Abstract
An efficient heavy metal absorbent, amino-terminated hyperbranched polymer modified walnut shell (FWNS) was prepared. The adsorption behavior for Pb(II) ions from aqueous solution was also discussed. The FWNS was prepared using an amino-terminated hyperbranched polymer (ATHBP) as modifying agent and glycidyl methacrylate(GMA) as the grafting bridge. The FWNS was characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and Scanning electron microscope (SEM). The adsorption capacity of FWNS for Pb(II) was investigated at different adsorbent dosages (0.25–2.5gL−1) and pH (from 2.0 to 6.0). The result indicated that the absorbent had good adsorption capacity over a wide pH range (2.0–6.0). The maximum Pb(II) ions adsorption capacity (Qm) obtained by a Langmuir fitting model was 1250.00 mg/g at 293 K. The adsorption kinetics fitted the pseudo-second-order equation. The adsorption of FWNS for Pb(II) ions was an endothermic reaction and spontaneous process. Furthermore, the result shows FWNS has excellent regeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, with the development of modern industry, groundwater resources have been polluted by heavy metal wastewater emissions, posing a serious threat to people’s daily life and health (Liu et al. 2013a, b). Therefore, it is crucial to seek effective treatment methods to reduce heavy metal concentration in wastewater. The conventional heavy metal wastewater treatment method (Kim et al. 2013; Googerdchian et al. 2012; Lee et al. 2012; Li et al. 2011; Jin et al. 2011) is a chemical method. It is a simple process with low cost but it is easily to produce large amounts of sediment and secondary pollution. Membrane filtration, which has often been used in recent years, has a good effect on removal of heavy metal ions, but the processing efficiency of membranes greatly decrease with use, and need to be replaced regularly. Polymeric adsorbent have a stronger ability to adsorb heavy metals, but the monomers are toxic, refractory and expensive.
A number of studies have described modified natural polymers as heavy metal adsorbent (Lo et al. 2012, Vaghetti et al. 2009, Xie et al. 2011, Chen et al. 2012, Cao et al. 2014, Altun et al. 2012). These heavy metal adsorbents have many advantages, such as a wide variety of raw materials, low cost, low monomer toxicity, simple synthesis process as well as environmental friendly. Some functional molecules including diethylenetriamine (Cao et al. 2014), citric acid (Altun et al. 2012), thiosemicarbazide (Liu and Gao 2016) have been used as modified monomers in past studies, but the limited number of functional groups introduced onto natural polymer, weaken the adsorption capacity and adsorption efficiency. Amino-terminated hyperbranched polymers (ATHBP) have unique molecular structures and properties such as the absence of chain entanglement, many nanopores, low viscosity and high solubility (Zhang 2009; Eissa et al. 2015). In addition, amino-terminated hyperbranched polymers have a large number of functional groups, which can be effective in the adsorption of heavy metal ions. The adsorption sites of agricultural and forestry wastes can be increased significantly by the modification of amino-terminated hyperbranched polymers. On the other hand, the remarkable structure of hyperbranched polymer such as the absence of chain entanglement can make many adsorption sites expose on the molecular surface, which achieves full adsorption of heavy metal ions.
Walnut shells (WNS) is an abundant agricultural residue with good stability, large specific surface area, high mechanical strength, good chemical stability and excellent regenerative abilities. Structural analyses as indicated approximately 17% cellulose, 36% hemicelluloses and 36% lignin, were made as described in past study (Li et al. 2004). Walnut shells contain many functional groups such as hydroxyl, aldehyde group, methoxyl and so on. In this paper, the amino-terminated hyperbranched polymer (ATHBP) modified walnut shell (FWNS) was prepared and the adsorption behavior for Pb(II) ions in aqueous solution was also investigated.
Experimental
Materials
Walnut shell (WNS) was collected from Qinhuangdao, China. Glycidyl methacrylate (GMA), HCl, Pb(NO3)2, the emulsifier(OP-10), K2S2O8, 1,4-dioxane, sodium bisulfite were obtained from Tianjin Guangfu Technology Development Co., LTD (Tianjin, P. R. China). Hyperbranched Polyethylenimine (HPEI, 50% (wt) aqueous solution, \(\overline{{M_{n} }} = 8000\; \, g/mol\)) was obtained from Hangzhou Yajian Biotechnology Co., LTD (Hangzhou, P. R. China). All reagents were of analytic reagent grade and used without further purification.
