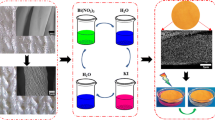
Abstract
Cotton fabrics with photodynamic performance (BP/CuPc-R/F) were prepared via a facile method and used as an effective photocatalytic system for wastewater treatment in this work. C.I. Reactive Blue 21 (a copper phthalocyanine derivative) and one kind of 3,3′,4,4′-benzophenone derivative (BPTCA) were covalently grafted onto cotton fabrics via conventional dyeing and finishing processes. The resultant photosensitive material (BP/CuPc-R/F) exhibited excellent hydroxyl-radical-generating activity under UVA light. Density functional theory calculation was used to investigate the photosensitizing mechanism and synergistic effect of copper phthalocyanine and benzophenone groups. BP/CuPc-R/F showed a stable activity in the decomposition of C.I. Reactive Black 5, and the degradation rate can still reach above 80% after 4 cycles. The degradation efficiency was enhanced in the condition of alkaline and high temperature (99% degradation at 45 °C in 10 min exposure). Furthermore, it also showed photo-induced antibacterial activity toward Staphylococcus aureus with bacterial reduction rate of 99.99% after 60 min of UVA light illumination. The photosensitive textile provides a facile and efficient approach for the removal of hazardous pollutants and microorganisms in water treatment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Functional textiles play an important role in environmental protection and sustainable development of industry in recent years, e.g. water treatment. Wastewater pollution is a serious environmental problem and is causing irreparable damage to the environment and human health. Organic dyes and auxiliaries from textile industries are sources of wastewater pollution (Hou et al. 2010). They pose various health hazards, e.g., they are teratogenic and carcinogenic (Mondal et al. 2020; Pazdzior et al. 2019; Song et al. 2022). The effluents from conventional dyeing processes are multicomponent, and eventually cause imbalances in ecosystems. Moreover, the industrial reuse of treated wastewater has attracted much attention because it saves energy and decreases discharges. Microbial pollution of water also poses environmental health risks (Carducci et al. 2020; Ren et al. 2019). Various strategies for waste effluent treatment have therefore been developed, e.g. traditional physical techniques such as adsorption and coagulation (Jv et al. 2019; Qi et al. 2020; Xie et al. 2011; Zhang et al. 2020a, b), biochemical degradation (Mohyudin et al. 2021; Sathe et al. 2022), electrochemical treatment (Pan et al. 2019; Sun et al. 2021) and photocatalytic degradation (Fessi et al. 2019; Ma et al. 2021; Wang and Wang 2020).
The use of photocatalytic techniques, in which reactive oxygen species (ROS) mediated oxidation of organic compounds and microbials under light irradiation works, has become popular in wastewater treatment, gas purification and self-cleaning materials (Dong et al. 2020; Wang et al. 2022). Accordingly, a large number of photocatalysts have been reported. These include inorganic semiconductor materials and their composites, e.g., TiO2 (Yamaguchi et al. 2020), ZnO/TiO2 (Hou et al. 2021), MnO2/g–C3N4 (Wu et al. 2019), and photoactive materials (Marin et al. 2012; Zhao et al. 2021) based on organic photosensitive compounds such as phthalocyanine (Chen et al. 2021), benzothiadiazole (Han et al. 2021) and anthraquinone (Yi et al. 2020a, b). Inorganic semiconductor nanoparticles can effectively generate strong oxidizing electron–hole pairs, but the photocatalytic applications of many types of nanoparticles are limited by their complex preparation processes and high rates of electron–hole pair recombination (Boyjoo et al. 2016; Dingenen et al. 2021; Shkrob et al. 2004; Yang et al. 2017). Fixing such nanoparticles in powder form on supports is also challenging, and leads to recycling problems and secondary pollution (Bhatt et al. 2011). The development of durable, stable, and highly efficient photocatalytic systems for wastewater treatment is therefore important.
Organic photoactive materials have drawn much attention in the fields of self-cleaning, water purifications and biomaterials because of their excellent photo-induced ROS-generating abilities, and their potential for immobilization on various supports (Huang et al. 2017; Shen et al. 2016; Yi et al. 2020a, b; Yi et al. 2018). Metallophthalocyanine derivatives, which absorb strongly in the UV- and visible-light ranges, have been widely used to broaden the light-absorption range and improve the photocatalytic efficiency (Ahmad et al. 2019; Shi et al. 2019; Zhang et al. 2011). The benzophenone group, which has excellent photoactive properties, occurs widely in biological medical materials (Hou et al. 2015; Hu et al. 2019). These organic compounds are mostly used for photosensitizing nanoparticles in wastewater treatment, but there has been little research on the use of combinations of organic photosensitive compounds for photocatalysis.
