Abstract
Cotton fabric can be modified to obtain additional functionality such as hydrophobic property, antibacterial property and flame retardant property. The traditional coating method often has adverse influence on the strength and permeability of cotton fabric. Polyhedral oligomeric silsesquioxanes-(poly(trifluoroethyl methacrylate)8) copolymer was modified on the surface of cotton fabric with three-dimensional (3D) ordered porous structure via the breath figure. A key factor of solution concentration was studied to regulate the formation of 3D porous structure. The morphology and chemical composition of the modified fabric were characterized. The hydrophobic and the dynamic hydrophobic property of the modified fabrics with different copolymer concentrations were studied. The self-cleaning properties, oil/water separation, rubbing durability and bacterial adsorption on the modified fabrics were examined. It was showed that the modified fabric had self-cleaning, oil/water separation capabilities, rubbing durability and an effective inhibitory effect on the biofilm formation of bacteria. In addition, compared to the unmodified fabric, the comfortable capability and breaking strength of the modified fabric were investigated.
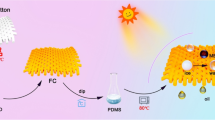
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabric becomes one of the most extensively used textiles due to its softness, comfort and breathability. However, cotton fabric is easily stained by dirt, particles, oil and unclean water and thus loses its original advantages. Cotton fabric is also easily adhered by bacteria, molds, and viruses to become a carrier for disease transmission. Some studies have reported that the hydrophobic self-cleaning functionalization of cotton fabric can avoid bacteria adhesion, water wettability, and dust contamination (Kong et al. 2020; Li et al. 2015a; Wu et al. 2016). The available methods for introducing hydrophobic functionality into textile surfaces include spraying (Fu et al. 2017; Li et al. 2019; Wang et al. 2014), ultrasound irradiation (Hao et al. 2013; Yazdanshenas and Shateri-Khalilabad 2013), in-situ synthesis (El-Naggar et al. 2018; Li et al. 2018, 2022a), deposition (Li et al. 2022b; Ou et al. 2016; Soto et al. 2018), and grafting polymerization (Castelvetro et al. 2007; Li et al. 2021; Liang et al. 2016). By these technologies, hydrophobicity can be effectively modified onto the surfaces of textiles, which then can be used in some special environments with new functions. However, a thick coating on the cotton fabric surface would affect its softness and breathability, and would lose tensile strength due to these modification processes. At present, there are a lot of studies concerned with multi-functionality on hydrophobic fabrics with anti-fouling, self-cleaning and reducing bacterial adsorption to prevent the formation of biofilms, while the original performance of cotton fabrics could not be maintained. Therefore, it is necessary to develop a modification method for cotton fabric that combines both functional property of coated materials and well-maintained inherent performance of cotton fabrics.
Micro/nano scale honeycomb porous films with the advantages of uniform pore size and high roughness, have some applications in separation membranes, biological materials, micro reactors, hydrophobic interface and template materials (Chen et al. 2018; Huang et al. 2020; Sun et al. 2015). Compared with the common porous structure preparation method, the breath figure method is a simple, efficient and inexpensive method, using water droplet as a template (Bunz 2006; Chen et al. 2018; Wan et al. 2014; Zhang et al. 2015). In addition, there is no need to remove the template during the preparation process. The morphology of the film can be adjusted by controlling the structure of the polymer, the type of solvent, concentration, substrate and film forming factors (Escalé et al. 2012; Hernández Guerrero and Stenzel 2012; Li et al. 2014; Liu et al. 2020; Yang et al. 2022). The breath figure method was reported for planar solid substrates (silicon wafer, glass, mica, ice) (Cong et al. 2012; Li et al. 2017; Yu et al. 2015) and nonplanar solid substrates (TEM grid, uniform microparticles) (Bai et al. 2013; Hwangbo et al. 2011; Zhang et al. 2013), and liquid substrates (water, ethyl alcohol, ethanol, glycerol) (Li et al. 2015b; Wan et al. 2012; Yin et al. 2013). Xu et al. used soft substrates such as cotton and polyester fabrics to prepare a porous film for self-powered sensor devices (Gong et al. 2017; Liu et al. 2018), which could prepare 3D porous surfaces.
Research has found that a smooth surface cannot inhibit bacterial adhesion (Yin et al. 2013). Therefore, it is possible to use a thin film with a rough surface with porous structure to solve the bacterial adhesion problem. Shiratori et al. (Manabe et al. 2013) built a porous membrane for the adhesion of Pseudomonas aeruginosa by the breath figure method. With the bacterial reproduction test, it was found that the porous membrane effectively prevented the adhesion and growth of bacteria when the pore size range was 5–11 μm, and the bacteria was reduced to 0.59%. Tang et al. (Heng et al. 2013) established a porous film with aggregation-induced luminescence properties. Uterine cancer cells and liver cancer cells cannot grow and adhere to the surface of a porous structure. On the contrary, the planar structure was conducive to cell growth. The wettability was the main reason that the porous membrane was not conducive to cell growth (Kong et al. 2021).