Preparation of functional walnut Shell (FWNS)
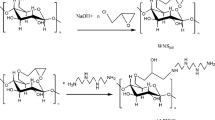
Before modification, Walnut shells were boiled for 60 min and rinsed thoroughly with distilled water, and dried for more than 24 h at 110 °C. Then, the walnut shell was ground by a high-speed grinder until a powder of size smaller than 100 μm was obtained. The chemical modification of WNS was carried out as follows (Cao et al. 2014): Firstly, 4.0 g of WNS was putted into the solution with water containing an emulsifier (0.3 g) for 30 min at 40 °C. Then, 7.0 g GMA, 5.4 g K2S2O8 and 3.6 g NaHSO3 were added and the mixture was stirred using a constant temperature magnetic heater stirrer for 60 min at 15 °C. Finally, the resulting solid was washed with acetone and ethanol and dried to constant weight at 80 °C, WNS-g-GMA was obtained.
6.0 g HPEI and 1.0 g WNS-g-GMA were added to 50 mL mixed solution (VWater: V1,4-dioxane = 1:3) and stirred at constant temperature 85 °C for 30 h. The reaction mixture was filtered and extracted with deionized water for 48 h, and the solid was dried at 80 °C in vacuum for 3 h. FWNS was obtained. These chemical modifications are illustrated in Fig. 1.
Characterization methods
A Nicolet380 Spectrometer System (Nicolet Company, U.S.A.) was used for Fourier transform infrared (FTIR) analysis to confirm the presence of ATHBP on WNS.
The XRD measurement was taken by a Rigaku D-max-2550/PC diffractometer (Rigaku Inc., Tokyo, Japan). The XRD pattern was obtained using Cu Kα radiation with an incident wavelength of 0.1542 nm under a voltage of 40 kV and a current of 200 mA. The scan rate was 4°/min.
The zeta potential of FWNS at various acidities was measured by solid addition method (Kiefer et al. 1997).
Scanning electron microscopy(SEM) images were observed on a Hitachi S-3400 N scanning microscope (Hitachi, Japan). The WNS samples were suspended in a bottle with a small amount of ethanol using ultrasound. The sample was mounted on glass plates and the ethanol removed from the sample surface, followed by application of a thin coating of gold in a vacuum.
Adsorption experiment
The desired dose of FWNS was added to a series of 40 mL tube containing the desired concentration of Pb(NO3)2 in aqueous solution, and the adsorption experiments were then performed in a shaking bath. The effect of initial pH of the solution on the removal of Pb(II) ions was determined over the range of 2.0–6.0. The solution pH was adjusted with 0.1 M NaOH or 0.1 M HCl as appropriate. The influences of adsorbent doses (from 0.25 to 2.5 g L−1) on removal of Pb(II) ions were also investigated. Additionally, the isothermal adsorption experiment were determined at different initial Pb(II) ions concentrations (50–700 mg/L) at pH = 6.0 and thermodynamic studies were investigated at different temperatures of 293 K, 303 K, 313 K, 323 and 333 K. Kinetics experiments were carried out at pH = 6.0 and 298 K. During the adsorption experiments, the tube with adsorbent and adsorbate were stirred at 200 rpm for 2.0 h in a water bath constant temperature oscillator (SHZ-82, Jintan jingbo Experimental Instrument Company, China). Afterwards, the residual adsorbate concentrations were measured by atomic absorption spectrometer (Z-2000, Hitachi Limited Company, Japan). All experiments were carried out with three replicates (Cao et al. 2014).
The adsorption capacity (Q) of FWNS and WNS were calculated from the following expressions:
where C0 is the initial metal ions concentration (mg/L), Ce is the remaining metal ion concentration (mg/L) and V(L) is the volume of solution, M (g) is the mass of adsorbent, and Qe(mg/g) is the equilibrium adsorption capacity.
Adsorption and desorption
In order to explore the recyclability of FWNS adsorbent, regeneration experiments were performed. The FWNS containing adsorbed Pb(II) was placed into 0.1 M HCl solution, and then was shaken at 20 °C for 2 h. Thereafter, the sorbent was removed from the solution and washed 3 times with deionized water. Five consecutive adsorption–desorption experiments were performed under the same conditions.