Considering the effectiveness of photo-driven oxidation methods and the advantages of photosensitve compounds, we used a combination of benzophenone and metallophthalocyanine group to develop an efficient, durable, and reusable photocatalytic textile for water treatment. Textiles have been widely used for photocatalyst supports in these years because such fabrics have porous structures and the potential for strong particle fixation. Cotton fabrics are considered to be the most suitable support because they are cheap, and they have good adsorption properties, mechanical strength and a large number of hydroxyl groups (Hou et al. 2009; Xie et al. 2009; Zhang et al. 2020a, b). In our previous work (Hu et al. 2018), C.I. Reactive Blue 21, which is a derivative of copper phthalocyanine (CuPc), was incorporated into cotton fabrics via a covalent anchoring to give material with light-induced antibacterial activity. A benzophenone derivative (BPTCA) which exhibited excellent photoactivity was also obtained and reported before (Hou et al. 2015). In this study, a cotton fabric-supported phthalocyanine and benzophenone photocatalytic system (BP/CuPc-R/F) was developed by simply incorporating C.I. Reactive Blue 21 and BPTCA onto cellulose fibers (Fig. 1). Hydroxyl-radical-scavenging experiments and density functional theory (DFT) calculations were performed to investigate the hydroxyl radical-generating abilities and photoactivity mechanisms. The decolorization of C.I. Reactive Black 5 and the antibacterial performance against Staphylococcus aureus (S. aureus) were used to evaluate the photocatalytic properties of the photoactive material.
Materials and methods
Materials
Cotton fabric was obtained from the Jinqiu Textile and Finishing Co., Ltd. (Shaoxing, China). C.I. Reactive Blue 21 and C.I. Reactive Black 5 were obtained from the Wujiang Taoyuan Dyestuff Co., Ltd. (Wujiang, China). The C. I. Reactive Black 5 was desalted and purified before use. 3,3′,4,4′-Benzophenonetetracarboxylic dianhydride (BPTCD) and p-nitrosodimethylaniline (p-NDA) were purchased from the Sun Chemical Technology Co., Ltd. (Shanghai, China). All other chemicals were purchased from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without purification.
Fabrication of BP/CuPc-R/F system
Copper phthalocyanine-grafted cotton fabrics, named as CuPc-R/F, were prepared by covalently grafting C.I. Reactive Blue 21 with various concentrations (0.5%, 2%, 5%, 8%, on weight the fabric (o.w.f)) on fabrics according to our previously reported method (Hu et al. 2018).
The C.I. Reactive Blue 21-modified cotton fabrics were then treated by different concentrations of BPTCA solutions (0, 10, 30 g/L). BPTCA was obtained by hydrolyzing BPTCD in deionized water in a certain concentration at 70–80 °C. Sodium hypophosphite monohydrate was added as a catalyst to the BPTCA solutions based on a molar ratio of catalyst/BPTCA = 1: 2. The C.I. Reactive Blue 21-grafted cotton fabrics were impregnated in the mixed solution, followed by padding through two dips and two nips to reach an average wet pickup of 120%, and then dried at 90 °C for 3 min and cured at 180 °C for 3 min. At last, the treated fabrics were washed with deionized water and dried at ambient condition (Fig. 1a). The cotton fabric treated with C.I. Reactive Blue 21 (8%, o.w.f) and BPTCA (30 g/L) was denoted by BP/CuPc-R/F. The grafting masses of C.I. Reactive Blue 21 and BPTCA on the fabrics were calculated according to the method reported before (Gao et al. 2017; Hu et al. 2018).
Instrumentation
Fourier-transform infrared (FT-IR) spectra were recorded with a PerkinElmer Spectrum Two Spectrometer (PerkinElmer Co., Ltd., USA). The visible absorption spectra of the fabrics were recorded with a Datacolor 650 spectrophotometer (Datacolor Co., Ltd., USA). UV–visible absorption spectra were recorded with a Hitachi U-3310 spectrophotometer (Hitachi Co., Ltd., Japan). Scanning electron microscopy (SEM) was performed with a Hitachi S-4700FEG microscope (Hitachi Co., Ltd., Japan). Raman spectra were recorded with a HORIBA Scientific LabRAM HR Evolution spectrometer (HORIBA Co., Ltd., France). X-ray photoelectron spectroscopy (XPS) was performed with a Thermo Scientific K-Alpha spectrometer (Thermo-Fisher Co., Ltd., USA). Thermogravimetry analysis (TGA) was conducted on a TG 209 F3 Tarsus analyzer at a heating rate of 10 °C/min (Netzsch Co., Ltd., Germany). Electron paramagnetic resonance (EPR) spectra were recorded with a Bruker EMX PLUS spectrometer (Bruker Co., Ltd., Germany).
Determination of hydroxyl radicals
Photoactivities of the materials were evaluated by determining their hydroxyl-radical-generating abilities. The amount of generated hydroxyl radicals was determined according to our previously published photometric method (Hu et al. 2019). In this method, p-NDA was used as a hydroxyl-radical scavenger, and the formation of other ROS was neglected (Bors et al. 1979).
EPR spectroscopy was used to verify the generation of hydroxyl radicals by BP/CuPc-R/F system. 5,5-Dimethyl-1-pyrroline N-oxide (DMPO) was used as the hydroxyl-radical-trapping agent. The mixture was illuminated for 0 or 15 min. The EPR conditions were as follows: power, 6.325 mW; center field, 3502.00 G; microwave frequency, 9.82 GHz; and modulation frequency, 100.00 kHz.