Polyhedral oligomeric silsesquioxane (POSS) with the nanometer-sized inorganic cores are encompassed by organic functional groups, which have excellent thermal and mechanical properties (Foorginezhad and Zerafat 2019; Raftopoulos and Pielichowski 2016; Zhang et al. 2017). Fluorinated polymers have superior chemical stability, thermal stability and low surface energy which play a major role in developing hydrophobicity and antifouling functional materials. Based on the advantages of POSS and fluorinated polymers, fluorine-containing POSS block copolymer can be synthesized, which used as the material to construct porous film with excellent performance.
Herein, the fluorinated POSS block polymer with ordered porous structure was loaded onto cotton fabric substrate through the breath figure. The hydrophobic properties were obtained from adjusting the polymer concentration. The surface structure and chemical constitution of the fabrics were characterized. The features of the fabric such as contact angle and water impact behavior were measured. Adhesion performance of dirt and bacteria were investigated for applications as self-cleaning and antifouling materials. The absorption capacity and reusability of the hydrophobic fabrics were studied as oil/water separation materials. In addition, the rubbing durability, softness, moisture permeability, dynamic air permeability and breaking strength of the porous fabrics were evaluated.
Experimental
Materials
Cotton fabrics (Guandong Textile Dyeing Garment Co., Ltd) were cleaned with deionized water before the modification. POSS-(PTFEMA)8 was synthesized as previous study (Kong et al. 2021). The molecular weight of the polymer was 13,450 g/mol, and the PDI was 1.43. Anhydrous ethanol, chloroform (CHCl3), dodecane were purchased from Sinopharm Chemical Reagent Co., Ltd. ANOSET®Yellow 4GN dye and ANOSET® Orange 4GN dye were provided by Shanghai Anoky Group Co., Ltd. Disperse Red 60 and Disperse Blue 219 were provided by the J&K Scientific Co., Ltd. E. coli O157:H7 ATCC 43,895 was bought from Shanghai Vita Chemical Reagent Co., Ltd.
Preparation of porous structure on cotton fabric surface
The polymer solutions with different concentrations (10, 30, 60, 90 and 120 mg/mL) were received by dissolving POSS-(PTFEMA)8 in CHCl3. Cotton fabric was infused into the solution to completely wet and put to humidity (90%) atmosphere. When the solvent was completely evaporated, the porous cotton fabric was prepared and further characterized.
Oil absorption capacity and reusability
The modified fabric was dried at 50 °C in a vacuum. The sample was dipped into 20 mL dodecane, and subsequently the sample removed from the solution and weighed. The absorption capacity (Cm) was the mass of absorbed dodecane (g) per unit mass of dry modified cotton fabric (g). The Cm was calculated as follow:
where m0 and m1 are the weight of the dried and absorbed hydrophobic cotton fabric, respectively. The reusability of the cotton fabric was evaluated by rinsing the sample with dodecane. Sample was subsequently dried under vacuum at 45 °C and weighed.
Bacterial adsorption
The adsorption of the modified cotton against E. coli O157:H7 (ATCC43895) were measured consulting to the literature (Manabe et al. 2013). The bacterial suspension (1 × 108 cells mL−1) was placed on the cotton fabric and cultured for 30 min. The free bacteria were removed from cotton fabric with phosphate buffer (PBS). The bacteria were immobilized on cotton surface with glutaraldehyde solution (2.5%) at 4 °C. When the glutaraldehyde was removed, the bacteria were dehydrated with alcohol solution, and then dried at 37 °C in a vacuum. Additionally, the sample was treated with bacteria for 30 min, subsequently added to the solution of PBS (10 mL). The sample was sonicated for 10 min to disperse the attached bacteria in PBS and serially diluted, and then spotted onto agar plate. After the plate was incubated at 37 °C for 24 h, the quantity of bacterial colonies was recorded.
Characterization
FTIR spectra (Thermo Is5, Nicolet Instrument Corporation, USA) of cotton and modified cotton fabrics were obtained. The morphologies of cotton fabric were scanned by a scanning electron microscope (SEM) (HITACHI, SU-1510, Japan) after gold sputtering. The surface morphology and roughness were observed through atomic force microscope (AFM) (Dimension FastScan, Bruker, Germany). The chemical element characterization of cotton and modified cotton were carried out by X-ray photoelectron spectroscopy (XPS) (Kratos, England). X-ray diffraction (XRD) curves of cotton fabrics were obtained by an X-ray diffraction at 4° min−1 in the scanning angle range of 2θ = 10–90° (D2 Phaser, Brucker, Germany). The contact angles (CA) were tested ten times (PT-602A, Dong Guan Precise Test Equipment Co., Ltd). To evaluate the droplet impact, a 12 μL water droplet was dropped on the cotton surface or modified cotton at a height of 40 mm. The surface morphology variation of the water droplet was taken by high speed CCD (American Trouble Shooter HR, 10000 FPS). To test the rubbing durability, the modified fabric and unmodified cotton fabric were fixed onto the stainless steel column and used as the rubbing partner, respectively. Then, the sample was subjected to a roundtrip with a distance of 200 mm (one cycle). Breaking strength was measured with a YG (B) 026D-250 electronic fabric strength tester in accordance with GB/T 3923-1997. Moisture permeability was quantitatively analyzed by taking the fabric to 25 g calcium chloride aqueous solution and monitoring the total weight in the saturated humidity environment (YG601H-II). The air permeability was tested on a YG(B)461E automatic gas permeability tester according to GB/5453-1997. Fabric hand properties including resilience score and softness score were measured by the PhabrOmeter Model 3 fabric evaluation system according to AATCC 202-2012.
