Abstract
Most enzymes used in industry originate from microorganisms, such as endoglucanases that degrade cellulose. In this context, Thermothelomyces heterothallicus is a thermophilic filamentous fungus capable of producing substantial amounts of this enzyme. This study aimed to produce, purify, and characterize an endoglucanase from the novel strain of T. heterothallicus PA2S4T, utilizing orange peel as a carbon source, and applying this enzyme to denim fabric. Optimization of enzymatic production through experimental design yielded the best results for endoglucanases at the lowest temperature (28 °C) and shortest cultivation time (60 h), using the highest concentration of orange peel (4.2%), resulting in an endoglucanase activity of 61 U.mL−1. Following purification steps, sample isolation was confirmed by observing a single band on SDS-PAGE at a molecular weight of 36.3 kDa. The enzymatic reaction exhibited an optimal pH of 7.0, with stability over a wide pH range (from 3.0 to 8.0). The enzyme showed optimal activity at 50 °C and remained thermally stable up to this temperature. Application of the enzyme in the bio-polishing step of denim jeans manufacturing, at concentrations of 1–5 U.mL−1, resulted in weight losses of 2–5%. The best weight loss results were found after 12 h of treatment, at 50 ºC and pH 5.0. Additionally, the scanning electron microscopy showed that 2 and 5 U.mL−1 of enzyme improved appearance of denim fabric by removing protuberances and irregularities compared to untreated fabric.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulases, enzymes responsible for cellulose degradation, are categorized into three primary groups: endoglucanases, exoglucanases, and β-glucosidases (Sohail et al. 2022; Alves et al. 2021; Santos et al. 2021). Ranked as the third most utilized industrial enzyme globally in 2021, cellulases are synthesized by microorganisms and are utilized across various sectors including paper, beverage, food, and textiles. (Ejaz et al. 2023a; Cerqueira et al. 2022; Drula et al. 2022; Santos et al. 2021; Gaete et al. 2020).
Enzymes provide an environmentally friendly alternative to conventional chemical processes in the textile industry, reducing the environmental impact associated with chemical residues from denim manufacturing (Ranjan et al. 2023; Ejaz et al. 2023b; Besegatto et al. 2018; Chen et al. 2017). Their integration into biopolishing processes helps minimize the use of harmful finishing agents (Andreaus et al. 2014; Cavaco-Paulo 1998; Madhu and Chakraborty 2017). Endoglucanases, in particular, are extensively utilized in the textile industry to enhance the properties of cellulose-based products, offering several advantages over traditional methods, such as enhanced fabric durability and targeted removal of loose fibers (Ranjan et al. 2023; Esfandiari et al. 2014). Thermophilic filamentous fungi of the phylum Ascomycota, particularly those thriving at 50 °C, are prominent producers of this enzyme (Cooney and Emerson 1964; Leo et al. 2019).
While limited research exists in the literature regarding the biotechnological applications of endoglucanase produced by the thermophilic fungus Thermothelomyces heterothallicus (also known as Myceliophthora heterothallica) (Schoch et al. 2020), its inherent properties, such as thermal stability and high catalytic activity, render it highly attractive for various applications. In line with this, orange peels, an abundant and often underutilized byproduct of the citrus industry, have been demonstrated to induce the production of valuable enzymes such as xylanases, chitinases, and pectinases by filamentous fungi (Uday et al. 2017; Qadir et al. 2020; Pommer et al. 2021). Utilizing this waste for endoglucanase production presents a sustainable solution, both environmentally and economically, by converting waste into valuable resources and contributing to the development of a circular economy.
Therefore, the objective of this study is to produce, purify, and characterize the biochemical parameters of an endoglucanase produced by the novel strain T. heterothallicus PA2S4T from orange peel waste, with a specific focus on its application in denim fabric treatment.
Material and methods
Maintenance of the strain and growth in submerged fermentation
In this study, the filamentous fungus T. heterothallicus strain PA2S4T was utilized, which is deposited in the Microbial Biochemistry Laboratory at Unioeste. This strain was recently isolated from soil samples collected in Nova Aurora, Paraná, Brazil (24°30′9" S 53°15′18" W) and taxonomically identified at the species level (Pommer et al. 2021). The strain was incubated on PDA medium at 40 °C for 10 days prior to the cultivation and optimization experiments and maintained under refrigeration, with replications carried out over a period of 30 days. Subsequently, 1 mL of a suspension consisting of 0.8% NaCl and 0.5% Tween 80, containing 2.0 × 106 U.mL−1 spores as determined by Neubauer chamber counting, was inoculated into 125-mL Erlenmeyer flasks containing 25 mL of liquid Khanna medium (Khanna et al. 1995), supplemented with 1% orange peel (Citrus sinensis var. pear) previously dried at 50 °C for 48 h, ground in a knife mill, and sieved to mesh size 18. At the conclusion of each assay, the cultures were filtered using a vacuum pump, Büchner funnel, and filter paper, yielding a cell-free crude enzymatic extract.