Photodegradation of C.I. Reactive Black 5
The degradation efficiency of C.I. Reactive Black 5 ( Scheme 1 ) was evaluated by using UV spectroscopy to monitor the dye concentration change. Pieces of modified fabrics of area 3 cm × 5 cm were placed in test tubes containing C.I. Reactive Black 5 aqueous solution (10 mL). The tubes were exposed to UVA light for 10, 20, 30, 60, and 90 min in a LY-GHX-V photoreactor (Shanghai Bilang Instrument Manufacturing Co., Ltd., China). After each exposure, the UV–vis spectra of the dye solutions and the absorption intensity values at the maximum absorption wavelength (596 nm) were recorded. The concentration of dye remained in the solution after different exposure time was calculated via a calibration method. The removal efficiency of the solution was calculated as.
where C0 is the initial concentration of the dye and Ct is the concentration of the dye after t (min) of illumination.
Photo-induced antibacterial performance
The photo-induced antibacterial activities against S. aureus of the photoactive cotton fabrics were evaluated. Two pieces of fabric of area 3.5 cm × 3.5 cm were placed on separate sterile Petri dishes and co-cultured with a bacterial suspension (105 CFU/mL, 300 μL). The samples were placed under UVA light or in a dark environment for 60 min, and then sequentially washed with sterilized phosphate-buffered saline solution at different dilutions, and dripped and incubated on an agar plate for 18 h. Bacterial reduction rates of the fabrics were determined based on the numbers of colony-forming units.
Results and discussion
Fabrication and characterization of BP/CuPc-R/F
C.I. Reactive Blue 21 (0.5%, 2%, 5%, and 8%, o.w.f) was incorporated into cotton fabrics via a reactive dyeing process. The grafted amount was affected by the dye amount in the dyeing bath. BPTCA can be grafted onto the cotton fabric via an ester reaction. The cotton fabrics that had been dyed with C.I. Reactive Blue 21 were treated with different concentrations of BPTCA (0, 10, 30 g/L). The preparation process of BP/CuPc-R/F is shown in Fig. 1a.
The surface morphologies of the grafted and pristine cotton fabrics are shown in Fig. 2a. The modified fabrics have a turquoise blue color, which is provided by the copper phthalocyanine group. SEM images show that the pristine fabric surface was smooth and intact. The surface of BP/CuPc-R/F was also smooth, except for some slight cracks. The diameter distributions of cotton fibers of the pristine and BP/CuPc-R/F fabrics were calculated based on SEM images. The distributions show that the diameters of pristine fibers and BP/CuPc-R/F were both distributed in the range of 15–22 μm. These results illustrate that the chemical treatment had just a little effect on the cellulose fibers.
Visible absorption spectroscopy was used to determine whether the photoactive compounds were successfully grafted onto the cellulose fibers. The visible absorption spectra of the grafted fabrics are shown in Fig. 2b. The obtained fabrics had strong absorption in the 600–700 nm range, and the absorption gradually increased with increasing concentration of C.I. Reactive Blue 21. These results confirm the presence of copper phthalocyanine in the fabrics. The electronic absorption spectrum of C.I. Reactive Blue 21 (Fig. 2c) shows strong absorption bands in the UV region at 300–400 nm and the visible region at 550–700 nm, corresponding to π–π* transitions of copper phthalocyanine. BPTCA had strong absorption in the UV region.
The chemical structures of BP/CuPc-R/F were investigated by FT-IR, Raman spectroscopies, and XPS (Fig. 3a–c). The band at 697 cm−1 in the FT-IR spectra (Fig. 3a) arises from the vibrations of Cu–N groups of copper phthalocyanine. The new peak at 1702 cm−1 was attributed to vibrations of the C = O group of BPTCA, and the peak at 1640 cm−1 moved to 1663 cm−1 after treatment with BPTCA. The intensity of the cellulose C–O stretching band at 1028 cm−1 decreased gradually after treatment with C.I. Reactive Blue 21. This indicates successful grafting. The Raman spectra in Fig. 3b show that the signals in the range of 400–600 cm−1, which are attributed to the ring deformation vibrations of the glucopyranose skeletons, clearly changed after reacting with the photosensitizers. This indicates transformation of the cellulose configuration. The intensity of the C–O–C glycosidic link stretching vibration at 1096 cm−1 decreased slightly. The new peaks observed at 1338, 1465, and 1535 cm−1 are respectively attributed to the stretching vibrations of the Cα–Cβ, Cu–N, and C = N groups of copper phthalocyanine. The signal at 1595 cm−1 corresponded to the frequency of C = O stretching band of benzophenone. Figure 3c shows XP survey spectra of the pristine cotton fabric and BP/CuPc-R/F. They contained C 1 s, O 1 s, N 1 s, and S 2p peaks. The distributions of N, S, and Cu elements on the surface of the grafted fabric were low. This may be because of their low contents, and some of them were distributed inside the fibers. All these results confirm the incorporation of copper phthalocyanine species and benzophenone groups into the cotton fabrics. TG analysis was conducted to study the thermal performance of pristine and modified fabrics. As is shown in Fig. 3d, the thermal degradation of blank cotton fabrics occurred at approximately 320 ℃, and CuPc-R/F had a similar degradation onset. The decomposition onset of BP/CuPc-R/F was at 293 ℃, and the maximum thermal decomposition rate had a slight increase compared with that of pristine fabric, which may be because of the pad-dry-cure finishing process. These results reveal that the two sequential modifications slightly affected the thermal stability.
