Abstract
Solid-state 13C nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for identifying the various allomorphs of cellulose, for example quantifying the proportions of cellulose Iα and Iβ allomorphs in the crystalline domains of various native celluloses. While marine invertebrate animals known as tunicates produce nearly pure cellulose Iβ, solid-state NMR reveals that bacterial cellulose is dominated by the Iα allomorph, but also has additional signals arising from a secondary crystalline form that are normally attributed to cellulose Iβ. However, in this paper we show that the 13C chemical shifts and correlation patterns in 2D NMR spectra for this secondary crystalline form in bacterial cellulose are not the same as those found in cellulose Iβ spectra reported for tunicate cellulose. In order to reach this conclusion, it was necessary to ensure all spectra were correctly set to the same chemical shift scale. In doing so, it became apparent there were spectral features for bacterial cellulose that were entirely consistent with previously reported spectra of bacterial cellulose but did not match those of the Iβ allomorph found in tunicate cellulose. Through a careful and detailed analysis of the 1D and 2D NMR spectra of three 13C-enriched bacterial cellulose samples, two sets of correlated 13C chemical shifts for this secondary crystalline form were identified. The fact that these chemical shifts and correlation patterns are different than those of tunicate cellulose suggests that there exists some sort of structural variation from the Iβ allomorph in bacterial cellulose since the 13C NMR spectrum closely resembles, but is not identical to, the 13C spectrum of the Iβ allomorph found in tunicate cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, the most abundant biopolymer on Earth, is a polysaccharide consisting of linear chains of D-glucose units connected via β (1 \(\to\)4) linkages. While this basic polymeric structure seems relatively straightforward, cellulose exhibits a surprising degree of polymorphism once these cellulose chains assemble into elementary fibrils and/or are subjected to various treatments (O’Sullivan 1997; French 2014). Native celluloses, which are produced by a wide range of living organisms (including bacteria, algae, some marine animals, and all plants), are usually referred to as cellulose I. In the elementary fibrils of cellulose I, the chains are all oriented in the same direction and assemble in a parallel fashion (Nishiyama et al. 2002, 2003). Cellulose that has been regenerated (dissolved in a solvent, then recrystallized) or mercerized (treated with concentrated sodium hydroxide solution) is referred to as cellulose II and has neighboring chains within a fibril oriented in an anti-parallel fashion (Langan et al. 2001). Cellulose allomorphs IIII (Wada et al. 2004a) and IIIII (Wada et al. 2009) are generated by treating cellulose I or II, respectively, with ammonia and allowing the excess ammonia to evaporate. Finally, cellulose has also been proposed to transform into allomorphs IVI and IVII when cellulose IIII or IIIII are subjected to heat treatment, although these structures are poorly characterized and may not actually be unique allomorphs (Wada et al. 2004b).
Solid-state 13C nuclear magnetic resonance (NMR) spectroscopy has played a key role in identifying the existence of cellulose allomorphs and in characterizing their structures (Atalla and Vanderhart 1999). In particular, the pioneering work by Vander Hart and Atalla employed solid-state 13C NMR spectroscopy to demonstrate that the crystalline regions of most native celluloses were actually composites of two allomorphs, referred to as cellulose Iα and cellulose Iβ (Atalla and Vanderhart 1984; VanderHart and Atalla 1984, 1987), with the relative proportions of the allomorphs depending on the source of the cellulose. Cellulose from algal and bacterial sources tend to be rich in cellulose Iα, while cellulose from plants and tunicate marine invertebrate animals tend to be rich in cellulose Iβ, with some tunicate cellulose samples being nearly pure cellulose Iβ (Belton et al. 1989; Larsson et al. 1995; Malm et al. 2010).
Crystal structures for the cellulose Iα (Nishiyama et al. 2003) and cellulose Iβ (Nishiyama et al. 2002) allomorphs have subsequently been determined from neutron and synchrotron X-ray diffraction data collected on oriented cellulose fibers. Both structures consist of parallel chains that assemble into sheets, with O–H···O hydrogen bonding between adjacent chains within the sheets, which stack and interact via weak van der Waals forces and weak C–H···O hydrogen bonds between sheets. The main differences between the cellulose Iα and Iβ allomorphs are the stacking pattern of these sheets and the fact that cellulose Iα has a triclinic P1 space group with one type of chain made up of two crystallographically distinct glucose units that alternate (Nishiyama et al. 2003), while cellulose Iβ has a monoclinic P21 space group with two types of chains, each made up of repeating glucose units that are related by the P21 screw axis symmetry (Nishiyama et al. 2002).
In agreement with the reported crystal structures, the solid-state 13C NMR spectra of cellulose Iα and Iβ allomorphs both have two signals for each carbon of the glucose monomers, arising from the two crystallographically distinct glucose units in each of the cellulose Iα and Iβ crystal structures. Through selective 13C-enrichment (Kono et al. 2002) and two-dimensional (2D) NMR correlation experiments (Kono et al. 2003a), sets of correlated 13C chemical shifts assigned to the crystallographically distinct glucose units of the cellulose Iα and Iβ allomorphs have been proposed.
The more recent solid-state NMR work by Wang et al. (2016) on plant primary cell walls raises some interesting questions about whether or not the long-standing assumption that the crystalline regions of native celluloses are limited to a combination of the Iα and Iβ allomorphs. They reported 2D solid-state NMR correlation experiments on the primary cell walls of 13C-enriched plants that indicate the cellulose in plant primary cell walls has polymorphic structures that are not a simple superposition of Iα and Iβ allomorphs. They observed 5 sets of correlated 13C chemical shifts that were assigned to cellulose in the interior of the fibrils (referred to as celluloses a through e) and 2 sets of chemical shifts assigned to surface cellulose (celluloses f and g). Since these sets of 13C chemical shifts arising from interior cellulose were found to differ significantly from the chemical shifts reported for cellulose Iα and Iβ, they suggested that plant primary-wall cellulose may have different conformations, packing, and hydrogen bonding from the cellulose of other organisms, most likely arising from the lower crystallinity and extensive interactions with the matrix polysaccharides present in the cell walls (Wang et al. 2016). Similar deviations from cellulose Iα and Iβ allomorphs have also been observed in subsequent solid-state NMR studies of plant secondary cell walls (Kang et al. 2019; Kirui et al. 2022).