Results and discussion
Cotton fabrics with porous surface
The schematic illustration of the porous film on the fabric surface via breath figure is exhibited in Fig. 1a. The fabric was dipped into the POSS-(PTFEMA)8/CHCl3 solution and dried in a moist atmosphere, and the solvent volatilized quickly to generate gradient of temperature. The temperature of the cotton fabric surface dropped sharply, which caused the congelation of water vapor in the high humidity environment. As the volatilization–condensation process continued, a droplet with a diameter in the nanometer range was formed in the rapid nucleation process. Then the droplets grew and formed an ordered array. At the same time, the droplets were covered by the POSS-(PTFEMA)8 solution to separate them from each other and ensure the uniformity of the droplet size. Thus, the water droplets served as pattern plates for the porous structure formation (Fig. 1b) (Zhang et al. 2015). When the water and solvent completely volatilized, the regular porous microstructures were formed on fabric surface.
Characterization of the modified fabrics
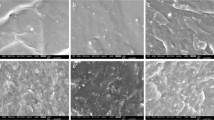
The SEM photographs of cotton and the modified fabrics with different POSS-(PTFEMA)8 copolymer concentrations are shown in Fig. 2. The unmodified fabric surface was smooth as exhibited in Fig. 2a. Significant differences on the surfaces were found between the fabric and modified fabrics. The formation of the porous structure on the cotton fabric was more complicated than that on a planar substrate through the breath figure. In planar substrates (glass and silicon wafer), 2D porous film was obtained in a high humidity environment from 1 to 60 mg/mL. If these conditions were not given, the porous film could not be prepared or the porous structure would not be regular.
Compared with glass or silicon wafer, cotton fabric substrate had 3D morphology. Under the same breath figure condition, random irregular porous microstructures were found from 0 to 60 mg/mL. Also, ordered porous structures cannot be prepared on the cotton fabric within the conventional concentration through breath figure. Compared with the planar substrates, the 3D architectures of textile surfaces were quite different such as loose, flexible, largely specific surface area and uneven (Guan et al. 2020). On the other hand, textile substrates could be wetted by the copolymer solution by dipping into the coating solution. It was found that the conventional solution concentration to form porous film on the planar surface was not suitable for preparing the equivalent on textile substrates. The polymer concentration was clearly important attribute to specific surface area of the textile. Therefore, increase the polymer concentration was a simple way of conjecture verification.
It could be seen that the honeycomb arrangement with smaller pore sizes on the cotton fabrics surface were formed when the concentrations were added from 60 to 120 mg/mL. At lower concentrations (10 and 30 mg/mL), only irregular patterns were discovered. At 90 mg/mL, ordered porous structures could be achieved as exhibited in Fig. 2e. The unique fabric texture features were also ensconced when the copolymer concentration was 120 mg/mL (Fig. 2f). The pore size was decreased which was mainly ascribed to the largely increased viscosity. Therefore, the suitable concentration was 90 mg/mL.
The FTIR and XRD spectra of cotton and modified cotton are demonstrated in Fig. 3. FTIR spectra of the cotton and modified cotton are presented in Fig. 3a. After fabrics were modified with POSS-(PTFEMA)8, the peak of C–F was identified at 1280 cm−1. The peak of Si–O–Si was founded at 1170 cm−1. The C=O stretching vibration peak was appeared at 1760 cm−1. FTIR spectra indicated that POSS-(PTFEMA)8 block copolymer was loaded on cotton surface.
The XRD spectra of fabric and modified fabric are exhibited in Fig. 3b. The POSS block copolymer showed a broad peak at 18.9°. The unmodified cotton had diffraction peaks at 14.9°, 16.7°, 22.8° and 34.6° associated with cellulose structure (Kuang et al. 2016; Xie et al. 2009). Compared with the unmodified cotton fabric, the peaks at 14.9°, 16.7° and 22.8° were weaker after the coating process. These results showed that the POSS block copolymer had little influence on the crystal structure of cotton (Lu et al. 2018), which might be attribute to the low concentration of the copolymer.
The XPS spectra and high resolution C 1 s spectra of unmodified and modified fabrics are exhibited in Fig. 4. Two peaks of C and O are tested on the unmodified cotton in Fig. 4a. After modification, new peaks of Si (3.37%) and F (14.43%) appeared in the modified fabric, indicating that fabric was covered with POSS-(PTFEMA)8. The high resolution C 1 s peak demonstrated four distinct peaks at 284.8 eV of C–C and C–H bonds, 286.1 eV of C=O bonds, 288.2 eV of O–C=O bonds and 293.2 eV of C–F bonds for modified cotton fabrics, which further provided evidence that the fabric was coated by the POSS block copolymer.