Detection of hydroxyl radicals and clarification of photochemical mechanism
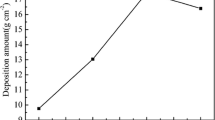
The treating concentrations in the baths could affect the masses of photosensitizers grafted on the cotton fabric, therefore affecting the amounts of hydroxyl radicals (·OH) generated, which leads to different photoactivities. With the increase of the concentrations, the quantity of the photosensitizers fixed on the fabrics increased. For BP/CuPc-R/F, the grafted mass of Reactive Blue 21 was determined to be 23.42 mg/g, and the mass of BPTCA was determined to be 20.10 mg/g. The amounts of hydroxyl radicals generated under UVA light by the cotton fabrics treated with various concentrations of BPTCA and C.I. Reactive Blue 21 were determined (Fig. 4a). The results show that the hydroxyl radical content increased with increasing BPTCA concentration in the range 0–30 g/L. They also show that the hydroxyl radical content of the fabric increased with increasing Reactive Blue 21 concentration in the range of 0–8% (o.w.f). The highest amount of hydroxyl radicals was produced by the cotton fabric dyed with 8% (o.w.f) of C.I. Reactive Blue 21 and treated with 30 g/L of BPTCA, i.e., BP/CuPc-R/F; this is considered to be a photocatalytic system. In addition, the amount of hydroxyl radicals produced by a fabric co-modified with copper phthalocyanine and benzophenone groups was greater than the sum of those produced by fabrics grafted with Reactive Blue 21 alone or BPTCA alone. This shows that there was a synergistic effect between copper phthalocyanine and benzophenone groups.
a Generation of hydroxyl radicals by fabrics treated with different concentrations of C.I. Reactive Blue 21 and BPTCA; b in situ EPR determination of hydroxyl radicals of BP/CuPc-R/F; c Jablonski diagrams representing excitation process; d photoactive mechanism of benzophenone and copper phthalocyanine derivatives; e mechanism responsible for photocatalytic activity of BP/CuPc-R/F system
EPR spectroscopy was used to verify the generation of hydroxyl radicals under light irradiation. The signals arising from trapping of hydroxyl radicals generated by BP/CuPc-R/F in DMPO solution were recorded at 3450–3550 G (Fig. 4b). For 0 min of UVA light irradiation (dark condition), there was no signal. However, a clear DMPO–·OH signal was observed after illumination for 15 min. This illustrates that the BP/CuPc-R/F system can effectively generate hydroxyl radicals on exposure to light.
A mechanism that can explain the photoactivities of the cellulose-fiber-supported photoactive system is shown in Fig. 4c and d. Photosensitive compounds (Ps), e.g., copper phthalocyanine and benzophenone derivatives, can be excited to the first singlet excited state 1(Ps) * (S1) after absorbing light of an appropriate wavelength. Various processes can occur from this state. The S1 state can undergo intersystem crossing (ISC) to reach the triplet excited state 3(Ps) * (T1). The triplet excited state can directly react with a substrate such as a fabric, pollutant, or water via an electron-transfer reaction to give the reduced form of the photosensitizer (type I). The reduced form of the photosensitizer can react with oxygen via electron transfer to give the Ps ground state and a superoxide anion radical; a series of reactions can then occur. Alternatively, the triplet excited state can react with oxygen to give singlet oxygen (1O2) (type II) (Klausen et al. 2020). ROS with antimicrobial abilities and the ability to decompose toxic compounds can be produced by these reactions.
DFT calculations were performed with Gaussian 09W software to verify the synergistic effect between copper phthalocyanine and benzophenone groups (Sun et al. 2011). The geometries of benzophenone derivative and copper phthalocyanine were optimized, and the excitation energies were calculated using time-dependent-DFT at the B3LYP/6-31G(d) level (for BPTCA) or the B3LYP/6-31G(d)/LANL2DZ level (for copper phthalocyanine). The results are shown in Fig. 4e. For BPTCA, the transition from the highest occupied molecular orbital (HOMO-4) to the lowest unoccupied molecular orbital (LUMO) was the major process involved in the S1 states, and the HOMO-8 to LUMO transition was the major process involved in the T3 state. The S1–T1 energy gap of BPTCA was less than 1, which indicates excellent photoactive ability (Yang et al. 2016). Figure 4e shows that after ISC process, benzophenone reached to its triplet excited state (T3, T1). Subsequent reactions with water, oxygen or another substrate gave ROS and a reduced form of BP (BP*−). The excitation energy of the T1 state (2.79 eV) of BP was greater than that of the S1 state (2.08 eV) of copper phthalocyanine, therefore copper phthalocyanine has electron-accepting abilities. The produced BP*− can transfer an electron to the S1 state of copper phthalocyanine, which enhances ISC process. This means that benzophenone can photosensitize the copper phthalocyanine group. The excited state of the copper phthalocyanine group can also instigate production of ROS. These theoretical results confirm the excellent ROS-producing abilities of the photoactive cotton fabrics, and the synergistic effect between copper phthalocyanine and benzophenone groups.
