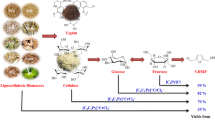
Abstract
An one-pot process has been developed for the conversion of lignocellulosic bio-mass and carbohydrates to 5-hydroxymethylfurfural (5-HMF), following a highly specific approach. First time, raw sugarcane bagasse and corn-cob biomasses were mechanically grinded to fine powder and further applied as feedstock for 5-HMF and furfural production in one-pot and scalable synthesis. A synergistic role of mixed organic and inorganic acids such as oxalic acid, AlCl3 and HCl, charcoal, and solvent system was critically investigated on cellulose with their proportion, which is responsible for the fruitful conversion to 5-HMF. Crucial role of charcoal was investigated under this study and noticeable improvement of yield ~ 16% was observed. The optimized process further tested up to 0.5 kg scale biomass conversion to 5-HMF production successfully. The scope of the process further extended for conversion of waste raw potato, corn powder, starch, glucose and fructose to 5-HMF production with high specificity and conversion. After solvent extraction, avoiding tedious column chromatography, the UPLC purity of 5-HMF was measured to 87–95%.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The alarming depletion tendency of fossil fuel reserves has stimulated the world-research towards utilization of cellulosic biomass as a feedstock for production of fine chemicals, industrial polymers and transportation fuels (Mika et al. 2018). The blind consumption of such reserves is also enhancing the environmental concerns very rapidly over the greenhouse gas emissions. In this regard, biomass is considered as the suitable candidate as the global annual bio-mass production is approximately 170 billion metric tons; majorly generating from crop straws, sugarcane bagasse, corn stalk etc. (Zhang et al. 2017b). The abundantly available biomass highly demands for development of environmentally and economically viable technologies to convert them into platform intermediates as alternatives of fossil-fuel resources. In context to this subject, 5-HMF, a six-carbon furan analogue, recognized as a platform chemical and bridge between bio-resources and bio-chemicals or fuels (Roman-Leshkov et al. 2007; Yi et al. 2016; Putten et al. 2013).
The structural composition of lignocellulosic biomass (cellulose, hemicellulose, lignin etc.) and their mutual arrangements within them make it a very complex and rigid in nature. In addition, the cellulose itself is having strong β(1 → 4) glycoside linkages which are hard to cleave selectively for glucose and further 5-HMF synthesis. Besides, glucose itself reacts very slowly and results 5-HMF in lower yield than fructose. Therefore, the conversion of cellulosic biomass is not as much as susceptible towards industrial production of 5-HMF than fructose. Moreover, the valorisation of lignocellulosic biomass needs acidic/basic pre-treatment process for softening and then further extraction or separation of cellulose from lignin and further hydrolysis of cellulose to carbohydrate monomers as initial feedstock. Further, in most of the bio-mass, carbohydrates remain as a polymeric mixture of different form of glucose/xylose monomer depending upon the nature of biomass therefore, development of a process that could be able to convert mixture of polymeric carbohydrates to 5-HMF in a specific manner following hydrolysis, isomerization and dehydration in a single-system is a challenging task (Yu and Tsang 2017). Not only 5-HMF production, but it’s in situ stabilization to reduce polymerization is also a challenging task and most of the scale-up process only suffers due to this issue (Yu et al. 2017). The conversion of cellulose to 5-HMF generally involves the isomerization of glucose to fructose. This step is considered to be the key for this transformation as it is an equilibrium limited step and directly affect the dehydration reaction of fructose to 5-HMF. Hence, the in situ isomerization of glucose to fructose needs specific metal salts in combination with other acidic environment, otherwise which can enhance the possibility of side reactions. As per literature, CrCl3 and Sn-beta zeolites are the most effective and well known catalysts for this conversion. Finally, the separation and purification of 5-HMF from the reaction media is very tedious, energy-expensive and time-consuming processes. Although, the lab scale specificity of 5-HMF is good in various ionic liquids and inorganic acidic catalysts conditions but these processes are not possible to apply for scale-up production (Zhang et al. 2017b).
The production of 5-HMF generally produced through effective pre-treatment of biomass followed by hydrolysis to corresponding hexose sugar units. Further, such sugar units underwent a catalytic dehydration to corresponding 5-HMF product (Fig. 1) (Putten et al. 2013; Questell-Santiago et al. 2018; Peterson 2008; Climent et al. 2011; Xu et al. 2020). In the earlier development, Raines et al. reported N,N-dimethylacetamide and lithium chloride as a particular solvent system for cellulose conversion to 5-HMF in 54% yield (Binder and Raines 2009). Subsequently, the development of an extraordinary Brønsted-Lewis surfactant combined heteropolyacid Cr[(DS)H2PW12O40]3 and very recently cobalt based catalyst also procured 5-HMF in 53 and 33% yields respectively (Zhao et al. 2011; Liu et al. 2020).
