fire incidents; a lower FGR value is correlated with enhanced material safety and superior flame-retardant efficacy. As illustrated in Fig. 7d, the FGR value for the fabric decreased substantially, decreasing from 6.58 kW/m2/s for RF to 3.22 kW/m2/s for G-3, indicating a 51.06% reduction. This implies that in the event of a fire, the enhanced safety margin is elevated by 51.06% relative to that of original untreated fabric, thereby substantially mitigating the potential damage inflicted by fire. These findings suggest that GPTMS-PEI/PSP is effective in mitigating the risk of RF fires (Ge et al. 2022).
The emission of CO and CO2 gases throughout the combustion process of a material can also pose a greater hazard. As shown in Fig. 7 c, the CO2PR of G-3 decreased by 0.05 g/s compared with that of RF, which demonstrates the ability of the flame-retardant coating to suppress CO2 emission. In addition, as shown in Table 2, AV-COY gradually increases from 0.02 (kg/kg) to 0.08 (kg/kg). This phenomenon arises from the dehydration of cellulose within the fabric at elevated temperatures, which in turn promotes fiber charring. This incomplete combustion results in rapid conversion of carbon in the fiber to CO when heated, inhibiting the conversion of CO to CO2 and indicating a potential gas-phase flame-retardant mechanism. Thus, the presence of the char layer hinders the complete combustion of the fabric. This incomplete combustion results in the rapid conversion of carbon in the fiber to carbon monoxide gas when heated (Shi et al. 2020). This also explains the gradual decrease in the G-3 combustion time with respect to RF. Therefore, this further leads to an increase in char residue from 3.04 wt% to 28.75 wt% for RF, which suggests that there is some condensed-phase flame retardancy in this flame retardant coating.
The formula for calculating the FGR is as follows: FGR (kW/m2/s) = \(\frac{\mathbf{p}\mathbf{H}\mathbf{R}\mathbf{R}}{\mathbf{T}\mathbf{p}\mathbf{H}\mathbf{R}\mathbf{R}}\), where T pHRR is the time to reach the pHRR.
Thermal stability of untreated and treated RF
The thermal degradation properties of the RF and G-3 fabrics were evaluated via the TG method. The TGA and DTG curves of the control and G-3 fabrics under a nitrogen atmosphere are shown in Fig. 8, and the corresponding data are summarized in Table 3.
T5% is defined as the temperature of onset of decomposition, the temperature at which 5% of the mass loss of RF occurs, and Tmax is the temperature at which the maximum rate of mass loss occurs. Rmax is the rate of mass loss at Tmax.
As shown in Fig. 8, G-3 has three thermal decomposition steps: the first step involves the volatilization of water vapor on top of the fabric; the second step involves the decomposition and release of CO2, hydrocarbons, and CO as the temperature increases; and the third step involves the thermo-oxidative degradation of the residual char. The T5 wt% of the sample fabrics decreases as the weight of the fabrics increases, from 286.66 °C for RF to 246.16 °C for G-3, which is mainly due to the early decomposition induced by the catalytic flame-retardant coating. Furthermore, the DTG curves, derived from the first-order derivatives of TG, demonstrate that the Rmax of the G-3 flame-retardant coated fabrics is lower than that of RF, with a notable decrease in Rmax from 2.41% to 1.22%. This behavior is consistent with the condensed-phase flame retardant mechanism: the GPTMS-PEI/PSP coating can form an insulating protective layer that inhibits heat penetration and slows the rate of mass loss, thus improving fire resistance (Li et al. 2020). Concurrently, the reduction in Tmax is ascribed to the formation of phosphoric acid and polyphosphoric acid due to the decomposition of phosphorus in PSP during thermal degradation (Qi et al. 2022a). This phenomenon indicates that the coating improved the thermal stability of RF and reduced mass loss. At 800 °C, the char residue of RF was only 9.72 wt%, whereas the char residue of the coated fabric G-3 increased to 30.34 wt%. Phosphoric acid promotes cellulose dehydration and carbonization, resulting in the formation of a protective layer with a dense char residue. This layer slows the rate of thermal decomposition and hinders further decomposition of the fabric, thereby reducing the production of flammable volatiles and leading to a substantial increase in the final residue.