Photocatalytic degradation of C.I. Reactive Black 5
The results discussed above show that the BP/CuPc-R/F system can generate large amounts of ROS when exposed to UVA light. C.I. Reactive black 5, which is a commonly used reactive dye, was selected as an example for the investigation of photocatalytic degradation. A photo-induced degradation test was conducted by placing a piece of modified fabric of area 3 cm × 5 cm in a test tube that contained 10 mL C.I. Reactive Black 5 solution (20 mg/L), and then exposing the test tube to UV light for various time periods. After different irradiation time, the absorption intensity values at 596 nm of the solutions were recorded (Fig. 5). Figure 5a shows that the absorption dropped by more than 100% during UV irradiation for 90 min of a dye solution containing BP/CuPc-R/F. This was far higher than the degradation efficiency of an aqueous solution containing the cotton fabric treated with 30 g/L BPTCA alone, which were in agreement with the hydroxyl-radical-generating abilities discussed in Sect. "Detection of hydroxyl radicals and clarification of photochemical mechanism". These results also confirm the synergism between those two photosensitive derivatives, and show that the BP/CuPc-R/F system can be used in wastewater treatment.
a Removal efficiency of C.I. Reactive Black 5 under different conditions; b time-dependent UV–vis spectral changes of C.I. Reactive Black 5 in presence of BP/CuPc-R/F system (BPTCA 30 g/L + C.I. Reactive Blue 21 8%; 25 °C, pH 6, 20 mg/L); effects of pH value (25 °C, 20 mg/L) c, temperature (pH 6, 20 mg/L) d, and initial concentration (25 °C, pH 6) e on degradation rate of C.I. Reactive Black 5 at different times; f cyclic catalytic oxidation of C.I. Reactive Black 5 by BP/CuPc-R/F system (25 °C, pH 6, 20 mg/L)
The time-dependent UV–vis spectral changes of C.I. Reactive Black 5 solutions show that the absorption at 596 nm decreased considerably with increasing exposure time to 60 min, and a new weak absorption peak at 515 nm was observed after 60 min of irradiation (Fig. 5b). After irradiation for 90 min, the solution showed almost no absorption peak in the visible region. The UV–vis spectral changes illustrate the breakages of conjugated system in the molecular skeleton of Reactive Black 5, and the conjugation structure was completely decomposed after 90 min irradiation.
The effects of pH value, temperature and initial concentration on the degradation rate were also investigated (Fig. 5c, d and e). Figure 5c shows that the degradation rate reached 100% within 30 min when the pH value was 2–11, whereas 90 min were needed to achieve complete decolorization under neutral conditions. This reveals that the degradation rate can be greatly improved in acidic or alkaline condition. Fixation of reactive dyes is generally performed under alkaline conditions (pH value of 10–11), which is beneficial in practical applications of the photosensitive system to treat industrial wastewater. The effects of pH value on photocatalytic degradation could be explained by the mechanisms shown in Eqs. (2)–(6).
Figure 5d shows that the degradation rate increased with increasing temperature, and it reached 100% within 10 min at 45 °C. This was attributed to the acceleration of the photocatalytic reaction as the temperature rose. The degradation rate decreased with the increase of initial concentration of Reactive Black 5 from 20 to 100 mg/L (Fig. 5e), which may be caused by a diminishing light transmission rate. These results show that the BP/CuPc-R/F system achieved enhanced catalytic oxidation of C.I. Reactive Black 5 under alkaline and high-temperature conditions, and show that it has advantages for use in the treatment of reactive dyeing wastewater.
The feasibility of using the BP/CuPc-R/F system in practical applications was investigated by performing cyclic catalytic oxidation experiments. The BP/CuPc-R/F system was placed in a Reactive Black 5 solution (25 °C, pH 6, 20 mg/L) and then exposed to UV light. After 90 min, the fabric was removed and placed in a fresh Reactive Black 5 solution for a new cycle. This process was repeated four times. The results in Fig. 5f show that the degradation rate remained above 80% even after four cycles. It illustrates that the photocatalyst is reusable. This may be because of the firm binding of the photosensitive compounds on the cotton fabrics.
Photo-induced antibacterial performance
The BP/CuPc-R/F system had excellent ROS-generating abilities. ROS are often considered to be responsible for photo-induced antibacterial activity. It was therefore conjectured that this photoactive system can also be used for microbial inactivation. The light-driven bactericidal performance of the BP/CuPc-R/F system was investigated by evaluating the antibacterial activities against S. aureus of cotton fabrics modified with different concentrations of BPTCA (0, 10, and 30 g/L) and C.I. Reactive Blue 21 (0–8%) under UVA irradiation or in a dark environment for 60 min. The results are shown in Fig. 6. Figure 6a shows that the fabric modified with 8% (o.w.f) of Reactive Blue 21 had a 70.55% antibacterial rate under UVA light, but had no antibacterial effect against S. aureus in a dark environment. Figure 6b shows that the antibacterial rate increased with increasing BPTCA concentration. More importantly, the antibacterial rate of BP/CuPc-R/F against S. aureus reached 99.99% under UVA light. Culture medium images show that no bacterium grew in the BP/CuPc-R/F co-cultured bacterial solutions after 60 min of exposure to UVA light. These results show that cotton fabric modified with benzophenone and copper phthalocyanine derivatives has photooxidative abilities.