Hydrophobic property of the modified cotton fabrics
The goal of modification had been to render the fabric hydrophobicity. Figure 5a shows the contact angles of the modified fabric. The static contact angle on the unmodified cotton fabric was 0°, indicating that the unmodified cotton was hydrophilic. After the introduction of POSS block copolymer with the increasing concentrations of 10, 30, 60, 90 and 120 mg/mL, respectively. The contact angles were 112.8°, 128.2°, 139.5°, 154.0° and 147.2°, respectively. The hydrophobic property on the modified fabric was result from the low surface energy of copolymer and hierarchically porous structures. The modified cotton fabrics contained F and Si elements leading to high contact angle with low surface energy. Surface roughness was also an important contributor to the wettability of cotton fabrics, besides surface chemistry. The water contact angle on the cotton fabric with 90 mg/mL POSS block copolymer without breath figure method was 136.8°. Due to the porous structure enhanced the surface roughness, the water contact angle was increased.
The AFM photographs of the cotton are shown in Fig. 6. The root mean square (RMS) value of the unmodified cotton with slippery surface was 11.7 nm (Fig. 6a). However, after modification a clear change was observed (Fig. 6b). Some rugged pores which were verified by SEM images in Fig. 2 were detected, and the RMS value increased to 140.0 nm. A higher RMS value represented larger roughness. Hence, the honeycomb structure on the fabric improved the roughness and further enhanced its hydrophobic performance. When droplets of ANOSET®Yellow 4GN solution were dripped onto the unmodified cotton fabric surface, the droplets would be quickly absorbed by the fabric and penetrate into the interior, causing a dyed spot on the fabric in Fig. 5b. However, the modified fabric exhibited excellent hydrophobicity and prevented the entrance of dye droplets into the interior, retaining the shape of the dye water droplets as shown in Fig. 5c.
The droplets impact on the fabric surface flows in two simultaneous yet separate ways, by either spreading or penetration, which occur on top and within the fabric. Figure 7a showed spreading and penetration processes of a water droplet on hydrophilic cotton fabric. When the droplet hit the fabric, it gradually spread and then immersed into the interior. At 32 ms, the fabric was completely soaked. As exhibited in Fig. 7b–f, immersion, spreading, retraction and oscillation occurred on the porous cotton fabrics surface. The water droplet was perfectly spherical at 0 ms, then it spread rapidly and retracted to its center. Subsequently, it rose up to a level and vibrated to the static state. During the whole process, the droplet maintained contact intact and had rebounded tendency. With the increase of the hydrophobic property, the bounce trend of water droplet became obvious on the modified cotton fabric. Therefore, the impact behavior could reveal the hydrophobic property.
D(t) was droplet contact diameter after hitting the surface divided by time, D0 was the initial droplet diameter. H(t) was the maximum height of the droplet divided by time after contact with the surface. As indicated in Fig. 7g, D(t)/D0 gradually increased with time to reach a maximum value and then nearly remained unchanged, and the droplet almost did not get smaller after spreading. As for the hydrophobic surface, D(t)/D0 gradually increased with impact time and reached a maximum value before decreasing. The droplet cannot leave the surface after colliding with the surface, and then the phenomenon of oscillation occurred.
After the maximum spreading state, the effects of surface structure on the D(t)/D0 values decreased with the increased water contact angles. This phenomenon occurred because viscous forces and interfacial were important to the spreading of the water droplet. Moreover, changes in surface energy influenced the droplet evolution on account of the surface structure. Especially, the Dmax/D0 value slightly increased with the decreased of contact angle on the surfaces.
From Fig. 7h, it can be found that the H(t)/D0 value was decreased to 0 on the hydrophilic cotton surface with the impact time of about 5 ms. However, the values of the hydrophobic fabric were fluctuated. The difference in degree of fluctuation indicated the difference in the degree of hydrophobicity. The cotton fabric with the highest water contact angle exhibited the highest H(t)/D0 value.
Self-cleaning property of the modified fabrics
Cotton fabrics were easily stained by contaminants. Once stained, a lot of detergent and water will be consumed, which will lead to pollution problem. Hence, the cotton fabrics with self-cleaning property were very important. Droplets of water, orange, milk, pomegranate juice, spinach juice, and coffee were dropped on the fabric surface, respectively. The photographs of different liquid droplets on the unmodified and modified fabrics and self-cleaning performance are revealed in Fig. 8. The unmodified fabric surface exhibited hydrophilic property and was contaminated by the droplets in Fig. 8a. All the droplets on the modified fabric surface exhibited the spherical shapes in Fig. 8b. The POSS-(PTFEMA)8 block copolymer and porous microstructures can endow cotton fabrics substrate with hydrophobic properties. ANOSET® Orange 4GN dye was applied as pollutant to study the self-cleaning property of the fabrics in Fig. 8c. The unmodified fabric was fouled promptly when the water dropped on the dye owe to good hydrophilic ability, while the modified cotton had orange spherical droplets attribute to high hydrophobic property. In addition, the soil particles was used as pollutant to demonstrate the self-cleaning of the modified cotton. As indicated in Fig. 8d, soil particles were spread, and water was joined onto the top of the soil particles. The dirt was carried away and cleared off by droplets due to the weak adhesion, which demonstrated that the modified fabric had excellent self-cleaning property.