Results and discussion
FTIR analysis of FWNS
The FTIR spectrum of original WNS, WNS-g-GMA and FWNS are shown in Fig. 2. The peak located at 3400 cm−1 is assigned to the O–H stretching vibration of hydroxyl groups. The bands at around 2935 cm−1 are assigned to C–H stretching vibrations in methylene and methyl groups, and the peak at 1257 cm−1 can be assigned to C–O stretching vibrations in phenols, ether, or alcohols (Cao et al. 2014). Comparing the original WNS spectrum with that in Fig. 2b, the peak in the range of 1730 cm−1 can be attributed to the -C=O and the peak at 905 cm−1, 846 cm−1 are assigned to epoxy group (Ding et al. 2014). The FTIR of FWNS is shown in Fig. 2c. The characteristic peak of epoxy groups at 905 cm−1, 846 cm−1 is no longer evident, which suggests that opening ring reaction between the epoxy groups and amino-terminated hyperbranched polymer has occurred confirming that WNS-g-GMA has grafted with amino-terminated hyperbranched polymer.
XRD analysis of FWNS
XRD patterns of WNS (a), WNS-g-GMA (b) and FWNS(c) are shown in Fig. 3. The original WNS (Fig. 3a) shows scattering at 2θ = 22.08°, which are taken as peaks characteristic of WNS. The WNS-g-GMA (Fig. 3b) shows scattering at 2θ = 18.28°, 27.48°, 36.36°, which are taken as peaks characteristic of PGMA. The absence of the peak at 2θ = 18.28°, 27.48°, 36.36° in FWNS (Fig. 3c) shows that crystallinity has decreased, which indicates that HPEI grafted on WNS-g-GMA destroyed ordered structure of WNS-g-GMA.
SEM image analysis
The surface morphology of WNS, FWNS and FWNS- Pb(II) were observed with SEM (Fig. 4). The original WNS particles (Fig. 4a) show a smooth surface, while the surfaces of FWNS (Fig. 4b) are shown to be uneven, with more pores increasing the surface area and facilitating the adsorption of lead ions. The surface morphology of FWNS-Pb(II) (Fig. 4c) has a surface to form a thick sheet structure (Fig. 4c), which shows that Pb(II) ions are adsorbed on FWNS.
Influence of pH
It is well known that the solution pH is the most important factor because of affecting the surface charge of adsorbents, the degree of ionization, and the speciation of adsorbates (Naghizadeh et al. 2011). The influence of pH on Pb(II) ions adsorption and Zero-point charge of FWNS are shown in Fig. 5. There is a significant increase in lead ions adsorption capacity with pH from 2 to 6. It’s probably because zeta potential varies in surface electric charge of the FWNS (Fig. 5B, pHpzc≈8.62) (Cao et al. 2014; Kiefer et al. 1997; Chen et al. 2010). The reduced metal uptake seen at low pH values may be attributed to the higher concentration of H+ ions. More –NH3+ on the surface of FWNS, enhanced electrostatic repulsion between –NH3+ with Pb(II) and chelation of –NH2 groups with Pb(II) weakened (Mohammod et al. 2011). But, these results indicate that FWNS has higher adsorption capacity at lower pH value than other heavy metal adsorbents (Xie et al. 2011; Altun and Pehlivan 2012; Liu and Gao 2016). The adsorption capacity of FWNS towards Pb(II) ions reached about 190.96 mg/g at pH = 6.0. A possible explanation could be that amino-terminated hyperbranched polymers have a large number of adsorption sites, weaken the influence of pH on the adsorption capacity, and causing the adsorbent to have high adsorption capacity over a wider pH range.
At higher pH values, the lower H+ concentration leads to more free -NH2 groups in the system, which makes it easier to chelate with lead ions.
Influence of adsorbent dosage
The influence of the dosage of the original WNS and FWNS on adsorption capacity of Pb(II) ions is shown in Fig. 6. Using FWNS, When the dosage of adsorbent was gradually increased from 0.25 to 2.5 g L−1, the capacity of adsorbent decreased sharply from 188.22 to 20.68 mg/g (Fig. 6). The possible reason for this is that the decrease in Pb(II) ions uptake at higher adsorbent dosage may be attributed to the competition of Pb(II) ions for available adsorption sites and an increase in the diffusion path length (Abdel et al. 2006). In addition, agglomeration of the adsorbent particles may also be the cause of the decrease of adsorption.