In continuation to this development, sugarcane bagasse bio-mass could be a very good feedstock for its conversion to furan compounds as it is found to be rich in cellulose, 34–36% and hemicellulose, 29–43% composition (Nassar 1998; Garcia-Perez et al. 2002). Chareonlimkun et al. applied a hot compressed water approach on sugarcane bagasse to result 5-HMF and furfural in 6–7 and 15% yields respectively. However, the method only limited up to 100 mg scale under harsh reaction conditions of temperature 250 °C and pressure 25 bar (Chareonlimkun et al. 2010b). Furthermore, the microwave irradiations of particular power under specific ionic liquid condition, utilized as noble heating mode for 5-HMF synthesis in laboratory scale (Dutta et al. 2012). During this development period, very recently, Catrinck et al. also reported a process for sugarcane bagasse conversion to furfural only in 83.1 g Kg−1 yield at 150 °C temperature for 24 h (Fig. 2) (Catrinck et al. 2020). In the same manner, corncob biomass (abundant in cellulose and hemicellulose) has also been exploited for 5-HMF and furfural synthesis at high reaction temperature. The process found its limitation in scale-up synthesis as enhancing corncob loading significantly reduced the yields of 5-HMF and furfural even in lab scale (Zhang et al. 2017a).
Strategies for scale-up synthesis of 5-HMF from various feed-stocks (Catrinck et al. 2020)
In addition to lignocellulosic biomass, the raw potato (abundant in carbohydrate content) could also be seen as a good and sustainable alternative for 5-HMF production. In India, every year 45.4 million tons potato was produced and among these, approximate 4.54–6.81 million tons get wasted due to lack of cold storage, bad quality and over production. Due to lack of disposal mechanism, the waste-potato creates huge environmental pollution. As we noticed, yet there is no process claimed for production of 5-HMF from raw waste potato. Therefore, this sustainable feedstock could also be exploited for 5-HMF synthesis. Moreover, the aluminum chloride (AlCl3) in combination with other organic or inorganic acids, under ionic liquid/organic medium also explored as a reagent system for 5-HMF synthesis, but the process was only tested for fructose, glucose, and very recently for corn starch (Liu et al. 2014; Zhou et al. 2017; Overton et al. 2020). Even, hydrochloric acid has also been utilized for hydrothermal conversion of cellulose to 5-HMF in 21% yield at a very high temperature i.e. 300 °C (Yin et al. 2011). Also, in earlier reports, oxalic acid found to be known for conversion of fructose, sucrose and inulin dehydration to 5-HMF synthesis (Rapp 1988; Asghari and Yoshida 2006; Haworth and Jones 1944) only for laboratory application and as per our experience only oxalic acid can’t be suitable for conversion of cellulose and lignocellulose to 5-HMF.
Presently, AVA Biochem, Swiss-based company, is the pioneer in the industrial production of 5-HMF using a water based hydrothermal processing technology only from fructose as initial feedstock (Kläusli 2014). The other prominent players in this area are Robinson Brothers, Penta manufacturer, NBB Company and Wutong Aroma Chemicals. Mostly, the industrial production of 5-HMF includes edible sources like fructose and glucose as initial feedstock. However, the use of such edible sources conflicts with food demand and not meet economical prospective. The industrial exploration of cellulosic biomass as initial feed for 5-HMF production is still missing in the literature. To the best of our knowledge, we have not found any industry or report, claiming commercial production of 5-HMF from lignocellulosic biomass without pre-treatment and following one-pot process.
Last few years, our group has been extensively using oxalic acid dihydrate for different organic transformations as a C1 source and we found its extraordinary applications for different purposes where the known reagents showed limitations (Ram et al. 2020; Shil et al. 2015; Thakur et al. 2018). Under this study, we have noticed its synergistic role to enhance reactivity and solve earlier problems associated with 5-HMF synthesis from lingocellulosic biomass under scale-up. Further, we have noticed a significant role of activated charcoal (Das et al. 2018) to improve 5-HMF yield by ~ 16% through its addition and solved number of problems noticed in scale-up synthesis. Therefore, we have majorly proposed conversion of ligocellulosic biomass following a convenient and scalable one-pot approach in a highly specific manner with negligible by-product formation. Furthermore, we have developed a process wherein the synergistic role of organic and inorganic acids and more specially activated charcoal, enhances the yield and specificity of the 5-HMF in biphasic solvent system at comparative low temperature. The present approach also applied up to 500-g scale for production of 5-HMF from different lignocellulosic biomass/carbohydrates.