We have recently shown that, unfortunately, the 13C chemical shifts reported for cellulose Iα and Iβ are often incorrectly reported due to inconsistent and sometimes incorrect chemical shift referencing (Brouwer and Mikolajewski 2023), including the 13C chemical shifts reported by Kono et al. (2003a, b). After correcting the chemical shift scales of numerous spectra taken from the cellulose solid-state NMR literature, we have resolved many of these inconsistencies and presented a definitive set of 13C chemical shifts for cellulose Iα and Iβ. In this same paper, we also presented a 2D double-quantum (DQ)/single-quantum (SQ) correlation spectrum collected on a Iα-rich bacterial cellulose sample that was used to confirm the corrected 13C chemical shifts and peak assignments for the cellulose Iα allomorph. This spectrum provided clear evidence that the assignment of the C2 signals for cellulose Iα by Kono et al. (2003a, b) was in error and was in need of correction as well.
This 2D NMR correlation spectrum also displayed some weaker correlations that were initially assumed to arise from the cellulose Iβ allomorph, since like most native celluloses, the crystalline regions of bacterial cellulose have long been assumed to be a superposition of cellulose Iα and Iβ based on the shapes of the C1 and C4 signals. However, with all of the cellulose 13C NMR spectra now on the same corrected chemical shift scale, as these weaker signals in the 1D and 2D spectra of bacterial cellulose were closely scrutinized, it became evident they did not have the same 13C chemical shifts and correlation patterns as the cellulose Iβ spectrum derived from tunicate cellulose. Through a careful and detailed analysis of 1D and 2D solid-state 13C NMR spectra of various 13C-enriched bacterial cellulose sample, this paper aims to show that these differences in the solid-state 13C NMR spectra suggest that the secondary crystalline form in bacterial cellulose, which appears at first glance to be cellulose Iβ, is somehow subtly different than the cellulose Iβ allomorph found in tunicate cellulose.
Materials and methods
Bacterial cellulose samples
Three bacterial cellulose samples were investigated in this work, each with different 13C enrichment schemes. The first sample, (U-13C6)-cellulose, was uniformly 13C enriched at each carbon site at about 25% by employing (U-13C6, 25%)-D-glucose (Sigma-Aldrich) as the carbon source. The preparation of this sample has been described elsewhere (Brouwer and Mikolajewski 2023). Solid-state NMR spectra (Figs. 1a, 2a, and 3) were collected on this sample while it was still moist.
Solid-state 13C CP MAS NMR spectra of 13C-enriched bacterial cellulose samples: a uniformly 13C enriched cellulose biosynthesized with (U-13C6, 25%)-D-glucose as the carbon source, b cellulose with 13C-enrichment primarily at carbons C1, C3, C4, C6 biosynthesized with (1,3-13C2)-glycerol as the carbon source, c cellulose with 13C enrichment primarily at carbons C1 and C4 on different glucose units biosynthesized from a carbon source with equal amounts of (1-13C)-D-glucose and (4-13C)-D-glucose. The numbers associated with each carbon signal indicate the percentage of its peak area to the total spectrum peak area, including both crystalline and non-crystalline signals, based on fitting model spectra to the experimental spectra (see Supporting Information)
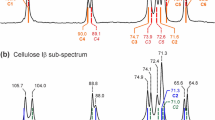
a13C CP MAS NMR spectra of the 25% uniformly 13C-enriched bacterial cellulose sample investigated in this work, b previously reported spectra of bacterial cellulose that have been digitized and corrected to be on the same chemical shift scale (with liquid TMS at 0 ppm), c idealized spectra of cellulose Iα and Iβ calculated from chemical shifts reported in (Brouwer and Mikolajewski 2023). The solid grey lines highlight the cellulose Iα signals that appear in all bacterial cellulose spectra, while the green and blue dashed lines highlight the C3 and C4 signals at 75.9 ppm and 88.7 ppm, respectively, that appear in all bacterial cellulose spectra but not in the spectra of cellulose Iα or Iβ. Adapted with permission from VanderHart DL, Atalla RH (1984) Macromolecules 17:1465–1472, Copyright (1984) American Chemical Society; Horii F, Hirai A, Kitamaru R (1987) Macromolecules 20: 2117–2120, Copyright (1987) American Chemical Society; Fink H-P, Purz HJ, Bohn A, Kunze J (1997) Macromolecular Symposia 120:207–217, Copyright (1997) John Wiley & Sons; Koch F-T, Prieß W, Witter R, Sternberg U (2000) Macromolecular Chemistry and Physics 201:1930–1939, Copyright (2000) John Wiley & Sons; Sternberg U, Koch F-T, Priess W, Witter R (2003) Cellulose 10:189–199, Copyright (2003) Springer Nature; Malm E, Bulone V, Wickholm K, Larrson PT, Iversen T (2010) Carbohydrate Research 345:97–100, Copyright (2010) Elsevier
a Experimental 1D 13C CP MAS NMR spectrum of 25% uniformly 13C-enriched bacterial cellulose, along with the best-fit calculated model spectrum which is composed of contributions from cellulose Iα, cellulose Iβ’ proposed in this work, and non-crystalline cellulose (see Fig. S1 and Table S1 in Supporting Information for details). b 2D 13C DQ/SQ correlation spectrum of 25% uniformly. 13C-enriched bacterial cellulose collected with a short dipolar recoupling time such that correlations are limited mainly to directly connected carbons. c Zoomed regions for each correlation of the crystalline cellulose regions. The upper plots have transparent colored circles indicating the SQ and DQ chemical shifts of the correlations arising from within each glucose unit of the various allomorphs. The lower plots have calculated DQ correlation intensities superimposed on the experimental contour plots. The chemical shifts are listed in Table 1
The second sample, (1,3,4,6-13C4)-cellulose, was enriched primarily at the C1, C3, C4, and C6 carbon sites by employing (1,3-13C2, 99%)-glycerol (Cambridge Isotope Laboratories) as the carbon source. The third sample, (1,4-13C2)-cellulose, was enriched primarily at the C1 and C4 carbon sites in different glucose units by employing a carbon source consisting of equal amounts of (1-13C, 99%)-D-glucose and (4-13C, 99%)-D-glucose (Cambridge Isotope Laboratories). These two samples were biosynthesized by growing Acetobacter xylinum (ATCC 23769) bacterial cultures at 30 °C in sterile Hestrin and Schramm medium (Hestrin and Schramm 1954) with the carbon sources described above. After 20 days, the cellulose pellicles were extracted, washed, and blended with 1 M NaOH. The resulting slurry was centrifuged and the supernatant was decanted. This was followed by two additional cycles of re-suspension in water and centrifugation. Solid-state NMR spectra (Figs. 1b, 1c, and 4) were collected on these samples after they had mostly dried out.