a Bactericidal efficiencies against S. aureus of blank fabric, CuPc-R/F, and BP/CuPc-R/F under UVA irradiation or in a dark environment (60 min); b bactericidal efficiencies of cotton fabrics treated with different concentrations of BPTCA (0, 10, 30 g/L) and C.I. Reactive Blue 21 (0%, 8%) under UVA irradiation
Conclusions
In this work, a copper phthalocyanine/benzophenone-decorated cotton fabric (BP/CuPc-R/F) with efficient and durable photoactivities was prepared via a facile dyeing process and a pad-dry-cure technique. The photosensitive system showed excellent activity in photo-induced production of hydroxyl radicals. DFT calculations showed that the photoactivity involved a synergistic effect between copper phthalocyanine and benzophenone groups. The target dye, C.I. Reactive Black 5, was efficiently removed from wastewater by the constructed photoactive BP/CuPc-R/F system. The recycling performance was excellent for at least four cycles. More importantly, the degradation efficiency was higher in alkaline solution (99% degradation in 30 min exposure, 25 °C, pH value of 11) or at high temperature (99% degradation in 10 min exposure, 45 °C, pH value of 7). In addition, the BP/CuPc-R/F system showed excellent photo-induced antibacterial activity against S. aureus (99.99% inactivation). These results provide a novel approach to construct an efficient and durable photocatalytic system by using organic photosensitive compounds. The scalable fabrication of photoactive cotton fabrics has good potential applications in environmental protection and medical field.
Data availability
The data in this article are reliable and are available from the corresponding author.
References
Ahmad I, Kan CW, Yao Z (2019) Photoactive cotton fabric for UV protection and self-cleaning. RSC Adv 9(32):18106–18114. https://doi.org/10.1039/c9ra02023c
Bhatt I, Tripathi BN (2011) Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 82(3):308–317. https://doi.org/10.1016/j.chemosphere.2010.10.011
Bors W, Michel C, Saran M (1979) On the nature of biochemically generated hydroxyl radicals, Studies using the bleaching of p-nitrosodimethylaniline as a direct assay method. Eur J Biochem 95:621–627. https://doi.org/10.1111/j.1432-1033.1979.tb13003.x
Boyjoo Y, Sun H, Liu J, Pareek VK, Wang S (2016) A review on photocatalysis for air treatment: from catalyst development to reactor design. Chem Eng J 310:537–559. https://doi.org/10.1016/j.cej.2016.06.090
Carducci A, Federigi I, Liu D, Thompson JR, Marco V (2020) Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res 179:115907. https://doi.org/10.1016/j.watres.2020.115907
Chen X, Ng D (2021) β-Cyclodextrin-conjugated phthalocyanines as water-soluble and recyclable sensitisers for photocatalytic applications. Chem Comm 57:3567–3570. https://doi.org/10.1039/d1cc00713k
Dingenen F, Blommaerts N, Van Hal M, Borah R, Arenas-Esteban D, Lenaerts S, Bals S, Verbruggen SW (2021) Layer-by-layer-stabilized plasmonic gold-silver nanoparticles on TiO2: towards stable solar active photocatalysts. Nanomaterials 11(10):2624. https://doi.org/10.3390/nano11102624
Dong S, Cui L, Zhang W, Xia L, Zhou S, Russell CK, Fan M, Feng J, Sun J (2020) Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem Eng J 384:123279. https://doi.org/10.1016/j.cej.2019.123279
Fessi N, Nsib MF, Chevalier Y, Guillard C, Dappozze F, Houas A, Palmisano L, Parrino F (2019) Photocatalytic degradation enhancement in pickering emulsions stabilized by solid particles of bare TiO2. Langmuir 35(6):2129–2136. https://doi.org/10.1021/acs.langmuir.8b03806
Gao A, Zhang H, Sun G, Xie K, Hou A (2017) Light-induced antibacterial and UV-protective properties of polyamide 56 biomaterial modified with anthraquinone and benzophenone derivatives. Mater Des 130:215–222. https://doi.org/10.1016/j.matdes.2017.05.071
Han J, Zhu Z, Li N, Chen D, Lu J (2021) Metalloporphyrin-based D-A type conjugated organic polymer nanotube for efficient photocatalytic degradation. Appl Catal B-Environ 291:120108. https://doi.org/10.1016/j.apcatb.2021.120108
Hou A, Zhou M, Wang X (2009) Preparation and characterization of durable antibacterial cellulose biomaterials modified with triazine derivatives. Carbohydr Polym 75:328–332. https://doi.org/10.1016/j.carbpol.2008.07.032
Hou A, Chen B, Dai J, Zhang K (2010) Using supercritical carbon dioxide as solvent to replace water in polyethylene terephthalate (PET) fabric dyeing procedures. J Clean Prod 18:1009–1014. https://doi.org/10.1016/j.jclepro.2010.03.001
Hou A, Feng G, Zhuo J, Sun G (2015) UV light-induced generation of reactive oxygen species and antimicrobial properties of cellulose fabric modified by 3,3′,4,4′-benzophenone tetracarboxylic acid. ACS Appl Mater Interfaces 7(50):27918–27924. https://doi.org/10.1021/acsami.5b09993