In total, when subjected to flame-retardant coatings, the RF coating induced changes in the thermal decomposition process, thereby increasing the thermal stability of RF at elevated temperatures. In addition, the elements Si, N and P accelerate fiber dehydration, resulting in the excellent retention of char in the fabric. This effectively isolates the fabric from external heat sources, thus slowing the thermal decomposition reaction.
Analysis of fabric char residues via Raman
Raman spectroscopy was employed as an analytical tool for the detection of residual char and for evaluating the influence of PEI/PSP on the char-forming properties of RF. Consequently, the results are represented in Fig. 9. Notably, the emergence of distinctive Raman peaks centered at 1346 cm−1 (D band) and 1581 cm−1 (G band) indicates the graphitic structure of the residual char (Qi et al. 2022a). For the quantification of the relative intensity ratios, the ID/IG value is utilized, where ID corresponds to the intensity of the D band, whereas IG signifies the intensity of the G band. Significantly, a decrease in the ID/IG value is correlated with enhanced graphitization within the char. Moreover, the formation of a high-quality char layer on a fabric is typically accompanied by a concomitant decrease in porosity and a decrease in pore structure. This reduction in porosity lowers the likelihood of oxygen penetration, subsequently reducing the potential for oxygen to interact with the char and slowing the char’s oxidation process (Wang et al. 2022). Importantly, given that oxygen acts as a catalyst in numerous thermal degradation and oxidation reactions, the successful isolation of oxygen significantly enhances the stability and longevity of the char layer (Zhang et al. 2021). In contrast, the graphitic structure inherent in high-quality char layers typically has a low thermal conductivity, leading to a diminished rate of heat transfer (Wang et al. 2023b). Therefore, this characteristic allows the char layer to effectively sustain a uniform temperature under high-temperature conditions, thus impeding the transfer of heat to adjacent areas. Evidently, the ID/IG value of the RF stood at 3.37. However, the increased weight of the coating layers notably increased char graphitization, culminating in a decrease in the ID/IG value to 1.78. The results indicate that increasing the number of coating layers can facilitate the formation of a superior char layer under fire conditions, thus acting as an effective flame retardant.
Surface morphology analysis of char residues
To gain deeper insights into the flame-retardant mechanism associated with the coating, SEM observations of the residual char after UL-94 were performed. To complement this, the elemental composition and distribution were determined via EDS, as illustrated in Figs. 10 and 11.
In Fig. 10, the residual char of RF suffered significant damage after combustion, displaying a more dispersed structure lacking a coherent fiber-like shape. In contrast, the SEM image of the G-3 residual char shown in Fig. 10 reveals that the fiber structure experienced only slight deformation, and the integrity of its fibers was maintained. Upon magnifying Fig. 10 tenfold for closer inspection, numerous protruding round formations were observed on the fiber surface. This phenomenon arises from the thermal decomposition and combustion reactions of the fabric, and the gases produced, including CO and CO2 are unable to completely dissipate. Consequently, the burning of the fabric resulted in the expansion of the surface (Chen et al. 2023; Zhao et al. 2021). These findings affirm that the flame-retardant coating enhances the charring process of the fabric, effectively isolating the fibers from oxygen. Consequently, this slows the transfer of heat to the external environment.
Additionally, as depicted in Fig. 11, EDS elemental analysis was conducted on the surface of the residual char of G-3. Notably, the P content on the burned G-3 surface was greater than that on the unburned surface. This is because phosphorus-based flame retardants do not volatilize easily at high temperatures, and their degradation products are mainly retained in the residue. Consequently, the relative concentration of phosphorus in the residue increases after burning. Finally, with the appearance of the protective layer of residual char, the combustion and volatilization of RF were greatly alleviated.
Flame-retardant mechanism
To better understand the flame-retardant mechanism of PEI/PSP, the thermal decomposition products of RF and G-3 were investigated via the TG-FIIR test, as shown in Fig. 12.