Zoomed regions of the main two-bond correlations in 2D 13C DQ/SQ correlation spectra of bacterial cellulose samples a 13C enriched at C1 and C4 showing correlations between adjacent glucose units across the C1–O–C4 glycosidic linkage b, c 13C-enriched at C1, C3, C4, and C6 showing correlations between b C1 and C3 within a glucose unit and c C4 and C6 within a glucose unit. The 1D spectra above are the experimental and calculated spectra with Iα and Iβ’ components (see Supporting Information for details). The upper correlation plots have transparent colored circles indicating the SQ and DQ chemical shifts of the relevant correlations. The lower plots have calculated DQ correlation intensities superimposed on the experimental contour plots
Solid-state NMR spectroscopy
The parameters for the solid-state NMR experiments on the bacterial (U-13C6)-cellulose sample (Figs. 1a, 2a, and 3) have been previously described (Brouwer and Mikolajewski 2023). Briefly, the experiments were carried out on a Bruker Avance III 850 MHz instrument (20.0 T, 213.8 MHz 13C Larmour frequency) using a 1.9 mm double resonance probe with 14.815 kHz MAS frequency, 2 ms cross polarization time, and SPINAL-64 1H decoupling (Fung et al. 2000) during acquisition. Approximately 5 mg of sample was packed into the rotor. The 1D 13C CP MAS spectrum was collected with 128 co-added transients and a 4 s recycle delay. The 2D 13C DQ/SQ correlation experiment employed a symmetry-based R1665 dipolar homonuclear recoupling sequence (Carravetta et al. 2000) with a recoupling time of 0.423 ms which primarily excites DQ coherences between nearest-neighbor 13C nuclei (approximately 1.5 Å).
The spectra for the (1,4-13C2)-cellulose sample (Figs. 1c and 4a) and the (1,3,4,6-13C4)-cellulose sample (Figs. 1b, 4b, c) were collected on a Varian InfinityPlus 300 MHz spectrometer (7.0 T, 75.40 MHz 13C Larmour frequency) using a 4 mm double resonance probe with a 5.500 kHz MAS frequency, 1 ms cross polarization time, SPINAL-64 1H decoupling (Fung et al. 2000) during acquisition, 2 s recycle delay, and either 64 (1D spectra) or 96 (2D spectra) co-added transients. Approximately 50 mg of sample was packed into the rotors. The 2D 13C DQ/SQ correlation spectra were acquired with the symmetry-based R26411 homonuclear dipolar recoupling sequence for DQ excitation and reconversion (Carravetta et al. 2000). The basic R element was a composite (90)0(270)180 pulse and 72 blocks of appropriately phase-shifted R elements were used for DQ excitation and reconversion (2.014 ms recoupling time). This recoupling time was found to be optimal for exciting DQ coherences between 13C nuclei that are separate by two bonds (approximately 2.5 Å). The indirect dimension was obtained with 256 t1 FIDs and employed the States method (States et al. 1982) to obtain pure absorption spectra.
For all spectra, the 13C chemical shift scale was referenced to liquid tetramethylsilane (TMS) at 0 ppm by setting the CH signal of adamantane to 38.48 ppm (Morcombe and Zilm 2003). The previously reported spectra of bacterial cellulose reproduced here in Fig. 2b were digitized from their original publications and had their chemical shift axes adjusted so that here they are correctly referenced with respect to liquid TMS at 0 ppm, following the protocol recently described by (Brouwer and Mikolajewski 2023). For a few of these digitized spectra, it was not clear from the corresponding papers how the chemical shift axis referencing was actually set; in these cases, the chemical shift axis was set so that the peak maxima for the C1 and C6 signals lined up with the other spectra.
Fitting of the experimental 1D and 2D spectra with model spectra was carried out with a spectrum fitting program written as a Mathematica notebook (Wolfram Research Inc 2021) which is provided in the Supporting Information along with the raw data for each spectrum.
Results and discussion
1D NMR spectra of bacterial cellulose
Figure 1 displays the 1D 13C CP MAS spectra of the three 13C-enriched bacterial cellulose samples investigated in this work (details on the models used to fit these spectra are available in the Supporting Information). The spectrum of the bacterial cellulose biosynthesized from 25% uniformly 13C-enriched D-glucose (Fig. 1a) clearly shows that all six of the carbon sites have been 13C-enriched to the same degree. For the bacterial cellulose sample biosynthesized from (1,3-13C2)-glycerol, its spectrum (Fig. 1b) displays strong C1, C3, C4, and C6 signals with weaker C2 and C5 signals, indicating quite selective enrichment at the C1, C3, C4, and C6 sites. Similarly, for the bacterial cellulose sample biosynthesized from a mixture of (1-13C)-D-glucose and (4-13C)-D-glucose, its spectrum (Fig. 1c) displays strong C1 and C4 signals, a slightly enhanced C3 signal, weak C2 and C5 signals, and a very weak C6 signal, indicating quite selective enrichment at the C1 and C4 sites. From the biosynthetic pathway for cellulose production by Acetobacter xylinus (Ross et al. 1991), it is known that isotopic label scrambling can take place, mainly through the pentose phosphate pathway (Arashida et al. 1993; Kai et al. 1994), but it seems that the scrambling was relatively minimal for these samples such that the vast majority of the 13C labels ended up in their intended locations.
It is worth commenting on the difference in resolution in Fig. 1a compared to Figs. 1b and 1c. The uniformly enriched sample was collected while still moist and the other two samples were collected in a mostly dry state. Consistent with previous investigations such as Fink et al. (1997), the resolution in the spectra for the moist sample is greater than the dried samples. While it certainly would have been better if all spectra were collected on samples that were moist, the main conclusions of this paper are not affected by this difference between the samples. Note that while there is quite a large difference in the peak widths of the non-crystalline signals between the moist and dry conditions, there is only a small difference in the peak widths attributed to the crystalline domains which are the focus of this paper. Based on model spectra calculated to fit these experimental spectra in which the chemical shifts were identical across all three samples (see Supporting Information), the average peak widths for the non-crystalline signals in Figs. 1a, b, and c varied quite a bit at 1.6 ppm, 3.2 ppm, and 2.6 ppm, respectively, while the average peak widths for the crystalline signals varied only a small amount at 0.56 ppm, 0.76 ppm, and 0.66 ppm, respectively.
Comparison to previously reported spectra
The 13C CP MAS NMR spectrum of the 25% uniformly 13C-enriched bacterial cellulose sample is presented again in Fig. 2a to allow for a focused discussion of its key features. At first glance, this spectrum contains many of the typical features expected for a native cellulose sample that is a superposition of the cellulose Iα and Iβ allomorphs. The C4 signal, as well as the C1 signal to a lesser extent, has the classic “triplet” peak shape that usually arises from the pair of signals for each of the cellulose Iα and Iβ allomorphs (Atalla and Vanderhart 1984; VanderHart and Atalla 1984, 1987). In the case of the C4 signal, one of the Iα signals and one of the Iβ signals usually overlap to give the central peak of this triplet that has the greatest intensity. In the case of the C1 signal, the central large peak of the triplet arises from the nearly identical chemical shifts of the Iα signals, while the lower intensity outer signals usually arise from the two different Iβ signals.