Hou J, Wang Y, Zhou J, Lu Y, Liu Y, Lv X (2021) Photocatalytic degradation of methylene blue using a ZnO/TiO2 heterojunction nanomesh electrode. Surf Interfaces 22:100889. https://doi.org/10.1016/j.surfin.2020.100889
Hu L, Zhang H, Gao A, Hou A (2018) Functional modification of cellulose fabrics with phthalocyanine derivatives and the UV light-induced antibacterial performance. Carbohydr Polym 201:382–386. https://doi.org/10.1016/j.carbpol.2018.08.087
Hu L, Hou A, Xie K, Gao A (2019) Light-induced production of reactive oxygen species by a novel water-soluble benzophenone derivative containing quaternary ammonium groups and it’s assembly on the protein fiber surface. ACS Appl Mater Interfaces 11(29):26500–26506. https://doi.org/10.1021/acsami.9b07992
Huang S, Xu Y, Liu Q, Zhou T, Zhao Y, Jing L, Xu H, Li H (2017) Enhancing reactive oxygen species generation and photocatalytic performance via adding oxygen reduction reaction catalysts into the photocatalysts. Appl Catal B-Environ 218:174–185. https://doi.org/10.1016/j.apcatb.2017.06.030
Jv X, Zhao X, Ge H, Sun J, Li H, Wang Q, Lu H (2019) Fabrication of a magnetic poly(aspartic acid)-poly(acrylic acid) hydrogel: application for the adsorptive removal of organic dyes from aqueou solution. J Chem Eng Data 64(3):1228–1236. https://doi.org/10.1021/acs.jced.8b01117
Klausen M, Ucuncu M, Bradley M (2020) Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 25:5239. https://doi.org/10.3390/molecules25225239
Ma D, Yi H, Lai C, Liu X, Yang L (2021) Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 275(3):130104. https://doi.org/10.1016/j.chemosphere.2021.130104
Marin ML, Santos-Juanes L, Arques A, Amat AM, Miranda MA (2012) Organic photocatalysts for the oxidation of pollutants and model compounds. Chem Rev 112(3):1710–1750. https://doi.org/10.1021/cr2000543
Mohyudin S, Farooq R, Jubeen F, Rasheed T, Fatima M, Sher F (2021) Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ Res 204:112387. https://doi.org/10.1016/j.envres.2021.112387
Mondal B, Bairagi D, Nandi N, Hansda B, Das KS, Edwards-Gayle C, Castelletto V, Hamley IW, Banerjee A (2020) Peptide-based gel in environmental remediation: removal of toxic organic dyes and hazardous Pb2+ and Cd2+ ions from wastewater and oil spill recovery. Langmuir 36(43):12942–12953. https://doi.org/10.1021/acs.langmuir.0c02205
Pan Z, Song C, Li L, Wang H, Pan Y, Wang C, Li J, Wang T, Feng X (2019) Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: recent advances and future prospects. Chem Eng J 376:120909. https://doi.org/10.1016/j.cej.2019.01.188
Pazdzior K, Bilinska L, Ledakowicz S (2019) A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J 376:120597. https://doi.org/10.1016/j.cej.2018.12.057
Qi L, Gong Y, Fang M, Jia Z, Cheng N, Yu L (2020) Surface-active ionic-liquid-encapsulated polyoxometalate nanospheres: construction, self-assembly, adsorption behavior, and application for dye removal. ACS Appl Nano Mater 3(1):375–383. https://doi.org/10.1021/acsanm.9b02012
Ren A, Zahid A, Fan D, Yang X, Imran MA, Alomainy A, Abbasi QH (2019) State-of-the-art in terahertz sensing for food and water security-a comprehensive review. Trends Food Sci Tech 85:241–251. https://doi.org/10.1016/j.tifs.2019.01.019
Sathe SM, Chakraborty I, Dubey BK, Ghangrekar MM (2022) Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: applications and challenges. Environ Res 204:112135. https://doi.org/10.1016/j.envres.2021.112135
Shen J, Steinbach R, Tobin J, Nakata MM, Bower M, Mccoustra M, Bridle H, Arrighi V, Vilela F (2016) Photoactive and metal-free polyamide-based polymers for water and wastewater treatment under visible light irradiation. Appl Catal B- Environ 193:226–233. https://doi.org/10.1016/j.apcatb.2016.04.015
Shi T, Li H, Ding L, You F, Ge L, Liu Q, Wang K (2019) Facile preparation of unsubstituted iron(II) phthalocyanine/carbon nitride nanocomposites: a multipurpose catalyst with reciprocally enhanced photo/electrocatalytic activity. ACS Sustain Chem Eng 7(3):3319–3328. https://doi.org/10.1021/acssuschemeng.8b05366
Shkrob IA Jr, Sauer MC (2004) Hole scavenging and photo-stimulated recombination of electron-hole pairs in aqueous TiO2 nanoparticles. J Phys Chem B 108(33):12497–12511. https://doi.org/10.1021/jp047736t
Song J, Deng Q, Huang M, Kong Z (2022) Carbon nanotube enhanced membrane distillation for salty and dyeing wastewater treatment by electrospinning technology. Environ Res 204:111892. https://doi.org/10.1016/j.envres.2021.111892
Sun H, Tian X, Wang J, Zhang J, Yuan Y, Sun Z (2011) Theoretical studies on molecular and structures of mono- and binuclear chromium carbazole derivatives for optoelectronics. J Phys Chem A 115(50):14495–14501. https://doi.org/10.1021/jp2066452