As shown in Fig. 12a, b, the thermal decomposition products of RF and G-3 are basically the same, and only the peak intensities are different, which is caused by the variation in the released gases (Rao et al. 2021). A comparison of the TG-FTIR curves corresponding to different temperatures can help us better analyze the thermal decomposition products. As shown in Fig. 12c, d, RF and G-3 show characteristic peaks of H2O (3750 cm−1), CxHy (2816 cm−1), CO2 (2432 cm−1), CO (2277 cm−1), carbonyl compounds (1734 cm−1) and ether groups (1108 cm−1) at 300 °C and 350 °C, respectively, which indicates that the fabric began to decompose. In addition, G-3 shows a characteristic NH3 peak at 3612 cm−1, which is due to the gas produced by the thermal decomposition of PEI.
As shown in Fig. 13, the absorption intensities corresponding to the distinctive peaks associated with ether groups at 1108 cm−1, carbonyl compounds at 1734 cm−1, and CxHy at 2816 cm−1 clearly exhibit a notable reduction in G-3. This reduction signifies a diminished production of volatile combustible gases during the thermal degradation process, suggesting a propensity for the retention of constituent components within the condensed-phase. In contrast, the intensities of the absorption peaks of H2O (3750 cm−1) and CO2 (2432 cm−1) increased significantly during the thermal decomposition of G-3 (Fig. 12c, d). The release of gases such as H2O, CO2 and NH3 can effectively dilute the oxygen concentration surrounding the fabric, thereby reducing the temperature. This phenomenon may indicate that a gas-phase flame-retardant mechanism plays a role.
We explain the flame retardant mechanism through the noncombustible gas dilution effect, high-energy radical capture mechanism, heat absorption and cooling effect, gas-phase and condensed-phase mechanism and other aspects. When the environment where the fabric is located reaches a certain temperature and oxygen concentration, the fabric begins to burn. Its combustion behavior is usually divided into four stages: heating, thermal decomposition, ignition and combustion. One of the most important stages is thermal decomposition. PEI, PSP and GPTMS play vital roles in N/P/Si flame retardant coatings. The amine groups in PEI exist mainly in the form of primary, secondary and tertiary amines (Cheng et al. 2021). When heated, primary amines generally leave in the form of gas, for example, decomposing nitrogen, ammonia and other noncombustible gases, reducing the concentration of oxygen around the fabric, thus slowing the combustion behavior and acting as a gas-phase flame retardant mechanism. The secondary amine and tertiary amine remain in the carbon layer through the reaction and play a flame retardant role in the condensed-phase.
In addition, owing to the high phosphorus content of PSP, it first decomposes into sodium dihydrogen phosphate and phosphoric acid when heated to a certain temperature. The hydroxyl groups of the fabric can be phosphorylated, resulting in cross-linking of the fibers through phosphate bonds (Passauer 2019). The thermal decomposition products of phosphoric acid or phosphate can inhibit the generation of levoglucosan, thereby accelerating the dehydration and carbonization of the fabric. This process helps isolate the fiber from external oxygen, effectively blocking thermal decomposition. In addition, the N in PEI can promote the production of phosphoric acid and may form P–N bonds (Cho et al. 2015). Moreover, phosphate decomposition products during the combustion process can capture high-energy free radicals, effectively interrupting the chain reaction of the combustion process, as shown in Fig. 14 (Yang et al. 2016b). The water vapor evaporated from the fabric under the action of phosphoric acid and phosphate can effectively absorb heat, thus playing a role in heat-absorbing cooling. Furthermore, the addition of silicon can make the char layer denser, thus providing a synergistic flame retardant effect (Wu et al. 2019; Xu et al. 2023).
Conclusion
In this study, RF with better flame retardancy was treated with the environmentally friendly material PEI/PSP via LBL. The GPTMS-PEI/PSP coated fabric G-3 had the LOI of 30.20% and self-extinguished within 10 s from the ignition source, with a damaged length of 6.13 cm. After 6 LCs, the LOI was 26.40%, indicating good durability of the flame-retardant coating bonded by covalent bonds. The results of the cone calorimeter test revealed a significant reduction in the pHRR of G-3 by 48.30% and a decrease in the FGR by 51.06%. These results clearly demonstrate that the application of the G-3 coating significantly enhances the fire safety of RF. In addition, the TG results revealed that G-3 was more thermally stable, with a residue of 30.34 wt% at 800 °C, which was much greater than the 9.72 wt% for the uncoated fabrics at the same temperature. The TG-FTIR results indicate a reduction in the generation of volatile combustible gases during thermal degradation, suggesting that the G-3 coating has a gas-phase flame-retardant effect. The above results also illustrate that the flame retardancy of GPTMS-PEI/PSP can enhance the dehydration and carbonization of fabric during the combustion process and produce a stable char layer containing Si/N/P, which can effectively impede heat transfer. This study provides a feasible method for realizing durable flame-retardant RFs.