In addition, the spectrum has broad signals for C4 and C6 at lower chemical shifts than the crystalline signals which arise from non-crystalline disordered cellulose domains and/or cellulose chains on the surface. There is also a slightly broadened non-crystalline/surface signal for C1 underneath the sharper crystalline signals that make up the C1 triplet. Superimposed on the broad C4 and C6 signals are some low intensity, but sharper signals that may be attributed to cellulose chains on the surfaces of fibrils (Malm 2010) that adopt different conformations at the C6 position than the interior cellulose chains (Phyo et al. 2018).
Figure 2b displays a series of previously reported 13C spectra of bacterial cellulose (VanderHart and Atalla 1984; Horii et al. 1987; Fink et al. 1997; Koch et al. 2000; Sternberg et al. 2003; Malm et al. 2010) showing that the spectrum of our bacterial cellulose sample is very consistent with previously reported spectra and none of the features in our spectra are unique to our sample. It is important to note that in order to make this comparison, these spectra were digitized and corrected to be on the same chemical shift scale with respect to liquid TMS at 0 ppm, following the process described in our recent paper (Brouwer and Mikolajewski 2023).
Figure 2c displays idealized sub-spectra for cellulose Iα and Iβ calculated using the 13C chemical shifts reported in our recent paper (Brouwer and Mikolajewski 2023) which result after a variety of errors in the reporting and referencing of the 13C chemical shifts of cellulose Iα and Iβ are accounted for and corrected. The Iα sub-spectrum was derived from a 13C spectrum of a Iα-rich sample from Cladophora algae, while the Iβ sub-spectrum was derived from the 13C spectrum of a Iβ-rich sample from a Halocynthia tunicate marine organism (Kono et al. 2003a).
With all of these spectra correctly reported on the same 13C chemical shift scale, a peculiar anomaly can be observed: while the bacterial cellulose spectra all show signals that correspond to the cellulose Iα allomorphs (light grey lines in Fig. 2), there are several features in the spectra of bacterial cellulose that do not correspond to the expected signals for the cellulose Iβ allomorph, as highlighted by the dashed blue and green lines in Fig. 2. The upfield peak of the C4 triplet at 88.7 ppm (blue line) does not directly correspond to either of the C4 peaks of cellulose Iβ and there is a C3 signal shifted downfield at 75.9 ppm (green line) that does not correspond to either of the C3 signals of cellulose Iβ. Importantly, a close inspection reveals that these anomalous features are present in all of the previously reported bacterial cellulose spectra reproduced here (Fig. 2b). To our knowledge, these deviations from the expected cellulose Iβ chemical shifts have not been noticed or commented on before. These anomalous 13C signals suggest that bacterial cellulose does not contain exactly the same Iβ structure as in tunicate cellulose, but rather some sort of subtle structural modification from the cellulose Iβ allomorph. From here on, we refer to this different cellulose Iβ form in bacterial cellulose as cellulose Iβ’ to differentiate it from the cellulose Iβ allomorph found in tunicate cellulose.
2D NMR spectra of bacterial cellulose
To further characterize this unexpected cellulose Iβ’ form in bacterial cellulose, a series of 2D 13C DQ/SQ correlation experiments were carried out on the 13C-enriched bacterial cellulose samples. Figure 3 displays the full 2D 13C correlation spectrum for the 25% uniformly 13C-enriched bacterial cellulose sample collected with a short dipolar recoupling time such that correlations between covalently-bonded carbons in close spatial proximity are emphasized. Pairs of signals appear as correlations at their respective isotropic chemical shifts in the direct SQ dimension and at the sum of their chemical shifts in the indirect DQ dimension. It should be noted that this is the same 2D spectrum reported previously in Brouwer and Mikolajewski (2023), but with more contour levels shown at lower intensities.
Figure 4a displays the main correlations observed in a 2D 13C correlation spectrum for the bacterial cellulose sample 13C-enriched at positions C1 and C4 while Figs. 3b, c display the main correlations observed for the bacterial cellulose sample enriched at positions C1, C3, C4, and C6. For all 2D spectra in Fig. 4, a longer dipolar recoupling time was used in order to excite DQ correlations involving carbons separated by two bonds: C1–C4 across the C1–O–C4 glycosidic linkages between adjacent glucose units and C1–C3 and C4–C6 within the glucose units.
These 2D correlation spectra provide additional spectral resolution and valuable correlation information that allow for the identification and assignment of the 13C signals in this bacterial cellulose Iβ’ form. Before getting into the detailed analysis of these spectra, it is worth mentioning that these 2D correlation spectra also provide additional evidence that this bacterial cellulose Iβ’ form differs from cellulose Iβ in tunicate cellulose. Figs. S4 and S5 in the Supporting Information show these 2D spectra overlaid with the expected correlations based on the 13C chemical shifts for the Iβ sub-spectrum derived from tunicate cellulose. It is quite clear, especially from the correlations involving C3 and C4, that the tunicate cellulose Iβ 13C chemical shifts are not fully consistent with these 2D correlation spectra. Therefore, we set out to identify the set of 13C chemical shifts and peak assignments of this bacterial cellulose Iβ’ form from these 1D and 2D NMR spectra. In the paragraphs that follow, we describe our analysis of these spectra which led to the final model with a set of 13C chemical shifts and peak assignments for this bacterial cellulose Iβ’ form reported in Table 1.
Analysis of 1D and 2D NMR spectra
The goal of this analysis was to find the set of 13C chemical shifts for this bacterial cellulose Iβ’ form and to group the signals into two sets that correspond to the two crystallographically distinct glucose units that appear to be present in this structure. There were three main criteria that needed to be met to reach a satisfactory analysis: (1) the calculated 1D spectra based on the chemical shifts had to be in good agreement with the experimental 1D spectra (see tops of Figs. 3a and 4); (2) the positions of the correlations in both the direct SQ dimension and the indirect DQ dimension had to match the observed correlations in the experimental 2D spectra; (3) the calculated DQ correlation intensities in the 2D spectra had to be in good agreement with the observed intensities and the “shapes” of the correlations in the experimental 2D spectra.
The 2D correlation spectrum of the 25% uniformly 13C-enriched bacterial cellulose sample (Fig. 3) was first analyzed. Since the dominant form in bacterial cellulose is the cellulose Iα allomorph, the cellulose Iα chemical shifts were first established by carefully matching the Iα chemical shifts to the most intense correlation signals in this spectrum (Brouwer and Mikolajewski 2023). These are represented by the orange and red correlations in Fig. 3.