Adsorption isotherms
In this part, adsorption isotherms were investigated. The influence of initial Pb2+ concentration on Pb(II) ions adsorption by FWNS is shown in Fig. 7. Langmuir and Freundlich models were used (Ding et al. 2013; Parab and Sudersanan 2010). The relevant isotherm equations are listed as:
Langmuir isotherm:
where Qe (mg/g) is the corresponding adsorption capacity and Ce (mg/L) is the ultimate concentration of Pb(II) ions at equilibrium. Qm (mg/g) is the theoretical maximum adsorption capacity of adsorbent for Pb(II) ions, and KL (L/mg) is the Langmuir isotherm coefficient. A plot of Ce/Qe versus Ce is shown in Fig. 8B, which shows that Ce/Qe and Ce have a good linear relationship. The values of KL and Qm, obtained from Fig. 8B are listed in Table 1.
Freundlich isotherm:
where KF ((mg/g) (L/mg)1/n) and 1/n are the adsorption constants of Freundlich model. A plot of \(\ln Q_{e}\) versus \(\ln C_{e}\), is shown in Fig. 8A and the corresponding parameters are also listed in Table 1.
The results indicated the Langmuir model is a good fit for the equilibrium adsorption as indicated by the high values of R2 (0.9939), and The calculated maximum adsorption capacity (Qm) of FWNS for Pb(II) ions was 1250.00 mg/g, close to experiment value (1072.8 mg/g). The results of Langmuir model suggests that a homogeneous monolayer of Pb(II) ions was adsorbed onto FWNS particles (Cao et al. 2014; Yuvaraja et al. 2014). In addition, the adsorption of Pb(II) ions on FWNS was a dynamic chemisorption reaction, which depends on the adsorption affinity of the surface functional groups of.
FWNS and the bond energy (Cao et al. 2014; Feng et al. 2013). The adsorption capacity of FWNS is significantly larger than that of other modified natural polymers. The adsorption capacity of several modified natural polymers in the literature was listed in Table 2(Liu et al. 2013a, b; Cheng et al. 2021; Liu and Gao 2016; Liu et al. 2019). On the other hand, the remarkable structure of hyperbranched polymers and the absence of significant chain entanglements can increase the availability of surface adsorption sites.
A separation factor (RL) to evaluate the suitability of an adsorption process, was defined as the following equation (Xia et al. 2011):
where C0 (mg/L) is the initial Pb (II) ion concentration in solution and KL(L/mg) is the Langmuir isotherm coefficient related to the free energy of adsorption. The values of RL (0.1247–0.6309) at 298 K were in the range of 0–1, which implies that adsorption of Pb(II) ions on FWNS is a favorable uptake (Bhaumik et al. 2013).
Adsorption kinetics
In this section, In order to find out how rapidly the adsorption progress was and whether the adsorption process is controlled by chemical or physical adsorption, the adsorption dynamics were investigated (Chen et al. 2010). Figure 9 showed the influence of time on Pb(II) ions adsorption by FWNS.
Pseudo-first-order and pseudo-second-order diffusion models were used to discuss the relationship between adsorption capacity of adsorbate and adsorption time. The two equations are as follows:
Pseudo-first-order kinetic model:
where Qe (mg/g) and Qt (mg/g) refer to the adsorption capacity of Pb(II) ions by FWNS at equilibrium and at time t (min), respectively. K1 (1/min) is the equilibrium rate constant of pseudo-first-order adsorption.
Pseudo-second-order kinetic model:
where K2 (g/(mg/min)) is the equilibrium rate constant for pseudo-second-order adsorption. The applications of the models to the experimental data are shown in Fig. 10. The constants determined from the experimental data and correlation coefficients (R2) are listed in Table 3.
The adsorption of Pb(II) ions on FWNS probably do not obey the pseudo-first-order kinetic model because obtained Qe(58.72 mg/g) is quite different from the experiment value(195.53 mg/g) and the R2 (0.9276) is low. By contrast, the results of pseudo-second-order equation are reasonable because of the high R2 (0.9999) and good agreement with the calculated Qe (Qe,exp = 195.53 mg/g). This result indicated that chemisorption was the rate-limiting step in adsorption process.
Thermodynamics of adsorption
The influence of temperature on the adsorption of Pb(II) ions on FWNS was investigated. Figure 11 showed the influence of temperature on Pb(II) ions adsorption by FWNS. ΔH and ΔS were obtained by the Eq. (6) (Lin et al. 2011):
where KD (L/mol) is thermodynamic adsorption equilibrium constant and obtained by Eq. (7), and.
R is the gas constant valued 8.314 J/(mol K). Plots of \(\ln K_{D}\) versus 1/T are shown in Fig. 12. The result shows \(\ln K_{D}\) versus 1/T have a good linear relationship. The values of ΔH and ΔS, obtained from Fig. 12 are listed in Table 4.
where Qe is the equilibrium Pb(II) concentration on the adsorbent (mg/L) and Ce is the equilibrium Pb(II) concentration in solution (mg/L).