Data availability
No datasets were generated or analysed during the current study.
References
Aghayan M, Alizadeh P, Keshavarz M (2021) Multifunctional polyethylene imine hybrids decorated by silica bioactive glass with enhanced mechanical properties, antibacterial, and osteogenesis for bone repair. Mat Sci Eng C-Mater 131:112534. https://doi.org/10.1016/j.msec.2021.112534
Alebeid OK, Zhao T (2017) Review on: developing UV protection for cotton fabric. J Text I 108:2027–2039. https://doi.org/10.1080/00405000.2017.1311201
Alongi J, Colleoni C, Malucelli G, Rosace G (2012) Hybrid phosphorus-doped silica architectures derived from a multistep sol-gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym Degrad Stabil 97:1334–1344. https://doi.org/10.1016/j.polymdegradstab.2012.05.030
An Q, Aamir M, Mao S, Liu Y, Wang Y, Zheng P, Liu W (2022) Current pollution status, spatial features, and health risks of legacy and emerging halogenated flame retardants in agricultural soils across China. Sci Total Environ 803:150043. https://doi.org/10.1016/j.scitotenv.2021.150043
Chen MJ, Lazar S, Kolibaba TJ, Shen R, Quan Y, Wang Q, Chiang HC, Palen B, Grunlan JC (2020) Environmentally benign and self-extinguishing multilayer nanocoating for protection of flammable foam. Acs Appl Mater Inter 12:49130–49137. https://doi.org/10.1021/acsami.0c15329
Chen X, Ding F, Zhang S, Liu Y, Hou X, Ren X (2023) Flame-retardant, antibacterial and hydrophobic multifunctional coatings on cotton fabrics via layer-by-layer self-assembly. Cellulose 30:6679–6694. https://doi.org/10.1007/s10570-023-05287-5
Cheng X, Tang R, Yao F, Yang X (2019) Flame retardant coating of wool fabric with phytic acid/polyethyleneimine polyelectrolyte complex. Prog Org Coat 132:336–342. https://doi.org/10.1016/j.porgcoat.2019.04.018
Cheng XW, Zhang C, Jin WJ, Huang YT, Guan JP (2021) Facile preparation of a sustainable and reactive flame retardant for silk fabric using plant extracts. Ind Crops Prod 171:113966. https://doi.org/10.1016/j.indcrop.2021.113966
Cho SY et al (2015) Carbonization of a stable β-sheet-rich silk protein into a pseudographitic pyroprotein. Nat Commun 6(1):7145. https://doi.org/10.1038/ncomms8145
Fang Y, Wu J, Chen Y, Wu L (2023) Durable flame retardant and anti-dripping of PET fabric using bio-based covalent crosslinking intumescent system of chitosan and phytic acid. Prog Org Coat 183:107785. https://doi.org/10.1016/j.porgcoat.2023.107785
Ge X, Ma S, Zhang X, Yang Y, Li G, Yu Y (2020) Halogenated and organophosphorous flame retardants in surface soils from an e-waste dismantling park and its surrounding area: distributions, sources, and human health risks. Environ Int 139:105741. https://doi.org/10.1016/j.envint.2020.105741
Ge Y, Qi Z, Sha D, Hu X, Liu S (2022) Durable flame-retardant cotton fabric modified by water-soluble C-N-P intumescent flame retardant. J Appl Polym Sci 139:e53070. https://doi.org/10.1002/app.53070
Hu Z, Ma Y, Chen H, Wei L, Zhu G, Liu L, Yao J (2023) Efficient, durable, and breathable flame retardant cotton fabric via a feasible surface finishing. Appl Surf Sci 615:156314
Jenny A, Claudio C, Giulio M, Giuseppe R (2012) Hybrid phosphorus-doped silica architectures derived from a multistep sol–gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym Degrad Stabil 97:1334–1344. https://doi.org/10.1016/j.polymdegradstab.2012.05.030
Jiang Z, Li H, He Y, Liu Y, Dong C, Zhu P (2019) Flame retardancy and thermal behavior of cotton fabrics based on a novel phosphorus-containing siloxane. Appl Surf Sci 479:765–775. https://doi.org/10.1016/j.apsusc.2019.02.159