Absorption capacity and reusability of the modified cotton fabrics
As shown in Fig. 9a–d, dodecane was adsorbed from the oil/water mix solution by using the modified fabric. Once the modified fabric came in contact with dodecane that settled on the surface of the water, dodecane was quickly adsorbed until the solution became completely transparent without any pollution. It was obvious that the modified fabric selectively absorbed dodecane from solution and exhibited good oil/water separation ability.
a–d Removal of dodecane (Disperse Red 60 dyed) from water with the modified fabric. e Sorption capacity of the modified fabric [POSS-(PTFEMA)8 is 90 mg/mL]. (1)–(3) Absorbing dodecane (Disperse Blue 219 dyed), (4) modified cotton fabric after rinsing with anhydrous ethanol. f Absorption capacity (Cm) and reusability after up to 10 rinsing-absorption cycles
For purpose of evaluate the repeatability of the modified fabric, the fabric was soaked with dodecane and washed with anhydrous ethanol (Fig. 9e). It was found that the blue color vanished. Its absorption capacity (Cm) was estimated after 10 rinsing absorption cycles (Fig. 9f). The reason for the good absorption ability could be result from the high surface area and porosity of the porous structure on cotton fabric. The Cm value was about 1.6 g/g. In brief, the modified cotton fabric exhibited remarkable absorptive capacity, which indicated that it could potentially be reused.
Bacterial adsorption of the modified cotton fabrics
Textiles with excellent antibacterial property have a wide application prospect. The adsorption of E. coli O157:H7 on cotton fabric before and after modification are exhibited in Fig. 10. The biofilms of E. coli O157:H7 was formed unmodified on cotton fabric in Fig. 10a, b. The modified cotton fabric surface showed less adsorption of E. coli O157:H7 than unmodified cotton (Fig. 10c, d). The hydrophobic ability of cotton fabric confirmed the bacterial adsorption. Clearly the E. coli O157:H7 biofilms were only formed on the hydrophilic fabric surface but not on the modified sample due to its hydrophobicity.
The bacterial count on unmodified and modified cotton after different contact times are exhibited in Table 1. The bacterial count on the fabric after 30 min was 7.5 × 107 CFU/cm2, and increased to 1.4 × 108 CFU/cm2 after 120 min. In contrast, the bacterial count on the modified fabric was lower, and only increased to 2.8 × 107 CFU/cm2 after 120 min. Thus, the modified cotton fabric could prevent the bacterial adhesion and the reproduction on the surface.
Rubbing durability of the modified cotton fabrics
The rubbing durability of the modified fabrics was investigated, and the contact angles of the modified cotton fabrics after rubbing treatment were shown in Fig. 11. The water contact angles of the modified cotton surfaces did not show obvious decrease after 100, 200 and 300 cycles of rubbing.
Comfortable capability and breaking strength of the modified fabrics
The comparative softness of unmodified and modified fabric is exhibited in Table 2. Softness is one of the elements to determine the wear comfort. The fabric is soft when the softness value is small. The modified cotton fabric had a larger value (80.02) than the unmodified fabric (64.85) and thus was slightly harsher (Kan and Wong 2015).
The moisture permeability of the modified fabric was also studied. The moisture permeability of fabric with the porous structure was decreased slightly from 9577 g m−2 30 min−1 to 8518 g m−2 30 min−1. It certified that the modified cotton fabric could maintain the inherent moisture permeability.
The conventional coating method often causes a thick and solid layer on the fabric surface, which is deprived of air permeability. The dynamic air permeability of the fabric and modified fabrics were 158.0 mm s−1 and 134.7 mm s−1, respectively, which indicated that the modified fabric still kept high air permeability (85.3%).
The breaking strength of the modified fabric was increased compared with unmodified cotton due to the enhanced intermolecular forces between adjoin fibers or yarns (Guan et al. 2020). The external force caused the relative drift among molecular chains was reduced (Wang and Wang 2009). On the other hand, the mild coating conditions did not significantly damage the fabric. Therefore, the modified fabric kept the inherent toughness of the fabrics and enhanced their breaking strength.