Materials and methods
Chemicals and materials
All chemicals and solvents used in the present process were of analytical grade and used without any further purification. The bulk abundance, lack of proper disposal mechanism, environmental concerns and potential to replace fossil resources made the cellulosic biomasses to be get explored more thoroughly and comprehensibly. Therefore, in our study, we have chosen sugarcane bagasse, corncob, raw potato and corn powder as raw materials for 5-HMF and furfural synthesis. For this study, sugarcane bagasse was collected from the local market, after removal of sugarcane juice. For easy handling of the biomass under reaction condition, it was dried and grinded through mechanical/electric grinder to fine powder. Before utilizing sugarcane bagasse as initial feedstock, initially it was mechanically grinded to fine powder and then underwent particular experimental procedures to result 36% cellulose, 26.3% hemicellulose, 16.8% lignin, 5.8% water and 2.2% ash content (refer to ESI for more information). This fine powder bagasse then directly utilized as initial feed for 5-HMF and furfural synthesis without any pre-treatment. Similarly, under this study, the corncob was collected from the local farmer area after harvesting the crop. Initially, it was mechanically grinded into fine powder similarly as that for sugarcane bagasse and then further the contents of cellulose, hemicellulose, lignin, moisture and ash were quantified and found to be 36.8, 22.4, 15.2, 7.8, and 0.6% respectively (refer to ESI for more information). The prepared fine powder without any pre-treatment directly utilized in the process. In the same manner, raw potato waste was brought from local vegetable shop, chopped into slices and dried in an oven in order to make it totally free from water molecules otherwise would create problem. Further, to avoid drying of potato, we have also tested raw potato-paste after squeezing to remove as much as water is possible and applied for the same reaction. The moisture and ash content of potato powder after drying calculated to be 9.2 and 3.8% respectively. The prepared potato powder then straightforwardly applied under reaction conditions. Corn powder was brought from local market and used before any further treatment. Cellulose, starch, fructose, aluminium chloride anhydrous (AlCl3), 2-butanol and hydrochloric acid (35–38%) were purchased from Central Drug Health (P) Ltd. Oxalic acid dihydrate, activated charcoal, sodium sulphate anhydrous, formic acid (85%), dimethylsulphoxide (DMSO) and methylisobutylketone (MIBK) were purchased from SD fine-chem. Ltd. 5-Hydroxymethylfurfural, glucose and deuterated chloroform (CDCl3) were purchased from sigma Aldrich. Furfural was purchased from across organics. HPLC grade acetonitrile was purchased from fisher scientific. Millipore milli-Q water filtered through 0.22 μm PTFE filter was utilized during the analysis.
Experimental methods
Cellulose powder to 5-HMF Synthesis (1-g scale synthesis)
An oven dried round bottomed flask (50 mL) was charged with cellulose powder (1 g), oxalic acid dihydrate (1 g, 1 equiv.), AlCl3 (150 mg, 15 wt%), 4 N HCl (387 μL, 15 wt%), activated charcoal (200 mg, 20 wt%), DMSO (6 mL) and MIBK: 2-butanol (3:1) = (4.5:1.5) mL as extracting phase solvent at 130 °C for 6 h under reflux condition. The progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the crude reaction mixture was filtered through the pad of celite (Celite 545). The filtrate then diluted by distilled water and MIBK to a certain volume. Then, the samples of both aqueous and organic layers were taken for UPLC quantification. Finally, the compound 5-HMF was extracted from the reaction mixture by ethyl acetate in repeated iterations, dried over Na2SO4 and concentrated under reduced pressure system, and finally complete dried in freeze-drying. The final product analyzed by UPLC chromatogram based on UV wavelength and retention time (compared with standard), and further reconfirmed by NMR and ESI–MS. 1H NMR (600 MHz, CDCl3) δ 9.37 (s, 1H), 7.12 (d, J = 3.5 Hz, 1H), 6.39 (d, J = 3.5 Hz, 1H), 4.55 (s, 2H); 13C NMR (150 MHz, CDCl3) δ 177.65, 161.03, 151.72, 123.49, 109.71, 56.84; m/z MS (ESI) [M + 1]+ = 127, [M + 1-H2O]+ = 109, [M + 1-H2O-CO]+ = 81.