Jiang X, Li P, Liu Y, Wang J (2023) Flame-retardant ramie fabrics with APP: flame retardancy, flame-retardant mechanism and mechanical properties. Cellulose 30:1321–1334. https://doi.org/10.1007/s10570-022-04962-3
Khalili P, Blinzler B, Kádár R, Blomqvist P, Sandinge A, Bisschop R, Liu X (2020) Ramie fabric elium® composites with flame retardant coating: Flammability, smoke, viscoelastic and mechanical properties. Compos Part A Appl Sci Manuf 137:105986. https://doi.org/10.1016/j.compositesa.2020.105986
Lazar S, Eberle B, Bellevergue E, Grunlan J (2020) Amine salt thickening of intumescent multilayer flame retardant treatment. Ind Eng Chem Res 59:2689–2695. https://doi.org/10.1021/acs.iecr.9b06359
Li YC, Mannen S, Morgan AB, Chang SC, Yang YH, Condon B, Grunlan JC (2011) Intumescent all-polymer multilayer nanocoating capable of extinguishing flame on fabric. Adv Mater 23(34):3926–3931. https://doi.org/10.1002/adma.201101871
Li P, Wang B, Xu Y, Jiang Z, Dong C, Liu Y, Zhu P (2019a) Ecofriendly flame-retardant cotton fabrics: preparation, flame retardancy, thermal degradation properties, and mechanism. Acs Sustain Chem Eng 7:19246–19256. https://doi.org/10.1021/acssuschemeng.9b05523
Li SS, Ding F, Lin XH, Li ZG, Ren XH (2019b) Layer-by-layer self-assembly of organic-inorganic hybrid intumescent flame retardant on cotton fabrics. Fiber Polym 20:538–544. https://doi.org/10.1007/s12221-019-8914-z
Li P, Wang B, Liu Y, Xu Y, Jiang Z, Dong C, Zhang L, Liu Y, Zhu P (2020) Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohyd Polym 237:116173. https://doi.org/10.1016/j.carbpol.2020.116173
Li L, Qi P, Peng A, Sun J, Cui Z, Liu W, Li H, Gu X, Zhang S (2022) Preparation of durable flame retardant nylon-cotton blend fabrics by 3-glycidyloxypropyl trimethoxy silane associated with polyethyleneimine and phytic acid. Cellulose 29:7413–7430. https://doi.org/10.1007/s10570-022-04693-5
Liao Y, Chen Y, Zhang FX (2021) A biological reactive flame retardant for flame retardant modification of cotton fabric. Colloid Surf A 630:127061. https://doi.org/10.1016/j.colsurfa.2021.127601
Lu Y, Zhao P, Chen Y, Lu Y, Zhang G (2022) A novel polymer reactive flame retardant for the preparation of highly durable cotton fabrics. Int J Biol Macromol 223:1394–1404. https://doi.org/10.1016/j.ijbiomac.2022.11.033
Lu J, Shi Y, Guan J, Dang R, Yu L, Wang H, Hu N, Shen X (2023) Enhanced mechanical properties of ramie fabric/epoxy composite laminates by silicon polymer. Ind Crop Prod 199:116778. https://doi.org/10.1016/j.indcrop.2023.116778
Miao ZW, Yan DP, Zhang T, Yang F, Zhang SK, Liu W, Wu ZP (2021) High-efficiency flame retardants of a P-N-rich polyphosphazene elastomer nanocoating on cotton fabric. Acs Appl Mater Inter 13:32094–32105. https://doi.org/10.1021/acsami.1c05884
Ng LF, Yahya MY, Mustafa Z (2022) Exploration of novel fiber-metal laminates sandwich structures with cellulosic ramie woven core. Polym Composite 43:6667–6677. https://doi.org/10.1002/pc.26990
Palen B, Rabaey MG, Rodriguez Melendez D, Iverson ET, Kolibaba TJ, Grunlan JC (2022) Polymeric coacervate coating for flame retardant paper. Cellulose 29:4589–4597. https://doi.org/10.1007/s10570-022-04594-7
Pan Y, Liang Q, Du J, Zhang H, Zhang D, Zhao H, Lu T, Zhang Y (2023) Influences of boron and nitrogen co-doped carbon dot based coating fabricated via layer-by-layer self-assembly on the UV protection and flame retardancy of cotton fabric. Cellulose 30:11249–11259. https://doi.org/10.1007/s10570-023-05541-w