Next, some of the weak correlations involving the non-crystalline cellulose were used to establish an initial estimate of the chemical shifts for these broad signals. There are obvious correlations between C4–C3, C4–C5, and C5–C6 that are well-separated from the rest of the signals and some broadened features in the C1–C2 and C3–C2 correlations that allow for an initial estimation of these chemical shifts. These are represented by the light grey correlations in Fig. 3. It should be noted that the chemical shifts used here for the non-crystalline cellulose are very similar to those reported by Mori et al. (2012) from a 2D correlation spectrum of amorphous cellulose. In particular they also observe the C5 shift overlapping with the C3 shift, while the C2 shift is somewhat upfield from these signals.
Some of the weaker features thus far unaccounted for in the various correlations were then attributed to the bacterial cellulose Iβ’ form (blue and green correlations in Fig. 3). This was most straightforward for the C4–C3 correlation in which there was quite obvious signal intensity distinct from cellulose Iα, especially for the C3 signal (see Fig. 3c). However, for other correlations it was less straightforward; for these cases the chemical shifts were initially set to those of cellulose Iβ derived from tunicate cellulose.
After inspecting the one-bond correlations in Fig. 3, the two-bond correlations in Fig. 4 were then inspected to check for consistency of the proposed chemical shifts. For example, the C1–C4 correlation (Fig. 4a) between C1 and C4 across the C1–O–C4 glycosidic linkage quite clearly indicates that the Iβ’ C1 and C4 signals with lower chemical shifts (blue) correlate to each other, while the signals with higher chemical shifts (green) correlate to each other. A similar correlation pattern is observed for the C1–C3 correlation in Fig. 4b.
With a set of initial chemical shifts for the Iα, Iβ’, and non-crystalline contributions derived from the one-bond (Fig. 3) and two-bond (Fig. 4) 2D correlations, model 1D spectra were then calculated and fit to the experimental spectra (see Fig. 3a and top of Fig. 4). In this fitting process, the peak areas of the various carbon signals for each of the cellulose forms were constrained to be equal to each other for the spectrum in Fig. 3a. For example, all of the peaks for cellulose Iβ’ were constrained to have the same peak area in the fitting process, as were the peaks for cellulose Iα and non-crystalline cellulose, reflecting the assumption that the carbon sites within a given form have equal crystallographic populations and that the 13C CP MAS spectrum is sufficiently quantitative. The crystalline Iα and Iβ’ signals were modelled with Lorentzian peaks, while the non-crystalline signals were modeled with mixed Lorentzian/Gaussian peaks. The width of each peak was generally allowed to refine freely, except for closely overlapping or poorly resolved peaks which were constrained to be equal to each other. For example, the C6 peaks for both cellulose Iα and Iβ’ were constrained to all have the same peak width. Similarly, the closely overlapping C2 and C5 peaks for cellulose Iα and Iβ’ were also all constrained to have the same peak width. Importantly, the chemical shifts for the model spectra for all three samples with varying 13C enrichment were always kept identical in both the 1D and 2D spectra; only the peak widths and the peak areas were allowed to vary between samples. Furthermore, when fitting the 1D spectra in Fig. 4, the relative proportions of the Iα, Iβ’, and non-crystalline forms were fixed to those found when fitting the spectrum of the uniformly 13C-enriched sample in Fig. 3a.
Once fits to the 1D spectra were completed, the 2D correlation intensities were then modeled and fit to the experimental one-bond and two-bond 2D spectra (bottom of Figs. 3c and 4). The model 2D spectra employed the chemical shifts and peak widths established in the fits to 1D spectra and only the peak volumes and a single peak width parameter for the indirect dimension were varied. The peak volumes were constrained such that the ratio of Iα to Iβ’ established in the fits to the 1D spectra were preserved for each correlation.
The analysis of these spectra was an iterative process in which the model chemical shifts were adjusted to visually match the 2D correlations as best as possible, then a model 1D spectrum was calculated and fit to the experimental spectrum by allowing adjustments to the peak areas, peak widths, and some of the chemical shifts. The correlation intensities for the 2D spectra would then be calculated and compared to experimental 2D spectra. This process was iterated numerous times with small changes in the model until a satisfactory agreement between all calculated and experimental spectra was reached. Several peak assignment schemes were considered for bacterial cellulose Iβ’ and, in the end, a very good agreement between one peak assignment model and all experimental 1D and 2D spectra was found, as shown in Figs. 3 and 4. The chemical shifts for this model are reported in Table 1 while the full fitting parameters for each of the 1D spectra are provided in the Supporting Information.
According to the model fit to the 1D spectrum of the uniformly 13C-enriched bacterial cellulose sample, these bacterial cellulose samples are estimated to be 56% cellulose Iα, 23% cellulose Iβ’, and 21% non-crystalline cellulose (see Fig. S1 and Table S1 in the Supporting Information). Another way of expressing this would be to say that the samples are 79% crystalline with the crystalline regions being 71% cellulose Iα and 29% cellulose Iβ’. Note that this probably underestimates the crystalline domains and overestimates the non-crystalline domains since these spectra were collected with relatively short recycle delays (2 to 4 s) which can potentially lead to an enhancement of the non-crystalline signals with shorter T1 relaxation times over the crystalline signals which tend to have longer T1 relaxation times (Horii et al. 1984).
It is worth emphasizing how strongly constrained the chemical shifts are in this analysis of both 1D and 2D spectra. Not only are the calculated 1D model spectra required to agree well with experimental 1D spectra (with the peak areas of all signals of a particular form being equal to each other in Fig. 3a and the proportions of Iα, Iβ’, and non-crystalline cellulose being consistent across all samples), but there is a strong constraint on the chemical shifts in fitting the 2D spectra. In particular, two signals with chemical shifts δi and δj arising from carbons i and j that are in close spatial proximity will give rise to an indirect DQ chemical shift of δi + δj. For example, this means that the proposed chemical shift for a particular C4 signal (δ4) needs to be consistent with not only the 1D spectrum but also four correlations in the 2D spectra: the one-bond correlations in Fig. 3 involving C3 (δ3 + δ4) and C5 (δ4 + δ5), as well as with the two-bond correlations in Fig. 4 involving C1 (δ1 + δ4) and C6 (δ4 + δ6). Similarly, the chemical shifts for C1 needs to be consistent with a one-bond correlation involving C2 and two-bond correlations involving C3 and C4, while the chemical shifts for C3 needs to be consistent with one-bond correlations involving C2 and C4 and a two-bond correlation involving C1. Carbons C2, C5 and C6 each have correlations involving two other carbons.