Enzyme activity measurement and protein quantification
The enzymatic activity of endoglucanase (CMCase) was determined using the modified Miller method (1959). In this method, 50 µL of the crude extract was mixed with 50 µL of a 1% (w/v) carboxymethyl cellulose (CMC) substrate solution. The mixture was then incubated in a water bath at 40 °C for 5 min. After incubation, 100 µL of 3,5-dinitrosalicylic acid (DNS) reagent was added, followed by boiling for 5 min. The tubes were cooled, and 1 mL of distilled water was added to each tube. The absorbance was measured at 540 nm using a spectrophotometer. One enzymatic unit (U) was defined as the amount of enzyme required to release 1 µmol of product per minute under the assay conditions.
The quantification of proteins was determined using the Bradford method (1976), with bovine serum albumin (BSA—Bio-Rad®) as the standard.
Optimization of enzymatic production
An experimental factorial design 23 with the variables temperature (°C), time (hours), and orange peel concentration (%), employing a central composite design (CCD) and response surface methodology (RSM), was conducted to optimize endoglucanase production. The experiment comprised a total of 11 trials, with 3 trials at the central point. Upon completion of each assay, the cultures were filtered using a vacuum pump, a Büchner funnel, and filter paper, yielding a crude enzymatic extract that was evaluated for CMCase activity and protein quantity. Table 1 presents the variables utilized in the experimental design of the central composite design, along with their corresponding coded values (-1, 0, and + 1) and real values.
The response to the variables, where Y represents endoglucanase activity, can be approximated by the following equation:
where β0 is the intercept; β1, β2, and β3 are the linear coefficients; and β12, β13 and β23 are the interaction coefficients. The data analysis and graphical construction were performed using STATISTICA 7 software. The variability of the experimental data was assessed by determining the coefficient of determination (R2) and by analysis of variance (ANOVA).
Purification of endoglucanase
A 70 mL volume of crude extract was applied to a CM-Sephadex ion-exchange column pre-equilibrated with 20 mM Sodium Acetate buffer at pH 4.8. Elution was performed using a NaCl concentration gradient ranging from 50 mM to 1 M, at a flow rate of 2 mL.min−1. Fractions of 5 mL each were collected and monitored for enzymatic activity and protein quantity. Fractions exhibiting high enzymatic activity were combined and lyophilized at the end of the process. For size exclusion chromatography, a Sephacryl S-100 HR gel filtration column pre-equilibrated with 20 mM Sodium Phosphate buffer at pH 7.0 was utilized. The lyophilized sample, resuspended in 750 µL, was applied, and proteins were eluted at a flow rate of 0.4 mL.min−1. Fractions of approximately 2 mL each were collected, and those showing high enzymatic activity were combined for further enzymatic characterization. All purification steps were conducted at 4 °C.
Purity analysis by denaturing electrophoresis (SDS-PAGE) and zymogram
The purity analysis of the sample followed the methodology described by Laemmli (1970). SDS-PAGE was performed using a 10% resolving gel and a 6% stacking gel, with a molecular weight standard marker such as PageRuler Plus Prestained (Thermo ScientificTM) ranging from 18.4 to 116.0 kDa. After completion of the electrophoretic run, the gel was stained with Silver Nitrate to visualize the protein bands following the methodology described by Blum et al. (1987).
For the zymogram, the gel was prepared according to the methodology of Champasri et al. (2015), where 0.1% (w/v) CMC was incorporated into the 10% resolving gel. Subsequently, the gel was incubated in 200 mM Sodium Phosphate buffer at pH 7.0 at 50 °C for 1 h. It was then stained with 0.2% (w/v) Congo Red overnight and destained with 2 M NaCl until the appearance of a hydrolysis halo, demonstrating the enzymatic capacity to degrade CMC.
Investigating the impact of temperature and pH on the purified enzyme
For determining the optimum temperature, a modified version of Miller's method (1959) was employed, conducting the enzymatic reaction over a temperature range from 30 °C to 70 °C. Thermal stability was assessed by incubating the enzyme without the substrate at temperatures ranging from 40 °C to 70 °C. Aliquots were collected at time intervals of 0, 1, 5, 10, 15, 30, 60, and 120 min, followed by the determination of enzyme activity using Miller's method (1959).