Conclusions
We successfully prepared a porous film of POSS-(PTFEMA)8 block copolymer on cotton fabric surface through the breath figure. The three-dimensional porous structure can be regulated by controlling POSS-(PTFEMA)8 concentration. The water contact angle of hydrophobic fabric modified with the copolymer concentration of 90 mg/mL was 154.0°. The static contact angle and dynamic hydrophobic behavior indicated that the fabric had achieved superhydrophobic properties. The modified cotton fabric could remove oil from a dodecane/water mixture due to its hydrophobic property. Furthermore, it demonstrated remarkable absorptive capacity even after 10 rinsing/absorption cycles. The modified cotton fabric could prevent contaminants from permanently attaching and protect from the bacterial adhesion. Thus it could be considered self-cleaning. In addition, the modified cotton fabric exhibited rubbing durability after 300 cycles of rubbing. Overall it kept the outstanding inherent performances of modified fabric such as softness, moisture permeability, dynamic air permeability and high breaking strength to a large extent.
References
Bunz UHF (2006) Breath figures as a dynamic templating method for polymers and nanomaterials. Adv Mater 18:973–989. https://doi.org/10.1002/adma.200501131
Bai H, Du C, Zhang A, Li L (2013) Breath figure arrays: unconventional fabrications, functionalizations, and applications. Angew Chem Int Ed 52:12240–12255. https://doi.org/10.1002/anie.201303594
Castelvetro V, Geppi M, Giaiacopi S, Mollica G (2007) Cotton fibers encapsulated with homo- and block copolymers: synthesis by the atom transfer radical polymerization grafting-from technique and solid-state NMR dynamic investigations. Biomacromolecules 8:498–508. https://doi.org/10.1021/bm060602w
Chen S, Gao S, Jing J, Lu Q (2018) Designing 3D biological surfaces via the breath-figure method. Adv Healthc Mater 7:1701043. https://doi.org/10.1002/adhm.201701043
Cong H, Wang J, Yu B, Tang J (2012) Preparation of a highly permeable ordered porous microfiltration membrane of brominated poly(phenylene oxide) on an ice substrate by the breath figure method. Soft Matter 8:8835–8839. https://doi.org/10.1039/c2sm25706h
El-Naggar ME, Shaarawy S, Hebeish AA (2018) Multifunctional properties of cotton fabrics coated with in situ synthesis of zinc oxide nanoparticles capped with date seed extract. Carbohydr Polym 181:307–316. https://doi.org/10.1016/j.carbpol.2017.10.074
Escalé P, Rubatat L, Billon L, Save M (2012) Recent advances in honeycomb-structured porous polymer films prepared via breath figures. Eur Polym J 48:1001–1025. https://doi.org/10.1016/j.eurpolymj.2012.03.001
Foorginezhad S, Zerafat MM (2019) Fabrication of superhydrophobic coatings with self-cleaning properties on cotton fabric based on Octa vinyl polyhedral oligomeric silsesquioxane/polydimethylsiloxane (OV-POSS/PDMS) nanocomposite. J Colloid Interfaces Sci 540:78–87. https://doi.org/10.1016/j.jcis.2019.01.007
Fu Y, Jin B, Zhang Q, Zhan X, Chen F (2017) pH-induced switchable superwettability of efficient antibacterial fabrics for durable selective oil/water separation. ACS Appl Mater Interfaces 9:30161–30170. https://doi.org/10.1021/acsami.7b09159
Gong J, Xu B, Tao X (2017) Three-dimensionally conformal porous microstructured fabrics via breath figures: a nature-inspired approach for novel surface modification of textiles. Sci Rep 7:2364. https://doi.org/10.1038/s41598-017-02615-1
Guan X, Gong J, Xu B (2020) Three-dimensional conformal porous microstructural engineering of textile substrates with customized functions of brick materials and inherent advantages of textiles. ACS Appl Mater Interfaces 12:17967–17978. https://doi.org/10.1021/acsami.0c01557
Hao L, Wang R, Fang K, Liu J (2013) Ultrasonic effect on the desizing efficiency of α-amylase on starch-sized cotton fabrics. Carbohydr Polym 96:474–480. https://doi.org/10.1016/j.carbpol.2013.04.003
Heng L, Hu R, Chen S, Li M, Jiang L, Tang BZ (2013) Ordered honeycomb structural interfaces for anticancer cells growth. Langmuir 29:14947–14953. https://doi.org/10.1021/la403720s
Hernández Guerrero M, Stenzel MH (2012) Honeycomb structured polymer films via breath figures. Polym Chem 3:563–577. https://doi.org/10.1039/c1py00219h
Huang P, Wu F, Shen B, Zheng H, Ren Q, Luo H, Zheng W (2020) Biomimetic porous polypropylene foams with special wettability properties. Compos B: Eng 190:107927. https://doi.org/10.1016/j.compositesb.2020.107927
Hwangbo K, Kim MR, Lee C, Cho KY (2011) Facile fabrication of uniform golf-ball-shaped microparticles from various polymers. Soft Matter 7:10874–10878. https://doi.org/10.1039/c1sm06529g