Taking into consideration all of these expected correlations, it becomes apparent how constrained the set of chemical shifts is in order to provide good agreement with all of the 1D and 2D spectra. Given the high quality of agreement between the calculated and experimental spectra shown in Figs. 3 and 4, the confidence in the proposed chemical shifts reported in Table 1 is quite high. There is more confidence in the proposed chemical shifts for carbons C1, C3, and C4 due to the greater number of correlations involved and better separation from other signals, while there is slightly less confidence in the chemical shifts for C2, C5, and C6 due to fewer correlations and more spectral overlap with other signals. Of all the observed correlations, the correlation between C4 and C5 (see Fig. 3c) does not seem to be modeled as well as the others. While there is definitely weak intensity for the C4 signal that is well-matched by the blue correlation, its partner correlation for the C5 signal seems to not match as well, although this could be due to low signal intensity for this correlation. Other than this one weak correlation, all other aspects of the spectra are very well accounted for by the proposed model.
The correlation between C1 and C4 observed for the (1,4-13C2)-cellulose sample (Fig. 4a) is particularly noteworthy. Since this sample was biosynthesized from a mixture containing equal amounts of (1-13C)-D-glucose and (4-13C)-D-glucose as the carbon source, the vast majority of the DQ correlations between C1 and C4 signals arise from C1 and C4 carbons on different glucose across the C1–O–C4 glycosidic linkages. Cellulose Iα is known to have a crystal structure with the triclinic P1 space group, having one type of chain with alternating glucose units that are crystallographically distinct (Nishiyama et al. 2003). Therefore, it is expected that C1 from glucose 1 (red) will correlate with C4 from glucose 2 (orange) and C1 from glucose 2 (orange) will correlate with C4 from glucose 1 (red), as shown in Fig. 4a. The bacterial cellulose Iβ’ signals show a different pattern in that the C1–O–C4 correlations arise from glucose units of the same type: C1 from glucose 1 (blue) correlates with C4 also from glucose 1 (blue), while C1 from glucose 2 (green) correlates with C4 also from glucose 2 (green). This correlation pattern is what is expected from the cellulose Iβ crystal structure (Nishiyama et al. 2002) which has the monoclinic P21 space group with two crystallographically distinct chains each made up of identical glucose units that are related by twofold screw axis symmetry. This observation is one of the reasons this bacterial cellulose Iβ’ form is considered to be a structural modification of the Iβ allomorph.
Comparison to cellulose Iβ
Figure 5 provides a graphical comparison of the 13C chemical shifts for bacterial cellulose Iβ’ to cellulose Iβ derived from tunicate cellulose (see Table 1 for chemical shift values). At first glance, the spectra look quite similar, but a close comparison reveals several key differences, especially for the C1, C3, and C4 signals. The C4 signals for bacterial cellulose Iβ’ are each shifted by + 0.3 ppm compared to the tunicate cellulose Iβ signals, which is about half of the full width at half maximum (FWHM) of 0.7 ppm of the C4 signals (see Supporting Information) For the C3 signals, one of the peaks (blue) remains virtually unchanged, but the other (green) shifts quite dramatically by + 1.5 ppm, which is much greater the FWHM of 0.4 ppm for this C3 signal. For the C1 signals, it appears that the actual chemical shifts do not change that much, but the assignments of the signals are swapped between the two glucose units. It is worth noting that there is some ambiguity in the original assignment of the C1 signals of the tunicate cellulose Iβ spectrum (Kono et al. 2003a) which was based on the correlations between C1 and C2 signals in a 2D INADEQUATE spectrum. Since the C2 chemical shifts in the two glucose units of tunicate cellulose Iβ are nearly identical, it is not actually possible to uniquely assign the C1 signals to the glucose units based on these correlations alone. For the same reason, the one-bond correlation spectrum in Fig. 3 is also not sufficient to assign the bacterial cellulose Iβ’ C1 signals since the C2 signals also have nearly identical chemical shifts. However, the two-bond correlations involving C1 and C4 signals from symmetry-related glucose units across the glycosidic linkage (Fig. 4a) and between C1 and C3 signals within the same glucose units (Fig. 4b) provides convincing evidence for our proposed assignment of the bacterial cellulose Iβ’ signals. It is possible that the original peak assignment of the C1 signals in Kono et al. (2003a, b) is mistaken and if that is the case, there is actually little difference in the C1 chemical shifts for tunicate cellulose Iβ and bacterial cellulose Iβ’.
Comparison of a the 13C NMR spectrum of cellulose Iβ’ in bacterial cellulose proposed in this work to b the idealized. 13C NMR spectrum of cellulose Iβ derived from tunicate cellulose and reproduced from Brouwer and Mikolajewski (2023). The chemical shifts for both cellulose Iβ and Iβ’ are listed in Table 1. Note that it is possible that the assignments of the C1 signals in cellulose Iβ could be swapped (see text)
To summarize up to this point, this careful analysis of the 1D and 2D solid-state 13C NMR spectra of bacterial cellulose reveals that bacterial cellulose consists primarily of crystalline cellulose Iα, non-crystalline and/or surface regions, and a secondary crystalline form that appears at first glance to be cellulose Iβ, but turns out to be a subtly different form (referred to here as cellulose Iβ’) based on well-substantiated differences in some of the 13C chemical shifts, particularly for C3 and C4 signals and possibly the C1 signals. The 13C chemical shifts and peak assignments for the tunicate cellulose Iβ spectrum are not fully consistent with the experimental 1D and 2D solid-state NMR spectra of bacterial cellulose presented here, as well as other 1D spectra for bacterial cellulose reported in the literature over many years. Furthermore, these chemical shifts for bacterial cellulose Iβ’ also appear to be unique when compared to chemical shifts for other forms of cellulose. The sets of chemical shifts identified here do not match those of mercerized or regenerated cellulose II (Kono et al. 2004; Idström et al. 2016), cellulose IIII (Kono et al. 2003b), or any of the interior celluloses a to e proposed to be in primary and secondary plant cell walls (Wang et al. 2016; Kang et al. 2019; Kirui et al. 2022).
Possible structural variations from cellulose Iβ
At this point, it is difficult to identify what specific structural changes could cause the differences in the 13C chemical shifts of cellulose Iβ and Iβ’. However, based on which carbons have different chemical shifts, it is possible to at least propose where in the structure there might be structural differences. As described above, the largest differences in chemical shifts between cellulose Iβ and Iβ’ involve the C3 and C4 carbons (and possibly the C1 carbons, depending on whether the original assignment of C1 signals is correct or not). Upon examination of the proposed crystal structure for cellulose Iβ (Nishiyama et al. 2002), it can be seen that all of these carbons play an important role in the interaction between adjacent glucose units within a chain (see areas highlighted in blue in Fig. 6). C1 and C4 are involved in the C1–O1–C4 glycosidic linkage while C3 is connected to the intra-chain O3–OH3···O5 hydrogen bond from one glucose unit to its neighbor. It seems reasonable to expect that a change in this hydrogen bond geometry and/or the glycosidic linkage geometry could give rise to changes in the 13C chemical shifts for the C3, C4, and C1 signals. It should be emphasized that 13C chemical shifts are very sensitive to changes in the local structural environment, so it is likely that any structural differences would be quite small.