The optimal pH was determined by conducting the enzymatic reaction in McIlvine buffer (50 mM) at pH values ranging from 4.0 to 8.0 and Tris–HCl buffer (50 mM) at pH 8.0 and 9.0. Subsequently, enzyme activity was determined using Miller's method (1959). pH stability was assessed by adding 100 µL of enzyme, without the substrate, to 100 µL of McIlvine buffer (50 mM) at pH values ranging from 4.0 to 8.0 and Tris–HCl buffer (50 mM) at pH 8.0 and 9.0. The mixture was then incubated at 4 °C for 24 h. Following incubation, enzyme activity was assayed using Miller's method (1959), with a 1% substrate in a 200 mM buffer at the optimal pH of the reaction.
Application of the purified enzyme to denim jeans fibers
Following the methodology of Sahin et al. (2016), the fabric was cut into 1 × 1 cm fragments and weighed (w1). Subsequently, the fragments were treated with pure CMCase enzyme at concentrations of 1 U.mL−1 (0.04 U.mg−1 of fabric), 2 U.mL−1 (0,08 U.mg−1 of fabric), 3 U.mL−1 (0.12 U.mg−1 of fabric), 4 U.mL−1 (0.16 U.mg−1 of fabric) and 5 U.mL−1 (0,20 U.mg−1 of fabric) in 50 mM sodium phosphate buffer at pH 7.0, at 50 °C for 12 h under stationary conditions. The influence of temperature, time, and pH was evaluated by treating the fragments with 2 U.mL−1 CMCase at temperatures ranging from 30 to 70 °C, durations ranging from 30 min to 24 h, and pH values ranging from 5.0 to 9.0, respectively. The buffers used were McIlvine 50 mM at pH 5.0 to 7.0 and Tris–HCl 50 mM at pH 8.0 and 9.0. Following treatment, the enzyme was inactivated by boiling for 5 min, and the fragments were washed twice with deionized water. Subsequently, the fragments were placed in an oven at 50 °C for 48 h, cooled in a desiccator for approximately 6 h, and weighed again after this process (w2) to analyze the extent of fabric weight loss. As a control, the fabric was incubated with the buffer alone, without the presence of the enzyme. The fabric weight loss was determined by comparing the weight loss of the dried sample (before and after treatment) using gravimetry, following the methodology described by Csiszár et al. (2001). Equation 2 was utilized to calculate the weight loss (% by weight) as described by Aly et al. (2004):
where w1 and w2 represent the weights of the fabric before and after treatment, respectively. The TESCAN® VEGA3 scanning electron microscope (SEM) apparatus was utilized for visualizing the morphology of both treated and untreated fabric fibers.
Statistical analysis
All data represent the mean of at least three independent experiments. The data underwent analysis of variance (ANOVA) using OriginPro Learning Edition Software, and differences with p < 0.05 were considered statistically significant. Means were compared using Student's t-test.
Results
Optimization of enzymatic production using central composite design (CCD) response surface methodology (RSM)
The results of Central Composite Design (CCD) experiments for endoglucanase production, considering temperature (°C), time (hours), and orange peel concentration (%), are presented in Table 2. The optimal condition was identified in trial 2, where microbial growth occurred at 28 °C for 60 h using 4.2% orange peel as a carbon source. Under these conditions, enzymatic activity reached 61.4 U/ mL−1.
Trial 8, corresponding to microbial growth at 48 °C for 132 h with 4.2% orange peel, also demonstrated promising results, yielding an activity of 49.2 U.mL−1, indicating robust growth performance across a broad temperature range.
Figure 1 depicts the Pareto chart for the investigated enzyme, with significance considered for p-values < 0.05. The only variable showing significance was the carbon source (orange peel). Given its significance, it is understood as the sole factor capable of directly influencing the assay, whereby higher availability of the carbon source in the medium correlates with increased enzymatic production within the tested ranges in the experiment. Despite the optimal conditions being observed at the shortest time (60 h) and lowest temperature (28 °C), the Pareto Chart indicates that these variables were not significant for enzyme production (p < 0.05).