Cellulose powder to 5-HMF Synthesis (500-g scale synthesis)
A Radley’s made reactor (15 Ltr) was charged with cellulose powder (500 g) and oxalic acid dihydrate (500 g, 1 equiv.) followed by addition of AlCl3 (75 g, 15 wt%), activated charcoal (100 g, 20 wt%) and 4 N HCl (194 mL, 15 wt%). Then, the reaction mixture solvated by DMSO (2.5 Ltr) and MIBK: 2-butanol (3:1) = (1.87:0.62) Ltr. Finally, the reaction mixture allowed to stir at 130 °C for 6 h with the help of Julabo made temperature controller. The progress of the reaction monitored on TLC. After completion of the reaction, the reaction crude was filtered through the celite pad. The filtrate then diluted by distilled ice water and then final product was extracted by ethyl acetate in repeated iterations, dried over Na2SO4 and evaporated using 20 L rotary evaporator and complete dried under freeze-drying. The final product analyzed as 5-HMF by UPLC chromatogram based on UV wavelength and retention time (compared with standard), and further reconfirmed by NMR and ESI–MS. The amount of 5-HMF was measured to be 164 g. The spectral data were same as mentioned above in experimental description; Cellulose powder to 5-HMF Synthesis (1-g scale synthesis).
Sugarcane bagasse to 5-HMF and furfural synthesis (500-g scale synthesis)
The mixture of sugarcane bagasse, oxalic acid dihydrate, AlCl3, activated charcoal, 4 N HCl along with solvents DMSO and MIBK:2-butanol was charged in reactor in the same equivalency as mentioned in experimental “Cellulose powder to 5-HMF synthesis (500-g scale synthesis)” section. After stirring the reaction for 8 h at 130 °C and passing the reaction mixture through celite pad, the filtrate then diluted by distilled ice water and then final two products were extracted by ethyl acetate in repeated iterations. The ethyl acetate extract then dried over Na2SO4 and concentrated under reduced pressure system. The two products then separated through solvent extraction using hexane. The hexane extract then concentrated under reduced pressure and complete dried in freeze- drying. The final product analyzed as furfural by UPLC chromatogram based on UV wavelength and retention time, and further reconfirmed by NMR and ESI–MS. The amount of furfural was measured to be 68.94 g. 1H NMR (600 MHz, CDCl3) δ 9.65 (s, 1H), 7.69 (m, 1H), 7.25 (d, J = 3.5 Hz, 1H), 6.60–6.61 (m, 1H); 13C NMR (150 MHz, CDCl3) δ 177.87, 152.98, 148.08, 121.02, 112.58; m/z MS (ESI) [M + 1]+ expected mass = 97.0292; observed mass = 97.0285. In the same fashion, the other compound remained after hexane extraction dried in freeze-drying and finally analyzed as 5-HMF by UPLC chromatogram based on UV wavelength and retention time, and further reconfirmed by NMR and ESI–MS. The amount of 5-HMF was measured to be 54.27 g. The spectral data were same as explained above for cellulose conversion.
Analytical methods
The 5-HMF and furfural were analyzed through ESI–MS, UPLC and NMR spectroscopy. The ESI–MS spectra were determined by a Waters micro mass Q-TOF Ultima spectrometer. 1H and 13C NMR spectra were recorded using a Bruker Advance 600 MHz (1H) and 150 MHz (13C). All NMR spectra were recorded at 25 °C in CDCl3 [residual CHCl3 (δH 7.26 ppm) or CDCl3 δC 77.00 ppm)]. The UPLC method was developed for qualitative and quantitative analysis of 5-HMF and furfural on Water made Acquity UPLC® H Class with PDA detector (refer to SI for more details). The standard curves for 5-HMF and furfural were obtained with R2 = 0.9999 and 0.9989 respectively.
Results and discussion
From the introduction section, the identified challenges were first attempted will be explained stepwise following the role of acids, charcoal, solvent systems, and temperature. These parameters investigated very keenly to solve utmost possible to enhance specificity, vast application in one-pot system, high conversion, easy separation, and further to reduce polymerization to humin formation, and finally applicability in scale-up synthesis of 5-HMF. To understand the system and to optimize the conditions in clear manner, we have selected cellulose as a model substrate in 1-g scale and further applied for different complex biomass and scale-up reaction up to 500-g. The scopes for other carbohydrates were also examined under this study.