Passauer L (2019) Thermal characterization of ammonium starch phosphate carbamates for potential applications as bio-based flame-retardants. Carbohyd Polym 211:69–74. https://doi.org/10.1016/j.carbpol.2019.01.100
Qi L, Qiu S, Xi J, Yu B, Hu Y, Xing W (2022a) Construction of super-hydrophobic, highly effective flame retardant coating for cotton fabric with superior washability and abrasion resistance. J Colloid Interf Sci 607:2019–2028. https://doi.org/10.1016/j.jcis.2021.10.021
Qi P, Li Y, Yao Y, Sun J, Li L, Liu J, Gu X, Li H, Zhang S (2022b) Ultra washing durable flame retardant coating for cotton fabric by the covalent bonding and interface polymerization. Chem Eng J 452:139453. https://doi.org/10.1016/j.cej.2022.139453
Rao W, Shi J, Yu C, Zhao H, Wang Y (2021) Highly efficient, transparent, and environment-friendly flame-retardant coating for cotton fabric. Chem Eng J 424:130556. https://doi.org/10.1016/j.cej.2021.130556
Shi X, Chen L, Zhao Q, Long J, Li Y, Wang Y (2020) Epoxy resin composites reinforced and fire-retarded by surficially-treated carbon fibers via a tunable and facile process. Compos Sci Technol 187:107945. https://doi.org/10.1016/j.compscitech.2019.107945
Shi X, Liu Q, Li X, Du A, Niu J, Li Y, Li Z, Wang M, Wang D (2022) Construction phosphorus/nitrogen-containing flame-retardant and hydrophobic coating toward cotton fabric via layer-by-layer assembly. Polym Degrad Stabil 197:109839. https://doi.org/10.1016/j.polymdegradstab.2022.109839
Vanderveen I, Deboer J (2012) Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067
Vest NA, Afonso AO, Melendez DR, Ponis J, Smith DL, Iverson ET, Zhang Z, Marquez JAD, Banerjee S, Wang Q, Grunlan JC (2023) Polyelectrolyte complex for flame retardant silk. Polym Degrad Stabil 216:110491. https://doi.org/10.1016/j.polymdegradstab.2023.110491
Wang HQ (2018) An ultra-low free-formaldehyde durable flame-retarding finishing for ramie fabric. J Nat Fibers 16:545–554. https://doi.org/10.1080/15440478.2018.1428845
Wang H (2019) An ultra-low free-formaldehyde durable flame-retarding finishing for ramie fabric. J Nat Fibers 16:545–554. https://doi.org/10.1080/15440478.2018.1428845
Wang LL, Zhang T, Yan HQ, Peng M, Fang ZP (2013) Modification of ramie fabric with a metal-ion-doped flame-retardant coating. J Appl Polym Sci 129:2986–2997. https://doi.org/10.1002/app.39015
Wang HQ, Yan HQ, Fang ZP (2021) Fabrication and properties of PEI/APP layer-by-layer coated ramie fabric combined with low-temperature plasma treatment. Fire Mater 46:117–129. https://doi.org/10.1002/fam.2952
Wang Y, Liu L, Ma L, Yuan J, Wang L, Wang H, Xiao F, Zhu Z (2022) Transparent, flame retardant, mechanically strengthened and low dielectric EP composites enabled by a reactive bio-based P/N flame retardant. Polym Degrad Stabil 204:110106. https://doi.org/10.1016/j.polymdegradstab.2022.110106
Wang H, Hao C, Shu T, Li P, Yu T, Yu L, Yan N (2023a) Flame-retardant Janus ramie fabric with unidirectional liquid transportation, moisture-wicking, and oil/water separation properties. Chem Eng J 474:1267–1257. https://doi.org/10.1016/j.cej.2023.145518
Wang Y, Ma L, Wang H, Cheng C, Yin X, Zhu Z (2023b) Fabrication of a flame retardant, strong mechanical toughness and antimicrobial polylactic acid by chitosan Schiff base/ammonium polyphosphate. Polym Degrad Stabil 216:110492. https://doi.org/10.1016/j.polymdegradstab.2023.110492