Cellulose Iβ structure (Nishiyama et al. 2002) with potential regions of the structure where the Iβ’ structure may differ based on the changes in the 13C chemical shifts (see text). The blue regions highlight the intra-chain the O3–OH3···O5 hydrogen bonds and C1–O1–C4 glycosidic linkages between adjacent glucose units. The green region highlights the inter-chain C3–H3···O2 hydrogen bond
Another possible explanation for the change in 13C chemical shifts between cellulose Iβ and Iβ’, especially for the C3 signal, could be a change in the relative positions of the cellulose chains. An examination of the Iβ cellulose crystal structure (Nishiyama et al. 2002) reveals that the C3 carbon in the origin chain appears be involved in a weak C–H···O hydrogen bond with the O2 oxygen of the center chain (see region highlighted in green in Fig. 6). The H3···O2 distance is 2.52 Å and the C3–H3···O2 angle is 162°. Interestingly, the C3 carbon of the center chain is not involved such a hydrogen bond. Both the 1H and 13C chemical shifts are known to be sensitive to C–H···O hydrogen bonding when the H–O distance is less than 2.7 Å and the C–H···O angle is greater than 130° (Yates et al. 2005). It seems reasonable that the Iβ’ structure could have a subtle shift in the relative positions of the origin and center chains such that this C3–H3···O2 hydrogen bond is disrupted or weakened, leading to a change in its 13C chemical shift. The fact that one of the C3 signals experiences a much greater change in chemical shift than the other seems consistent with this possibility.
Conclusions
A careful analysis of 1D and 2D solid-state 13C NMR spectra of bacterial cellulose revealed that several features in these spectra are different from what is expected for the cellulose Iβ allomorph, suggesting that there exists some sort of subtle structural modification of the Iβ allomorph in bacterial cellulose (referred to here as cellulose Iβ’). Essential to identifying these differences in the 13C spectra was the capability of putting all 13C spectra on the correct chemical shift scale, as described in our previous work (Brouwer and Mikolajewski 2023). There is a great deal of variation in previously reported solid-state 13C NMR spectra of cellulose, due to inconsistent and sometimes incorrect referencing of the chemical shift scale, that has prohibited close comparisons of these spectra. The bacterial cellulose spectra presented here are entirely consistent with previously reported spectra of bacterial cellulose, once chemical shift scales were corrected. When the bacterial cellulose spectra were compared to (corrected) sub-spectra of cellulose Iα and Iβ, derived from Cladaphora algae and Halocynthia tunicate samples respectively, it became clear that while bacterial cellulose consists primarily of the cellulose Iα allomorph, the secondary crystalline form is related to but distinct from the cellulose Iβ allomorph. A detailed analysis of 2D DQ/SQ correlation spectra provided additional evidence for these differences and allowed for the assignment of sets of correlated 13C chemical shifts to the crystallographically distinct glucose units in bacterial cellulose Iβ’. The main chemical shift differences appear to involve carbons C3 and C4 (and possibly C1), suggesting that the structural differences between cellulose Iβ and Iβ’ involve these carbons. Two possible models for these structural differences were proposed. This work highlights the fascinating complexity of cellulose structure and demonstrates the valuable detailed information about local structure that solid-state NMR spectroscopy can continue to provide about this important material.
Data availability
The Supporting Information provides full details on the models used to calculate and fit the experimental 13C NMR spectra of the bacterial cellulose samples. It also provides comparisons of the 13C chemical shifts for the tunicate cellulose Iβ allomorph to the experimental 1D and 2D NMR spectra. The Mathematica notebook used for fitting the 1D and 2D spectra, as well as the raw data for each spectrum, is also provided in the Supporting Information.
References
Arashida T, Ishino T, Kai A et al (1993) Biosynthesis of cellulose from culture media containing 13C-labeled glucose as a carbon source. J Carbohydr Chem 12:641–649. https://doi.org/10.1080/07328309308019413
Atalla RH, Vanderhart DL (1984) Native cellulose: a composite of two distinct crystalline forms. Science 223:283–285. https://doi.org/10.1126/science.223.4633.283
Atalla RH, Vanderhart DL (1999) The role of solid state 13C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl Magn Reson 15:1–19. https://doi.org/10.1016/S0926-2040(99)00042-9
Belton PS, Tanner SF, Cartier N, Chanzy H (1989) High-resolution solid-state 13C nuclear magnetic resonance spectroscopy of tunicin, an animal cellulose. Macromolecules 22:1615–1617. https://doi.org/10.1021/ma00194a019
Brouwer DH, Mikolajewski JG (2023) Resolving the discrepancies in reported 13C solid state NMR chemical shifts for native celluloses. Cellulose 30:4827–4839. https://doi.org/10.1007/s10570-023-05186-9
Carravetta M, Edén M, Zhao X et al (2000) Symmetry principles for the design of radiofrequency pulse sequences in the nuclear magnetic resonance of rotating solids. Chem Phys Lett 321:205–215. https://doi.org/10.1016/S0009-2614(00)00340-7
Fink H-P, Purz HJ, Bohn A, Kunze J (1997) Investigation of the supramolecular structure of never dried bacterial cellulose. In: Macromolecular symposia vol 120, pp 207–217. https://doi.org/10.1002/masy.19971200121
French AD (2014) Idealized powder diffraction patterns for cellulose allomorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Fung BM, Khitrin AK, Ermolaev K (2000) An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson 142:97–101. https://doi.org/10.1006/jmre.1999.1896
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58:345–352. https://doi.org/10.1042/bj0580345
Horii F, Hirai A, Kitamaru R (1984) CP/MAS carbon-13 NMR study of spin relaxation phenomena of cellulose containing crystalline and noncrystalline components. J Carbohydr Chem 3:641–662. https://doi.org/10.1080/07328308408057922
Horii F, Hirai A, Kitamaru R (1987) CP/MAS 13C NMR spectra of the crystalline components of native celluloses. Macromolecules 20:2117–2120. https://doi.org/10.1021/ma00175a012