Optimization of the reaction conditions
The complex nature of cellulose structure makes it very difficult to undergo acidic hydrolysis of glycosidic bonds followed by isomerization and dehydration to yield 5-HMF as final product. Hence, very critical evaluation of the reaction parameters like reagents, solvents, temperature, reaction time and substrate loading were carried out to find out the best optimized reaction conditions for 5-HMF synthesis.
Synergistic effect of organic, Lewis and mineral acids for 5-HMF synthesis
Under this study, several acids and their combinations were tested in order to optimize the reaction conditions (Table 1). Among these, a combination of mixed acids such as oxalic acid dihydrate, AlCl3 and HCl was found to be highly efficient and responsible for specific conversion following hydrolysis, isomerization and dehydration steps in a single pot manner for its fruitful conversion of cellulose to 5-HMF synthesis. When the reaction of cellulose performed with only oxalic acid dihydrate under reaction conditions, then negligible amount of desired product formation was noticed (Table 1, entry 1). The AlCl3 was also checked and resulted with no conversion of 5-HMF (Table 1, entry 2). In both of the cases, majorly unreacted carbohydrate was recovered after reaction that reflect lower specificity of hydrolysis, which is the first step of this transformation. Even, the possibility of aluminum oxalate was also investigated but did not work under this condition (Table 1, entry 7). The mineral acid, HCl resulted with 15% yield of 5-HMF in existing protocol but still found less reactive (Table 1, entry 3). Further, in order to provide more clarity, the combination of oxalic acid dihydrate and AlCl3 was checked under established conditions and resulted with 27% yield of 5-HMF, however, the combination of oxalic acid dihydrate with hydrochloric acid reduced its yield by 7% (Table 1, entry 4, 5). In the same fashion, when the combination of AlCl3 and HCl was tested, then it offered the same yield as obtained earlier in case of oxalic acid dihydrate and AlCl3 (Table 1, entry 6). Moreover, under the mixed acid conditions of oxalic acid dihydrate (1 equiv.), AlCl3 (15 wt%) and HCl (15 wt%), the reaction ended with 32% yield of 5-HMF (Table 1, entry 8). Through this investigation, we assumed that oxalic acid dihydrate might be helping in providing the necessary acidic environment for hydrolysis of cellulose to glucose. Furthermore, as the Lewis acids are commonly well-known for glucose isomerization to fructose, thereby AlCl3 might have been played a key role in this transformation. Moreover, HCl might be anchoring the dehydration of fructose to 5-HMF as Bronsted acids are well recognized for such type of dehydration reactions. Although, these studies clearly indicated that individually these acids or their other combinations performed poorly but their right combination performed a synergistic role for this transformation with high specificity.

Role of activated charcoal
The role of activated charcoal was first investigated by our group to enhance the stability of the 5-HMF under reaction condition and reduce polymerization to humin formation. Moreover, the addition of charcoal positively influenced the reaction and resulted 5-HMF with high yield. The process was patented in 2018 emphasizing the critical role of activated charcoal for fruitful conversion to 5-HMF (Das et al. 2018). Under acid optimized conditions, the reaction yet resulted with only 32% yield of 5-HMF but addition of 20 wt% of activated charcoal enhanced the yield up to 16% and resulted with overall 48% yield of 5-HMF (Table 1, entry 9). The higher specificity of 5-HMF in the presence of activated charcoal explicitly suggested the key role of it for this conversion through restricting the formation of unwanted polymerization and humin. The activated charcoal might be playing a significant role to stabilize the 5-HMF through adsorption/binding force under the reaction condition and reducing its polymerization tendency to humin formation. Earlier, the most of the scalable processes suffer for 5-HMF separation and poor yield due to un-wanted polymerization and humin formation. In addition, the activated charcoal also reduces the light sensitivity of the reaction system in glass vessel reactor, which also enhance the stability of light sensitive 5-HMF molecules as minor effect.