The Gibbs free energy of adsorption (ΔG) subsequently obtained by Eq. (8), are also listed in Table 4 (Gong et al. 2008).
Seen from Table 4, ΔH value indicates the adsorption of Pb(II) ions on FWNS is endothermic. And the chelation between functional groups and Pb(II) ions on the surface of FWNS is also involved in the adsorption process. The higher the temperature is, the easier the adsorption is (Chen et al. 2010). The positive ΔS implied degrees of freedom of the liquid–solid interface increased during adsorption process of Pb(II) ions onto FWNS. Additionally, the adsorption reaction is a spontaneous nature because of negative values of ΔG. Furthermore, with the increase of temperature, the negative value of ΔG increases, which further proves that the temperature is favorable to the adsorption process.
Adsorption/desorption
In order to explore the possibility of recycling FWNS, the adsorption/desorption process was also explored. The efficiencies of removing lead ions from FWNS for five consecutive adsorption/desorption cycles are presented in Table 5. The results show the removal efficiency of lead ions from FWNS remains high, above 93%.
Conclusion
An efficient heavy metal adsorbent (FWNS) was prepared by grafting amino-terminated hyperbranched polymer onto walnut shell. Structural characterization using various instruments demonstrated that amino-terminated hyperbranched polymer was successfully grafted onto walnut shell. The results showed that FWNS had adsorption capacity over a wide pH range. The adsorption isotherms showed that the adsorption of Pb(II) ions on FWNS resulted in a homogeneous monolayer and was a dynamic chemisorption process. The adsorption behavior of Pb(II) ions is well described by the pseudo-second-order kinetic model. Furthermore, the adsorption process of Pb(II) ions by FWNS is spontaneous and endothermic. The higher temperature is favorable for the adsorption. The results demonstrated that FWNS can be reused. Finally, FWNS can be obtained from an agricultural byproduct, walnut shell, through a simple modification process at low cost, making it a competitive adsorption material in practice.
References
Abdel ASE, Gad YH, Dessouki AM (2006) Use of rice straw and radiation-modified maize starch/acrylonitrile in the treatment of wastewater. J Hazard Mater 129:204–212
Altun T, Pehlivan E (2012) Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem 132:693–700
Bhaumik M, McCrindle R, Maity A (2013) Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole-polyaniline nanofibres. Chem Eng J 228:506–515
Cao JS, Lin JX, Fang F, Zhang MT, Hu ZR (2014) A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies. Bioresour Technol 163:199–205
Chen SH, Yue QY, Gao BY, Xu X (2010) Equilibrium and kinetic adsorptions study of the adsorptive removal of Cr(VI) using modified wheat residue. J Colloid Interface Sci 349:256–264
Chen S, Yue Q, Gao B, Li Q, Xu X, Fu K (2012) Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: a fixed-bed column study. Bioresour Technol 113:114–120
Cheng S, Xing BL, Shi CL, Nie YH, Xia HY (2021) Efficient and selective removal of Pb(II) from aqueous solution by modification crofton weed: Experiment and density functional theory calculation. J Clean Prod 280(1):124407
Ding DH, Zhao YX, Yang SJ, Shi WS, Zhang ZY, Lei ZF, Yang YN (2013) Adsorption of cesium from aqueous solution using agricultural residue Walnut shell: equilibrium, kinetic and thermodynamic modeling studies. Water Res 47:2563–2571
Ding ZH, Yu R, Hu X, Chen YJ, Zhang YF (2014) Graft copolymerization of epichlorohydrin and ethylenediamine onto cellulose derived from agricultural by-products for adsorption of Pb(II) in aqueous solution. Cellulose 21:1459–1469
Eissa MM, Samy M, Ramadan AM (2015) Amino-terminated hyperbranched polymer for toughness improvement of epoxy/claynanocomposites. Polym Bull 72(12):3147–3168
Feng YF, Dionysiou DD, Wu YH, Zhou H, Yang LZ, Xue LH, He SY (2013) Adsorption of dye stuff from aqueous solutions through oxalic acid-modified swede rape straw: adsorption process and disposal methodology of depleted bioadsorbents. Bioresour Technol 138:191–197
Gong R, Zhu SX, Zhang D, Chen J, Ni SJ, Guan R (2008) Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: kinetic and thermodynamic profile. Desalination 230:220–228