Kan CW, Wong ML (2015) A study of paper towel hand properties. AATCC Rev 15:38–47. https://doi.org/10.14504/ar.15.5.3
Kong Q, Zhang Z, Li Z, Ren X (2020) Dynamic hydrophobic behavior of water droplets impact on the cotton fabrics coated with POSS block copolymer. Cellulose 27:1705–1716. https://doi.org/10.1007/s10570-019-02870-7
Kong Q, Li Z, Ding F, Ren X (2021) Hydrophobic N-halamine based POSS block copolymer porous films with antibacterial and resistance of bacterial adsorption performances. Chem Eng J 410:128407. https://doi.org/10.1016/j.cej.2021.128407
Kuang Y, Chen G, Ming S, Wu Z, Fang Z (2016) Solvent resistance of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) treated cellulose nanofiber film for flexible electronics. Cellulose 23:1979–1987. https://doi.org/10.1007/s10570-016-0906-1
Li Z, Ma X, Zang D, Shang B, Qiang X, Hong Q, Guan X (2014) Morphology and wettability control of honeycomb porous films of amphiphilic fluorinated pentablock copolymers via breath figure method. RSC Adv 4:49655–49662. https://doi.org/10.1039/c4ra08472a
Li S, Huang J, Ge M, Cao C, Deng S, Zhang S, Chen G, Zhang K, Al-Deyab SS, Lai Y (2015) Robust flower-like TiO2@ cotton fabrics with special wettability for effective self-cleaning and versatile oil/water separation. Adv Mater Interfaces 2:1500220. https://doi.org/10.1002/admi.201500220
Li Z, Ma X, Zang D, Hong Q, Guan X (2015) Honeycomb porous films of pentablock copolymer on liquid substrates via breath figure method and their hydrophobic properties with static and dynamic behaviour. RSC Adv 5:21084–21089. https://doi.org/10.1039/c5ra00066a
Li Z, Kong Q, Ma X, Zang D, Guan X, Ren X (2017) Dynamic effects and adhesion of water droplet impact on hydrophobic surfaces: bouncing or sticking. Nanoscale 9:8249–8255. https://doi.org/10.1039/c7nr02906c
Li N, Pranantyo D, Kang E-T, Wright DS, Luo H-K (2018) In situ self-assembled polyoxotitanate cages on flexible cellulosic substrates: multifunctional coating for hydrophobic, antibacterial, and UV-blocking applications. Adv Funct Mater 28:1800345. https://doi.org/10.1002/adfm.201800345
Li W, Wang H, Li Z (2019) Preparation of golf ball-shaped microspheres with fluorinated polycaprolactone via single-solvent electrospraying for superhydrophobic coatings. Prog Org Coat 131:276–284. https://doi.org/10.1016/j.porgcoat.2019.02.039
Li W, Wang X, Wu Y, Chen M, Li Z (2021) One-step spontaneous grafting via diazonium chemistry for the fabrication of robust bionic multifunctional superhydrophobic fabric. Surf Coat Technol 407:126802. https://doi.org/10.1016/j.surfcoat.2020.126802
Li W, Liu K, Zhang Y, Guo S, Li Z, Tan SC (2022) A facile strategy to prepare robust self-healable superhydrophobic fabrics with self-cleaning, anti-icing, UV resistance, and antibacterial properties. Chem Eng J 446:137195. https://doi.org/10.1016/j.cej.2022.137195
Li W, Zhang Y, Yu Z, Zhu T, Kang J, Liu K, Li Z, Tan SC (2022) In situ growth of a stable metal–organic framework (MOF) on flexible fabric via a Layer-by-Layer strategy for versatile applications. ACS Nano 16:14779–14791. https://doi.org/10.1021/acsnano.2c05624
Liang H, Sha L, Cordelia C, Chang G (2016) Constructing safe and durable antibacterial textile surfaces using a robust graft-to strategy via covalent bond formation. Sci Rep. https://doi.org/10.1038/srep36327
Liu S, Gong JL, Xu BG (2018) Three-dimensionally conformal porous polymeric microstructures of fabrics for electrothermal textiles with enhanced thermal management. Polymers. https://doi.org/10.3390/Polym10070748
Liu Q, Wu Y, Li Z (2020) Facile preparation of super-hydrophobic fabrics composed of fibres with microporous or microspherical coatings using the static breath figure method. Prog Org Coat 149:105938. https://doi.org/10.1016/j.porgcoat.2020.105938
Lu Y, Jia Y, Zhou Y, Zou J, Zhang G, Zhang F (2018) Straightforward one-step solvent-free synthesis of the flame retardant for cotton with excellent efficiency and durability. Carbohydr Polym 201:438–445. https://doi.org/10.1016/j.carbpol.2018.08.078
Manabe K, Nishizawa S, Shiratori S (2013) Porous surface structure fabricated by breath figures that suppresses Pseudomonas Aeruginosa biofilm formation. ACS Appl Mater Interfaces 5:11900–11905. https://doi.org/10.1021/am4035762
Ou J, Wang Z, Wang F, Xue M, Li W, Amirfazli A (2016) Washable and antibacterial superhydrophbic fabric. Appl Surf Sci 364:81–85. https://doi.org/10.1016/j.apsusc.2015.12.113
Raftopoulos KN, Pielichowski K (2016) Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog Polym Sci 52:136–187. https://doi.org/10.1016/j.progpolymsci.2015.01.003