According to the Analysis of Variance (ANOVA) presented in Table 3, it is evident that there was significance in the regression, as the calculated Fcal (230.57) exceeded the tabulated Ftab (10.13) with 5% significance, ensuring statistical reliability of the results obtained through the methodology. Despite exhibiting a higher lack-of-fit calculated F-value (1,117.12) compared to the tabulated F-value (18.51) at the 5% significance level, the linear model was selected for further analysis. This discrepancy can be attributed to the close proximity of replicate values obtained at the central point, resulting in a low sum of squares for pure error (SS < 0.25). Consequently, the calculated F-value becomes inflated, surpassing the tabulated value. Furthermore, the quadratic model demonstrated an inadequate fit to the experimental data, as evidenced by a coefficient of determination (R2) of only 71.3% (data not shown). Conversely, the linear model exhibited a superior fit with an R2 of 0.9463, indicating its ability to explain 94.63% of the data variability. Therefore, the linear model was considered the most suitable representation of the observed relationship.
The regression equation derived from the ANOVA test predicts the levels of endoglucanase as a function of the variables temperature (°C), time (hours), and orange peel concentration (%). The model, coded with 95% confidence, can predict enzymatic production (U.mL–1) as follows:
The response surface methodology plots for CMCase (Fig. 2) indicate that trials with a high concentration of carbon source led to increased enzyme production. The temperature vs. time plot illustrates minimal effects of these variables on the response (Fig. 2a). However, the carbon source vs. temperature and carbon source vs. time plots demonstrate that enzyme production is influenced by variations in the carbon source (Fig. 2b and c).
Response Surface Model for CMCase production by the fungus T. heterothallicus PA2S4T from the CCD. a Relationship between Temperature and Time; b Relationship between Temperature and Carbon Source; c Relationship between Carbon Source and Time; d Boxplot for CMCase production. Test 1: CCD CMCase—cultivation under conditions specified in trial 2 from CCD; Test 2: Repetition CMCase—cultivation with a duration of 60 h, an incubation temperature of 28 °C, and a carbon source concentration of 4.2% after CCD optimization. The microorganism was inoculated in Khanna medium under submerged fermentation and stationary conditions
Additionally, the result of a Student's t-test to validate the incubation temperature of 28 °C, cultivation time of 60 h, and carbon source concentration of 4.2% compared to the best CCD result reveals that both conditions are not statistically different (p = 0.087). The boxplot depicts the distribution of the data (Fig. 2d), where the first box (CCD CMCase) represents the CCD sample under optimal conditions and the second box (Repetition CMCase) represents the subsequent repetition of this analysis under optimal conditions. This demonstrates homogeneity of the data, with no outliers observed in any of the analyses.
Enzyme purification and zymogram
The crude extract was loaded onto a CM-Sephadex ion exchange column. Fractions exhibiting high activity resulted in 66 mL with a specific activity of 34.3 U.mg−1 (Table 4). Subsequently, when applied to the Sephacryl S-100 HR gel filtration column, the collected fractions with enzymatic activity amounted to 16 mL, displaying an activity of 86.0 U.mg−1, which were then concentrated for subsequent application in electrophoresis and zymographic analysis. Table 3 illustrates the steps involved in CMCase purification. The purification factor up to obtaining the pure enzyme was 3x, with a final recovery of 16% activity.
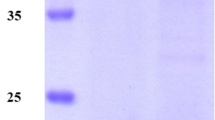
Figure 3a depicts the electrophoresis result alongside molecular weight markers, revealing a single protein band of approximately 36.3 KDa, thus confirming the enzyme's purity based on the distinctiveness of the band.
In Fig. 3b, analysis of the CMC zymogram reveals cellulolytic activity evidenced by the formation of a faint halo in the gel stained with Congo Red, indicating the enzyme's capability to hydrolyze CMC.
Biochemical characterization of CMCase
The optimal temperature for CMCase activity was determined to be 50 °C, as depicted in Fig. 4a. This temperature aligns with the characteristics of a thermophilic fungus. Regarding thermostability, as illustrated in Fig. 4b, the enzyme exhibited considerable stability during the 120-min assay period at 40 °C and 50 °C. However, at elevated temperatures of 60 °C and 70 °C, the enzyme showed a notable decrease in activity, with approximately 50% activity loss observed within the initial 30 min at 60 °C and up to 60% activity loss within the first 10 min at 70 °C.
The optimal pH for CMCase activity was determined to be 7.0, as depicted in Fig. 4c, although it displayed promising relative activity at lower pH values such as 3.0 and 4.0. Concerning pH stability, as illustrated in Fig. 4d, it is evident that the enzyme, while experiencing a partial decrease in activity, remains stable over a broad pH range, which is advantageous for industrial applications. The enzyme exhibited the highest stability at pH 4.0, yet it retained over 60% residual activity across the tested pH range.