Wu H, Zeng B, Chen J, Wu T, Li Y, Liu Y, Dai L (2019) An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem Eng J 374:1304–1316. https://doi.org/10.1016/j.cej.2019.06.027
Xu Y, Yan C, Du C, Xu K, Li Y, Xu M, Bourbigot S, Fontaine G, Li B, Liu L (2023) High-strength, thermal-insulating, fire-safe bio-based organic lightweight aerogel based on 3D network construction of natural tubular fibers. Compos Part B-Eng. https://doi.org/10.1016/j.compositesb.2023.110809
Yang J, Liao W, Deng S, Cao Z, Wang Y (2016a) Flame retardation of cellulose-rich fabrics via a simplified layer-by-layer assembly. Carbohyd Polym 151:434–440. https://doi.org/10.1016/j.carbpol.2016.05.087
Yang S, Wang J, Huo S, Wang J, Tang Y (2016) Synthesis of a phosphorus/nitrogen-containing compound based on maleimide and cyclotriphosphazene and its flame-retardant mechanism on epoxy resin. Polym Degrad Stabil 126:9–16. https://doi.org/10.1016/j.polymdegradstab.2016.01.011
Yang H, Li S, An Q, Zhai S, Xiao Z, Zhang L (2021) Facile transformation of carboxymethyl cellulose beads into hollow composites for dye adsorption. Int J Biol Macromol 190:919–926. https://doi.org/10.1016/j.ijbiomac.2021.08.229
Yang M, Yang Y, Shi J, Rao W (2023) Fabrication of eco-friendly flame-retardant and hydrophobic coating for cotton fabric. Cellulose 30:3267–3280. https://doi.org/10.1007/s10570-023-05051-9
Zhang T, Yan H, Wang L, Fang Z (2013) Controlled formation of self-extinguishing intumescent coating on ramie fabric via layer-by-layer assembly. Ind Eng Chem Res 52:6138–6146. https://doi.org/10.1021/ie3031554
Zhang AN, Zhao HB, Cheng JB, Li ME, Li SL, Cao M, Wang YZ (2021) Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem Eng J. https://doi.org/10.1016/j.cej.2020.128361
Zhao B, Kolibaba TJ, Lazar S, Grunlan JC (2021) Environmentally-benign, water-based covalent polymer network for flame retardant cotton. Cellulose 28:5855–5866. https://doi.org/10.1007/s10570-021-03874-y
Zheng X, Dong Y, Liu X, Xu Y, Jian R (2022) Fully bio-based flame-retardant cotton fabrics via layer-by-layer self assembly of laccase and phytic acid. J Clean Prod 350:131525. https://doi.org/10.1016/j.jclepro.2022.131525
Acknowledgments
This work was supported by the Startup Fund from Fujian Normal University, the Natural Science Foundation of Fujian Province of China (2021J01198), the Scientific and Technological Project of Quanzhou (2022C008R), and the Science and Technology Development Projects of the Central Committee Guidance Local (2023L3063).
Funding
This work was supported by the Startup Fund from Fujian Normal University, the Natural Science Foundation of Fujian Province of China (2021J01198), the Scientific and Technological Project of Quanzhou (2022C008R), and the Science and Technology Development Projects of the Central Committee Guidance Local (2023L3063).
Author information
Authors and Affiliations
Contributions
Wenjian Li: Conceptualization, Methodology, Validation, Formal analysis, Data Curation, Writing—Original draft, Visualization. Fubin Luo: Writing—Review & Editing. Yumei Dai: Investigation. Denglong Chen: Project administration. Hongzhou Li: Resources, Writing—Review & Editing, Supervision, Funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Luo, F., Dai, Y. et al. Fabrication of highly efficient flame-retardant and biocompatible ramie fabrics through covalent bonding and layer-by-layer assembly methods. Cellulose (2024). https://doi.org/10.1007/s10570-024-06147-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10570-024-06147-6