Soto D, Ugur A, Farnham TA, Gleason KK, Varanasi KK (2018) Short-fluorinated iCVD coatings for nonwetting fabrics. Adv Funct Mater 28:1707355. https://doi.org/10.1002/adfm.201707355
Sun J, Memon MA, Bai W, Xiao L, Zhang B, Jin Y, Huang Y, Geng J (2015) Controllable fabrication of transparent macroporous graphene thin films and versatile applications as a conducting platform. Adv Funct Mater 25:4334–4343. https://doi.org/10.1002/adfm.201501733
Wang X, Wang C (2009) The antibacterial finish of cotton via sols containing quaternary ammonium salts. J Sol-Gel Sci Technol 50:15–21. https://doi.org/10.1007/s10971-009-1914-5
Wan L, Li J, Ke B, Xu Z (2012) Ordered microporous membranes templated by breath figures for size-selective separation. J Am Chem Soc 134:95–98. https://doi.org/10.1021/ja2092745
Wan L, Zhu L, Ou Y, Xu Z (2014) Multiple interfaces in self-assembled breath figures. Chem Commun 50:4024–4039. https://doi.org/10.1039/c3cc49826c
Wang L, Xi GH, Wan SJ, Zhao CH, Liu XD (2014) Asymmetrically superhydrophobic cotton fabrics fabricated by mist polymerization of lauryl methacrylate. Cellulose 21:2983–2994. https://doi.org/10.1007/s10570-014-0275-6
Wu M, Ma B, Pan T, Chen S, Sun J (2016) Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv Funct Mater 26:569–576. https://doi.org/10.1002/adfm.201504197
Xie K, Zhang Y, Yu Y (2009) Preparation and characterization of cellulose hybrids grafted with the polyhedral oligomeric silsesquioxanes (POSS). Carbohydr Polym 77:858–862. https://doi.org/10.1016/j.carbpol.2009.03.010
Yang X, Liu Q, Guan J, Li Z (2022) The microstructure and surface characteristics of fluorine-based copolymer coatings deposited by the static breath method. Surf Interface Anal 54:918–930. https://doi.org/10.1002/sia.7105
Yazdanshenas ME, Shateri-Khalilabad M (2013) One-step synthesis of superhydrophobic coating on cotton fabric by ultrasound irradiation. Ind Eng Chem Res 52:12846–12854. https://doi.org/10.1021/ie401133q
Yin S, Goldovsky Y, Herzberg M, Liu L, Sun H, Zhang Y, Meng F, Cao X, Sun DD, Chen H, Kushmaro A, Chen X (2013) Functional free-standing graphene honeycomb films. Adv Funct Mater 23:2972–2978. https://doi.org/10.1002/adfm.201203491
Yu B, Cong HL, Li ZJ, Yuan H, Peng QP, Chi M, Yang SJ, Yang RX, Wickramasinghe SR, Tang JG (2015) Fabrication of highly ordered porous membranes of cellulose triacetate on ice substrates using breath figure method. J Polym Sci Polym Phys 53:552–558. https://doi.org/10.1002/polb.23667
Zhang Z, Hughes TC, Gurr PA, Blencowe A, Uddin H, Hao X, Qiao GG (2013) The behaviour of honeycomb film formation from star polymers with various fluorine content. Polymer 54:4446–4454. https://doi.org/10.1016/j.polymer.2013.06.033
Zhang AJ, Bai H, Li L (2015) Breath figure: a nature-inspired preparation method for ordered porous films. Chem Rev 115:9801–9868. https://doi.org/10.1021/acs.chemrev.5b00069
Zhang W, Camino G, Yang R (2017) Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: an overview of fire retardance. Prog Polym Sci 67:77–125. https://doi.org/10.1016/j.progpolymsci.2016.09.011
Acknowledgments
The authors gratefully acknowledge the financial support from the Fundings.
Funding
This work is supported by the Project funded by Central Government Guides Local Science and Technology Development Fund Project (2022BGE253), Jiangsu Province Industry-University-Research Cooperation Project (BY2022893), Jiangsu Province Vocational College Young Teachers Enterprise Practice Training Project (2022QYSJ015), Jiangsu Advanced Textile Engineering Technology Center Funds (XJFZ/2021/10, XJFZ/2021/19), Scientific Planned Projects for Jiangsu College of Engineering and Technology (GYKY/2021/6), Science and Technology Guiding program of China Textile Industry Federation (2022032) and the Opening Project of Key Laboratory of Clean Dyeing and Finishing Technology of Zhejiang Province (QJRZ2109).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by QK. The first draft of the manuscript was written by QK and all authors commented on previous versions of the manuscript. XR and ZL read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethics approval
This research work did not involve any human or animal participants.
Consent for publication
The authors hereby consent to publication of the present research work in this journal, if selected for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, Q., Ren, X. & Li, Z. Three-dimensional porous structure on cotton fabric through the breath figure method with functions of self-cleaning and oil/water separation. Cellulose 30, 3915–3930 (2023). https://doi.org/10.1007/s10570-023-05087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05087-x