Googerdchian F, Moheb A, Emadi R (2012) Lead sorption properties of nanohydro- xyapatite–alginate composite adsorbents. Chem Eng J 200–202:471–479
Jin X, Yu C, Li Y, Qi Y, Yang L, Zhao G, Hu H (2011) Preparation of novel nano-adsorbent based on organic-inorganic hybrid and their adsorption for heavy metals and organic pollutants presented in water. J Hazard Mater 186:1672–1680
Kiefer E, Sigg L, Schosseler P (1997) Chemical and spectroscopic characterization of algae surfaces. Environ Sci Technol 31:759–765
Kim SA, Kamala SK, Lee K, Park Y, Shea PJ, Lee W, Kim H, Oh B (2013) Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valentiron composite. Chem Eng J 217:54–60
Lee S, Laldawngliana C, Tiwari D (2012) Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated wastewaters. Chem Eng J 195–196:103–111
Li S, Xu S, Liu S, Yang C, Lu Q (2004) Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technol 85:1201–1211
Li N, Zhang L, Chen Y, Tian Y, Wang H (2011) Adsorption behavior of Cu(II) onto titanate nanofibers prepared by alkali treatment. J Hazard Mater 189:265–272
Lin JW, Zhan YH, Zhu ZL, Xing YQ (2011) Adsorption of tannic acid from aqueous solution onto surfactant-modified zeolite. J Hazard Mater 193:102–111
Liu GT, Gao YD (2016) Synthesis, characterization of aminothiourea modified walnut Shell and its adsorption for Pb(II) ions from aqueous solution. Polymer (korea) 40(2):194–200
Liu BJ, Lv X, Meng XH, Yu GL, Wang DF (2013a) Removal of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb(II) as imprinted ions. Chem Eng J 220:412–419
Liu Y, Chen M, Hao Y (2013b) Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem Eng J 218:46–54
Liu GT, Zhang W, Luo RS (2019) Synthesis, characterization of amino-modified walnut shell and adsorption forPb(II) ions from aqueous solution. Polym Bull 76(3):1099–1114
Lo SF, Wang SY, Tsai MJ, Lin LD (2012) Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbon. Chem Eng Res Des 90:1397–1406
Mohammod M, Sen TK, Maitra S, Dutta BK (2011) Removal of Zn from aqueous solution using castor seed hull. Water Air Soil Pollut 215:609–617
Naghizadeh A, Nasseri S, Nazmara S (2011) Removal of trichloroethylene from water by adsorption on tomultiwall carbon nanotubes. Iran J Environ Health Sci Eng 8:317–325
Parab H, Sudersanan M (2010) Engineering a lignocellulosic biosorbent–coir pith for removal of cesium from aqueous solutions: equilibrium and kinetic studies. Water Res 44:854–860
Vaghetti JCP, Lima EC, Royer B, Cunha BM, Cardoso NF, Brasil JL, Dias SLP (2009) Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II) from aqueous solutions. J Hazard Mater 162:270–280
Xia CL, Jing Y, Jia YZ, Yue DY, Ma J, Yin XJ (2011) Adsorption properties of congo red from aqueous solution on modified hectorite: kinetic and thermodynamic studies. Desalination 265:81–87
Xie GR, Shang XQ, Liu RF, Hu J, Liao SF (2011) Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd(II) ions from aqueous solution. Carbohydr Polym 84:430–438
Yuvaraja G, Krishnaiah N, Subbaiah MV, Krishnaiah A (2014) Biosorption of Pb(II) from aqueous solution by Solanum melongena leaf powder as a low-cost biosorbent prepared from agricultural waste. Colloids Surf b: Biointerfaces 114:75–81
Zhang F, Zhang DS, Chen YY, Lin H (2009) The antimicrobial activity of the cotton fabric grafted with an amino- terminated hyperbranched polymer. Cellulose 16(2):281–288
Acknowledgments
This work was financially supported by S & T Program of Hebei (Grant no. 21323602D), Science and Technology Research and Development Program of Qinhuangdao (Grant no. 202005A057) and the Breeding Research Subjects of Yanshan University (Grant no.16LGY020). We are thankful to Dr. Rudolph Winter, professor at Department of Chemistry&Biochemistry, University of Missouri- St Louis who made modification in English.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Zhang, L. & Luo, R. Preparation of efficient heavy metal adsorbent based on walnut shell and adsorption for Pb(II) ions from aqueous solution. Cellulose 29, 9819–9830 (2022). https://doi.org/10.1007/s10570-022-04869-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04869-z