Analysis of CMCase activity in denim
After purification, the enzyme was applied to denim fabric. To optimize cellulase activity, a series of experiments were conducted, focusing on critical variables: enzyme concentration, incubation time, temperature, and pH. These parameters were evaluated for their influence on enzymatic efficiency in weight loss during denim biopolishing. Remarkably, post-treatment with CMCase resulted in fabric weight loss ranging from 3–4.5% with 2–4 U.mL–1and approximately 5% with 5 U.mL–1 (Fig. 5a). Figure 5b illustrates time-dependent weight loss, with values around 2% for 60 and 120 min of treatment, and 4% for 12 and 24 h. Additionally, the effects of temperature and pH on denim treatment were investigated. As depicted in Fig. 5c and d, CMCase exhibited the highest weight loss at 50 °C and pH 5.0, respectively.
The maintenance of fiber integrity is evident through scanning electron microscopy for both treatments (2 U.mL−1 and 5 U.mL−1). Figure 6b illustrates that even with the lower enzyme concentration applied (2 U.mL−1), there is an improvement in the fabric's appearance, with impurities removed from the fibers compared to the control treatment (Fig. 6a). Furthermore, it was observed that the treatment with 5 U.mL−1 did not cause damage to the fabric fibers, indicating it as a safe concentration for application on jeans.
The scanning electron microscope analysis of the denim fabric after treatment with CMCase from T. heterothallicus PA2S4T is depicted in the following images: a represents the control sample, fabric treated with buffer; b represents fabric treated with 2 U.mL−1 of the enzyme; and c represents fabric treated with 5 U.mL−1 of the enzyme
Discussion
The central composite design (CCD) and response surface methodology (RSM) were employed to optimize endoglucanase production by T. heterothallicus PA2S4T using three variables: temperature, time, and carbon source concentration, with the objective of determining the optimal cultivation conditions. Since the optimal conditions were identified through a linear test, a Rotational Central Composite Design (RCCD), which includes axial points, was not necessary. The Analysis of Variance (ANOVA) presented in Table 3 indicated an R-squared value of 0.9463, demonstrating the adequacy of the linear model for data analysis. As the variables are not fixed, other factors can be investigated, as demonstrated by Silva et al. (2021), who studied variables such as CMC concentration, temperature, and inoculum concentration. Silva et al. (2021) employed a Rotational Central Composite Design and found that the quadratic temperature variable was significant, indicating a negative effect of temperature, with lower temperatures associated with better results, as suggested by the Pareto Chart in his study.
The carbon source selected for our investigation was orange peel, chosen due to the significant amount of waste generated during industrial fruit processing, where approximately half of the fruit weight remains unused, including the peels (Wilkins et al. 2007). Our analysis using CCD and RSM revealed that only the carbon source variable significantly influenced endoglucanase production (p < 0.05), while temperature and incubation time did not exhibit significant effects on the results. Optimal conditions for submerged fermentation were determined to be at 28 °C for 60 h, resulting in an activity of 61 U.mL−1. Following optimization, endoglucanase production increased by 4.3-fold compared to non-optimized conditions. In contrast, Silva et al. (2016) reported endoglucanase production of 244 U.g−1 under submerged fermentation of the same fungus, employing a 1% carbon source comprising cardboard, wheat bran, and sugarcane bagasse, after 168 h of cultivation at 45 °C. The discrepancy in production profiles could be attributed to differences in strains; however, the notable cellulase potential of the fungus T. heterothallicus remains evident.
The endoglucanase isolated in this study, when subjected to electrophoresis, exhibited a molecular weight of 36.3 kDa, consistent with the findings reported by Van Tilbeurgh and Claeyssens (1985), who suggested molecular weights for cellulases ranging from 34 to 250 kDa, with most falling between 25 and 50 kDa. Similarly, an endoglucanase from Botrytis ricini URM 5627, characterized by Silva et al. (2018), displayed a molecular weight of 39 kDa, falling within the range described by Van Tilbeurgh and Claeyssens (1985) and closely resembling the result obtained in this study.
The optimum temperature was determined to be 50 °C (Fig. 4a), with gradual loss of thermostability observed at temperatures exceeding this value, as depicted in Fig. 4b. The enzyme remained stable at temperatures of 40 °C and 50 °C. However, when the temperature was elevated to 60 °C and 70 °C, a gradual decline in activity was observed, with more than 50% of its activity lost within 30 min of reaction. A similar thermostability profile was reported by Rother et al. (2023) for the same fungus in a study on chitinase, where although the optimal temperature was found to be 65 °C, the thermostability above 50 °C appeared to be compromised, suggesting consistency across different enzymatic activities within the same fungal strain.