Sun M, An J, Pan Z, Feng G, Fan X, Song C, Wang T (2021) Enhanced organic wastewater treatment performance in electrochemical filtration process of coal-based carbon membrane via the simple Fe2+ addition. Sep Purif Tech 276:119418. https://doi.org/10.1016/j.seppur.2021.119418
Wang J, Wang S (2020) Reactive species in advanced oxidation processes: formation, identification and reaction. Chem Eng J 401:126158. https://doi.org/10.1016/j.cej.2020.126158
Wang X, Li Z, Zhang Y, Li Q, Du H, Liu F, Zhang X, Mu H, Duan J (2022) Enhanced photocatalytic antibacterial and degradation performance by p-n-p type CoFe2O4/CoFe2S4/MgBi2O6 photocatalyst under visible light irradiation. Chem Eng J 429:132270. https://doi.org/10.1016/j.cej.2021.132270
Wu B, Li Y, Su K, Tan L, Liu X, Cui Z, Yang X, Liang Y, Li Z, Zhu S (2019) The enhanced photocatalytic properties of MnO2/g-C3N4 heterostructure for rapid sterilization under visible light. J Hazard Mater 377:227–236. https://doi.org/10.1016/j.jhazmat.2019.05.074
Xie K, Yu Y, Shi Y (2009) Synthesis and characterization of cellulose/silica hybrid materials with chemical crosslinking. Carbohydr Polym 78:799–805. https://doi.org/10.1016/j.carbpol.2009.06.019
Xie K, Zhao W, He X (2011) Adsorption properties of nano-cellulose hybrid containing polyhedral oligomeric silsesquioxane and removal of reactive dyes from aqueous solution. Carbohydr Polym 83:1516–1520. https://doi.org/10.1016/j.carbpol.2010.09.064
Yamaguchi M, Abe H, Ma T, Tadaki D, Niwano M (2020) Bactericidal activity of TiO2 nanotube thin films on Si by photocatalytic generation of active oxygen species. Langmuir 36(42):12668–12677. https://doi.org/10.1021/acs.langmuir.0c02225
Yang W, Zhao J, Sonn C, Escudero D, Karatay A, Yaglioglu H, Küçüköz B, Hayvali M, Li C, Jacquemin D (2016) Efficient intersystem crossing in heavy-atom-free perylenebisimide derivatives. J Phys Chem C 120(19):10162–10175. https://doi.org/10.1021/acs.jpcc.6b01584
Yang H, Liu Z, Wang K, Pu S, Yang S, Yang L (2017) A facile synthesis of TiO2-CdS heterostructures with enhanced photocatalytic activity. Catal Letters 147:2581–2591. https://doi.org/10.1007/s10562-017-2151-0
Yi S, Zou Y, Sun S, Dai F, Si Y, Sun G (2018) Rechargeable photoactive silk-derived nanofibrous membranes for degradation of Reactive Red 195. ACS Sustain Chem Eng 7(1):986–993. https://doi.org/10.1021/acssuschemeng.8b04646
Yi S, Sun S, Fan Y, Zou Y, Dai F, Si Y (2020a) Scalable fabrication of rechargeable photoactive cellulose nanofibrous membranes for efficient degradation of dyes. Cellulose 27(9):5285–5296. https://doi.org/10.1007/s10570-020-03168-9
Yi S, Wu Y, Zhang Y, Zou Y, Dai F, Si Y (2020b) Antibacterial activity of photoactive silk fibroin/cellulose acetate blend nanofibrous membranes against Escherichia coli. ACS Sustain Chem Eng 8:16775–16780. https://doi.org/10.1021/acssuschemeng.0c04276
Zhang M, Shao C, Guo Z, Zhang Z, Mu J (2011) Hierarchical nanostructures of copper(II) phthalocyanine on electrospun TiO2 nanofibers: controllable solvothermal-fabrication and enhanced visible photocatalytic properties. ACS Appl Mater Interfaces 3(2):369–377. https://doi.org/10.1021/am100989a
Zhang H, Wang J, Xie K, Pei L, Hou A (2020a) Synthesis of novel green reactive dyes and relationship between their structures and printing properties. Dyes Pigm 174:108079. https://doi.org/10.1016/j.dyepig.2019.108079
Zhang X, Li Z, Lin S, Théato P (2020b) Fibrous materials based on polymeric salicyl active esters as efficient adsorbents for selective removal of anionic dye. ACS Appl Mater Interfaces 12(18):21100–21113. https://doi.org/10.1021/acsami.0c03039
Zhao Y, Li Y, Sun L (2021) Recent advances in photocatalytic decomposition of water and pollutants for sustainable application. Chemosphere 276:130201. https://doi.org/10.1016/j.chemosphere.2021.130201
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 22208310); Opening Project of Key Laboratory of Clean Dyeing and Finishing Technology of Zhejiang Province (Grant No. QJRZ2202 and QJRZ2204); Research Start-up Funding of Zhejiang Sci-Tech University (Grant No. 21202301-Y); and Postdoctoral Fellowship of Zhejiang Province (Grant No. ZJ2021109).
Author information
Authors and Affiliations
Contributions
LH: Methodology, Software, Investigation, Writing-Original Draft, Funding acquisition; ML: Methodology, Project administration, Funding acquisition; XS: Software, Data curation; KX: Writing-Review & Editing, Data Curation; AH: Methodology, Writing—Review & Editing, Resources.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that had appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, L., Song, X., Li, M. et al. Scalable fabrication of benzophenone/phthalocyanine-decorated photodynamic cotton fabrics for enhanced dye degradation and antibacterial performance. Cellulose 30, 4683–4696 (2023). https://doi.org/10.1007/s10570-023-05141-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05141-8