Idström A, Schantz S, Sundberg J et al (2016) 13C NMR assignments of regenerated cellulose from solid-state 2D NMR spectroscopy. Carbohydr Polym 151:480–487. https://doi.org/10.1016/j.carbpol.2016.05.107
Kai A, Arashida T, Hatanaka K et al (1994) Analysis of the biosynthetic process of cellulose and curdlan using 13C-labeled glucoses. Carbohydr Polym 23:235–239. https://doi.org/10.1016/0144-8617(94)90184-8
Kang X, Kirui A, Dickwella Widanage MC et al (2019) Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat Commun 10:1–9. https://doi.org/10.1038/s41467-018-08252-0
Kirui A, Zhao W, Deligey F et al (2022) Carbohydrate-aromatic interface and molecular architecture of lignocellulose. Nat Commun 13:1–12. https://doi.org/10.1038/s41467-022-28165-3
Koch F-T, Prieß W, Witter R, Sternberg U (2000) Calculation of solid-state 13C NMR spectra of cellulose Iα, Iβ and II using a semi-empirical approach and molecular dynamics. Macromol Chem Phys 201:1930–1939. https://doi.org/10.1002/1521-3935(20001001)201:15%3c1930::AID-MACP1930%3e3.0.CO;2-7
Kono H, Yunoki S, Shikano T et al (2002) CP/MAS C-13 NMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS C-13 NMR spectrum of the native cellulose. J Am Chem Soc 124:7506–7511. https://doi.org/10.1021/Ja010704o
Kono H, Erata T, Takai M (2003a) Determination of the through-bond carbon-carbon and carbon-proton connectivities of the native celluloses in the solid state. Macromolecules 36:5131–5138. https://doi.org/10.1021/ma021769u
Kono H, Erata T, Takai M (2003b) Complete assignment of the CP/MAS 13C NMR spectrum of cellulose IIII. Macromolecules 36:3589–3592. https://doi.org/10.1021/ma021015f
Kono H, Numata Y, Erata T, Takai M (2004) 13C and 1H resonance assignment of mercerized cellulose II by two-dimensional MAS NMR spectroscopies. Macromolecules 37:5310–5316. https://doi.org/10.1021/ma030465k
Langan P, Nishiyama Y, Chanzy H (2001) X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2:410–416. https://doi.org/10.1021/bm005612q
Larsson PT, Westermark U, Iversen T (1995) Determination of the cellulose Iα allomorph content in a tunicate cellulose by CP/MAS 13C-NMR spectroscopy. Carbohydr Res 278:339–343. https://doi.org/10.1016/0008-6215(95)00248-0
Malm E, Bulone V, Wickholm K et al (2010) The surface structure of well-ordered native cellulose fibrils in contact with water. Carbohydr Res 345:97–100. https://doi.org/10.1016/j.carres.2009.10.020
Morcombe CR, Zilm KW (2003) Chemical shift referencing in MAS solid state NMR. J Magn Reson 162:479–486. https://doi.org/10.1016/S1090-7807(03)00082-X
Mori T, Chikayama E, Tsuboi Y et al (2012) Exploring the conformational space of amorphous cellulose using NMR chemical shifts. Carbohydr Polym 90:1197–1203. https://doi.org/10.1016/j.carbpol.2012.06.027
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082. https://doi.org/10.1021/ja0257319
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125:14300–14306. https://doi.org/10.1021/ja037055w
O’Sullivan AC (1997) Cellulose: the structure slowly unravels. Cellulose 4:173–207. https://doi.org/10.1023/A:1018431705579
Phyo P, Wang T, Yang Y et al (2018) Direct determination of hydroxymethyl conformations of plant cell wall cellulose using 1H polarization transfer solid-state NMR. Biomacromolecules 19:1485–1497. https://doi.org/10.1021/acs.biomac.8b00039
Ross P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55:35–58. https://doi.org/10.1128/mr.55.1.35-58.1991
States DJ, Haberkorn RA, Ruben DJ (1982) A two-dimensional nuclear overhauser experiment with pure absorption phase in four quadrants. J Magn Reson 48:286–292. https://doi.org/10.1016/0022-2364(82)90279-7
Sternberg U, Koch F-T, Priess W, Witter R (2003) Crystal structure refinements of cellulose allomorphs using solid state 13C chemical shifts. Cellulose 10:189–199. https://doi.org/10.1023/A:1025185416154
VanderHart D, Atalla R (1987) Further carbon-13 NMR evidence for the coexistence of two crystalline forms in native celluloses. ACS Symp Ser 340:88–118. https://doi.org/10.1021/bk-1987-0340.ch005
VanderHart DL, Atalla RH (1984) Studies of microstructure in native celluloses using solid-state 13C NMR. Macromolecules 17:1465–1472. https://doi.org/10.1021/ma00138a009
Wada M, Chanzy H, Nishiyama Y, Langan P (2004a) Cellulose IIII crystal structure and hydrogen bonding by synchrotron X-ray and neutron fiber diffraction. Macromolecules 37:8548–8555. https://doi.org/10.1021/ma0485585
Wada M, Heux L, Sugiyama J (2004b) Polymorphism of cellulose I family: Reinvestigation of cellulose IVl. Biomacromol 5:1385–1391. https://doi.org/10.1021/bm0345357
Wada M, Heux L, Nishiyama Y, Langan P (2009) X-ray crystallographic, scanning microprobe X-ray diffraction, and cross-polarized/magic angle spinning 13C NMR studies of the structure of cellulose IIIII. Biomacromol 10:302–309. https://doi.org/10.1021/bm8010227
Wang T, Yang H, Kubicki JD, Hong M (2016) Cellulose structural polymorphism in plant primary cell walls investigated by high-field 2D solid-state NMR spectroscopy and density functional theory calculations. Biomacromolecules 17:2210–2222. https://doi.org/10.1021/acs.biomac.6b00441
Wolfram Research Inc (2021) Mathematica. version 13.0
Yates JR, Pham TN, Pickard CJ et al (2005) An investigation of weak CH⋯O hydrogen bonds in maltose anomers by a combination of calculation and experimental solid-state NMR spectroscopy. J Am Chem Soc 127:10216–10220. https://doi.org/10.1021/ja051019a
Acknowledgments
The authors thank the Department of Chemistry and Chemical Biology at McMaster University for access to the 850 MHz NMR spectrometer.
Funding
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada in the form of a Discovery Grant (funding reference number 03836) and an Undergraduate Student Research Award.
Author information
Authors and Affiliations
Contributions
Material preparation was carried out by JM. Data collection, analysis, and writing of the manuscript were carried out by DB. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brouwer, D.H., Mikolajewski, J.G. Solid-state NMR reveals a structural variation from the Iβ polymorphic form in bacterial cellulose. Cellulose 30, 11341–11356 (2023). https://doi.org/10.1007/s10570-023-05577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05577-y