The strain PA2S4T of T. heterothallicus exhibited an optimal pH of 7.0, with stability observed over a wide pH range tested, spanning from 3.0 to 8.0, albeit with some loss of activity at more extreme pH values. This trend aligns with findings reported for β-glucosidases from T. heterothallicus investigated by Silva et al. (2016), which demonstrated stability between pH 3.5 and 10.0, albeit with an optimal pH of 5.0. Similarly, in a study by Visser et al. (2011), where a strain initially identified as Chrysosporium lucknowense C1 was later recognized as a strain of the Myceliophthora genus, enzymatic activity was detected at pH levels above 7.0, resembling the optimal pH of 7.0 observed for the enzyme in this investigation.
The enzymes can be utilized to treat denim fibers, specifically jeans fabric, aiming to eliminate impurities, lint, and any residual fuzz that may adhere to the fabric (Csiszár et al. 2001). Their natural origin and non-damaging effects on the fabric are considered advantageous. These enzymes facilitate a biopolishing effect, enhancing the uniformity of the fabric's appearance and prolonging its lifespan (Csiszár et al. 2001). In this study, the endoglucanase from T. heterothallicus PA2S4T was applied to jeans. In industrial contexts, an acceptable weight loss during the biopolishing process typically ranges from 3 to 5% (Montazer and Harifi 2018; Choudhury 2017; Šimić et al. 2015). Treatment conditions meeting this standard were achieved with enzyme concentrations ranging from 2 to 5 U.mL−1 (Fig. 5). Therefore, considering cost-effectiveness, the lowest enzyme concentration was chosen for experiments investigating the effects of time, temperature, and pH. The optimal conditions identified were 12 h of incubation at 50 °C and pH 5. The parameters selected for scanning electron microscopy (SEM) were the most significant based on the weight loss tests: 2 U.mL−1 (the lowest enzyme concentration) and the highest enzyme concentration studied (5 U/mL), alongside a control treatment without enzyme addition. In the control group (Fig. 6a), the denim fabric fibers exhibited deformities, with protrusions and irregularities evident. Following the treatments, a polished appearance of the fabric is apparent (Fig. 6b and c), indicating the removal of surface deformities from the fibers. Although significant results were observed even with low enzyme concentrations (Fig. 6b), the most effective biopolishing and impurity removal were achieved with 5 U.mL−1 of enzyme treatment (Fig. 6c). Despite a slightly higher tissue weight loss, it remained within the ideal range reported in the literature, typically between 3 and 5% (Šimić et al. 2015). Bussler et al. (2021), employing a similar methodology but with a concentration of 1 U.mL−1 of Caulobacter crescentus cellulase in sodium citrate buffer (pH 5.5), achieved promising results in tissue biopolishing, with a weight reduction of 2.43%.
Conclusion
The recently identified strain of T. heterothallicus PA2S4T is a thermophilic fungus demonstrating potential for producing a novel endoglucanase utilizing orange peel to stimulate microbial growth. The purified endoglucanase derived from this strain was effectively applied in denim jeans treatment, yielding satisfactory outcomes in biopolishing by enhancing fabric homogeneity while minimizing weight loss. Hence, the endoglucanase from T. heterothallicus PA2S4T exhibits considerable promise for industrial applications. Moreover, this enzyme offers the added advantage of being sourced from crops supplemented with low-cost agro-industrial residues, promoting an environmentally sustainable approach that aligns with the increasing demand for eco-friendly biotechnological solutions in the industrial sector.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alves BA, Rotta IS, Ferreira-Machado AB, Paiva AD (2021) Bioprospection and characterization of cellulase-producing bacteria from Brazilian Cerrado soil. Res Soc Dev 10(8):e34010817426–e34010817426. https://doi.org/10.33448/rsd-v10i8.17426
Aly A, Moustafa A, Hebeish A (2004) Bio-technological treatment of cellulosic textiles. J Clean Prod 12:697–705. https://doi.org/10.1016/S0959-6526(03)00074-X
Andreaus J, Olekszyszen DN, Silveria MHL (2014) Processing of cellulosic textile materials with cellulases. In: Cellulose and other naturally occurring polymers, edn. Research Signpost, Kerala, pp 11–19
Besegatto SV, Costa FN, Damas MSP, Colombi BL, De Rossi AC, de Aguiar CRL, Immich APS (2018) Enzyme treatment at different stages of textile processing: a review. Ind Biotechnol 14(6):298–307. https://doi.org/10.1089/ind.2018.0018
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophor 8(2):93–99. https://doi.org/10.1002/elps.1150080203
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bussler L, Jacomini D, Corrêa JM, Kadowaki MK, Maller A, Simão RCG (2021) Recombinant cellulase of Caulobacter crescentus: potential applications for biofuels and textile industries. Cellulose 28:2813–2832. https://doi.org/10.1007/s10570-021-03700-5
Cavaco-Paulo A (1998) Processing textile fibers with enzymes: an overview.https://doi.org/10.1021/bk-1998-0687.ch015
Cerqueira KS, Silva KM, do Nascimento Silva G, da Silva Rodrigues JR, Lobato A, de Souza RR (2022) Isolation and selection of cellulolytic enzyme-producing microorganisms. Braz J Dev 8(4):28693–28699. https://doi.org/10.34117/bjdv8n4-386
Champasri C, Champasri T, Woranam K (2015) Purification, biochemical characterization of a macrotermesgilvuscellulase and zymogram analysis. Asian J Biochem 10(5):190–204. https://doi.org/10.3923/ajb.2015.190.204
Chen L, Wang L, Wu X, Ding X (2017) A process-level water conservation and pollution control performance evaluation tool of cleaner production technology in textile industry. J Clean Prod 143:1137–1143. https://doi.org/10.1016/j.jclepro.2016.12.006
Choudhury AKR (2017) Various ecofriendly finishes. Principles of textile finishing. Woodhead Publishing, pp 467–525
Cooney DG, Emerson R (1964) Thermophilic fungi. An account of their biology, activities, and classification. W. H. Freeman and Company, San Francisco
Csiszár E, Urbánszki K, Szakács G (2001) Biotreatment of desized cotton fabric by commercial cellulase and xylanase enzymes. J Mol Catal B Enzym 11:1065–1072. https://doi.org/10.1016/S1381-1177(00)00149-1
Drula E et al (2022) The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res 50(D1):D571–D577. https://doi.org/10.1093/nar/gkab1045
Esfandiari A, Firouzi-Pouyaei E, Aghaei-Meibodi P (2014) Effect of enzymatic and mechanical treatment on combined desizing and bio-polishing of cotton fabrics. J Text Inst 105(11):1193–1202. https://doi.org/10.1080/00405000.2014.880222
Ejaz U, Saleem F, Rashid R et al (2023a) Unveiling the genomic potential of a novel thermostable glycoside hydrolases producing Neobacillus sedimentimangrovi UE25. Antonie Van Leeuwenhoek 116:653–665. https://doi.org/10.1007/s10482-023-01835-y
Ejaz U, Moin SF, Sohail M, Mersal GAM, Ibrahim MM, El-Bahy SM (2023b) Characterization of a novel end product tolerant and thermostable cellulase from Neobacillus sedimentimangrovi UE25. Enzyme Microb Technol 162:110133. https://doi.org/10.1016/j.enzmictec.2022.110133
Gaete AV, Teodoro CEDS, Martinazzo AP (2020) Use of agro-industrial waste for cellulase production: a review. https://doi.org/10.33448/rsd-v9i8.5785
Khanna P, Sundari SS, Kumar NJ (1995) Production, isolation and partial purification of xylanases from an Aspergillus sp. World J Microbiol Biotechnol 11:242–243. https://doi.org/10.1007/BF00704661
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Leo VV, Zothanpuia L, Ramesh N, Singh BP (2019) Microorganisms as an efficient tool for cellulase production: availability, diversity, and efficiency. Elsevier B.V. https://doi.org/10.1016/B978-0-444-64223-3.00004-7
Madhu A, Chakraborty JN (2017) Developments in application of enzymes for textile processing. J Clean Prod 145:114–133. https://doi.org/10.1016/j.jclepro.2017.01.013
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Montazer M, Harifi T (2018) Nanofinishing of textile materials. Woodhead Publishing
Qadir F, Ejaz U, Sohail M (2020) Co-culturing corncob-immobilized yeasts on orange peels for the production of pectinase. Biotechnol Lett 42:1743–1753. https://doi.org/10.1007/s10529-020-02897-yy
Pommer V, Rother PDH, Rasbold LM, da Conceição Silva JL, Maller A, Simão RDCG, Kadowaki MK (2021) A novel Thermothelomyces heterothallicus PA2S4T fungus isolated from the soil induces chitinase production using orange peel flour. Sci Plena 17(9). https://doi.org/10.14808/sci.plena.2021.091501
Ranjan R, Rai R, Bhatt SB, Dhar P (2023) Technological road map of cellulase: a comprehensive outlook to structural, computational, and industrial applications. Biochem Eng J 198:109020. https://doi.org/10.1016/j.bej.2023.109020
Rother PDH, Pommer V, Zuppa ES, Maller A, Silva JLC, Simão RCGS, Kadowaki MK (2023) Shrimp shell bioconversion and prebiotic production by Chitinases from Thermothelomyces heterothallicus PA2S4T. Waste Biomass Valor 1–12. https://doi.org/10.1007/s12649-023-02214-4
Sahin S, Ozmen I, Biyik H (2016) Industrial applications of endoglucanase obtained from novel and native Trichoderma atroviride. Chem Biochem Eng Q 30:265–278. https://doi.org/10.15255/CABEQ.2014.2130
Santos FA, de Carvalho-Gonçalves LCT, de Carvalho Cardoso-Simões AL, de Melo Santos SF (2021) Evaluation of the production of cellulases by Penicillium sp FSDE15 using corncob and wheat bran as substrates. Bioresour Technol Rep 14:100648. https://doi.org/10.1016/j.biteb.2021.100648
Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Karsch-Mizrachi I (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database 2020:baaa062. https://doi.org/10.1093/database/baaa062
Silva RN, Melo LFA, Luna Finkler CL (2021) Optimization of the cultivation conditions of Bacillus licheniformis BCLLNF-01 for cellulase production. Biotechnol Rep (Amsterdam, Netherlands) 29:e00599. https://doi.org/10.1016/j.btre.2021.e00599
Silva TP, de Albuquerque FS, Dos Santos CWV, Franco M, Caetano LC, Pereira HJV (2018) Production, purification, characterization and application of a new halotolerant and thermostable endoglucanase of Botrytis ricini URM 5627. Bioresourcetechnology 270:263–269. https://doi.org/10.1016/j.biortech.2018.09.022
Silva VDCT, de Souza Coto, AL, Souza RC, Neves, MBS, Gomes E, Bonilla-Rodriguez GO (2016) Effect of pH, temperature, and chemicals on the endoglucanases and β-glucosidases from the thermophilic fungus Myceliophthora heterothallica F. 2.1. 4. Obtained by solid-state and submerged cultivation. Biochem Res Int 2016https://doi.org/10.1155/2016/9781216
Šimić K, Soljačić I, Pušić T (2015) Application of cellulases in the process of finishing. Tekstilec 58(1):47–56. https://doi.org/10.14502/Tekstilec2015.58.47−56
Sohail M, Barzkar N, Michaud P, Tamadoni Jahromi S, Babich O, Sukhikh S, Das R, Nahavandi R (2022) Cellulolytic and xylanolytic enzymes from yeasts: properties and industrial applications. Molecules 27(12):3783. https://doi.org/10.3390/molecules27123783
Van Tilbeurgh H, Claeyssens M (1985) Detection and differentiation of cellulase components using low molecular mass fluorogenic substrates. FEBS Lett 187(2):283–288. https://doi.org/10.1016/0014-5793(85)81260-6
Visser H, Joosten V, Punt PJ, Gusakov AV, Olson PT, Joosten R, Wery J (2011) Development of a mature fungal technology and production platform for industrial enzymes based on a Myceliophthora thermophila isolate, previously known as Chrysosporium lucknowense C1. Ind Biotechnol 7(3):214–223. https://doi.org/10.1089/ind.2011.7.214
Uday USP, Majumdar R, Tiwari ON, Mishra U, Mondal A, Bandyopadhyay TK, Bhunia B (2017) Isolation, screening and characterization of a novel extracellular xylanase from Aspergillus niger (KP874102. 1) and its application in orange peel hydrolysis. Int J Biol Macromol 105:401–409
Wilkins MR, Widmer WW, Grohmann K, Cameron R (2007) Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Biores Technol 98(8):1596–1601. https://doi.org/10.1016/j.biortech.2006.06.022
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Araucária Foundation, and Paraná Western State University (UNIOESTE).
Funding
This work was supported by National Council for Scientific and Technological Development (CNPq) (Grant numbers 458859/2014–1), Coordination for the Improvement of Higher Education Personnel (CAPES) and Araucária Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by B.D., V.M.D, W.J.S. and A.M. The first draft of the manuscript was written by B.D., M.K.K., J.L.C.S., R.C.G.S and A.M and all authors commented on previous versions of the manuscript. The review of the manuscript and experiments was carried out by W.J.S, T.D.B, M.R.S. and A.M. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to publication
All the authors consent for the publication of this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Detoni, B., Delai, V.M., da Silva, W.J. et al. Production and partial characterization of endoglucanase by Thermothelomyces heterothallicus PA2S4T and its application in biopolishing of denim jeans. Cellulose 31, 6989–7001 (2024). https://doi.org/10.1007/s10570-024-06002-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-06002-8