The role of solvent system for the reaction
The selected acidic systems were only effective under a particular solvent system. With the slight modification of solvent/s, the rate of reaction either changed assertively or ended with insoluble carbohydrates, polymerization and mixture of products. Based on the results, we have tried to tune the solvent system in a way that can hydrolyzed more rigid β(1 → 4) glycoside linkages of cellulose in a highly specific manner followed by isomerization and dehydration to achieve highest yield of 5-HMF. The reaction did not response for most commonly used reactive phase solvents like water, THF and water/THF mixture for 5-HMF synthesis from cellulose (Fig. 3). The high boiling point solvent DMF also checked, but not procured the desired product (Fig. 3). The effect of solvent system was found very predominant as the reaction only performed well in DMSO (6 mL), MIBK:2-butanol (3:1) = (4.5:1.5) mL biphasic solvent system and best compatible with our optimized acidic conditions. Even, when the reaction performed in single-phase solvent system, then the extraction of desired compound from the reaction media became very problematic with low specificity of the reaction (Fig. 3). This may be attributed to the insolubility of cellulose in the respective solvent systems and further in situ extraction of it in extracting phase at standard conditions. The stability of 5-HMF in DMSO may be the other reason for enhancement of the yield. This solvent system is so specific that the process does not require tedious purification to achieve highest yield of targeted product.
Solvent screening for cellulose conversion to 5-HMF synthesis. Reaction conditions: Cellulose powder (1 g), oxalic acid dihydrate (1 g, 1 equiv.), AlCl3 (150 mg, 15 wt%), activated charcoal (200 mg, 20 wt%), 4 N HCl (387 µL, 15 wt%), reactive phase solvent (6 mL), MIBK:2-butanol (3:1) = (4.5:1.5) mL as extracting phase solvent; amole percent yields were calculated with respect to glucose monomer; bTHF:Water = (4:2) mL; cextracting phase solvent was not used
Effect of reaction temperature on 5-HMF synthesis
The effect of the reaction temperature found very crucial as the reaction did not happen at 70 °C temperature and even trace of 5-HMF observed on TLC at 90 °C (Table 2, entry 1, 2). Although, the reaction started to happen at 110 °C as observed by TLC and the UPLC yield was 34% as shown in Table 2, entry 3. Hence, the suitable reaction temperature for this transformation concluded at 130 °C to get the highest yield of the product (Table 2, entry 4). Reaction temperature higher than standard protocol (150 °C) led to decrease the yield of the reaction very significantly and enhanced the formation of humins and polymerization of 5-HMF, and also mixture of unidentified by-products (Table 2, entry 5).

One-gram scale reaction for 5-HMF synthesis from carbohydrates and biomass
Under the optimized reaction conditions, initially cellulose powder was treated with the mixture of oxalic acid dihydrate, AlCl3, 4 N HCl and activated charcoal in DMSO and MIBK:2-butanol biphasic solvent system. The reaction was performed at optimized temperature and time under reflux condition as explained in experimental section. After completion of the reaction, the final product 5-HMF was obtained in 48 mol% and 34 wt% (UPLC) yield (334 mg) (refer to SI for UPLC experimental conditions) (Table 3, entry 1). The reaction is so specific that no need of tedious purification required to achieve the purity up to 95% (UPLC). After the exciting results, we have decided to apply the same process for sugarcane bagasse biomass, which showed problem in earlier reports (Chareonlimkun et al. 2010a; Catrinck et al. 2020). The complex lignin shelter encapsulated around cellulose and hemicellulose in biomass, generally demands chemical pre-treatment steps in order to valorise them. Under this study, our target was to develop a process that could be useful for chemically unprocessed lignocellulosic biomass direct conversion to 5-HMF synthesis. Therefore, under this study, now the critical investigation of reaction parameters on sugarcane bagasse revealed that lowering or enhancing the acid concentration did not improve the biomass conversion to 5-HMF and furfural as observed by TLC. Even, the variation in reaction temperature, not led to improve the yield of the reaction. Although, increasing reaction time to 8 h had positive impact on 5-HMF and furfural yields. Hence, the standard reaction protocol finalized for sugarcane bagasse powder was almost same as that for cellulose except reaction time. Finally, the sugarcane bagasse powder (1 g) was directly applied under the optimized reaction conditions and the progress of reaction was monitored on TLC. After completion of the reaction, the ethyl acetate extract was evaporated and dried under freeze-drying showed 55 mol% and 39 wt% (UPLC) yield (139.4 mg) of 5-HMF and 47 mol% and 30 wt% (UPLC) yield (79.8 mg) of furfural with respect to cellulose and hemicellulose contents respectively (Table 3, entry 2). The furfural was easily separated by hexane through solvent extraction from 5-HMF. Similarly, the hot water washed sugarcane bagasse after grinding into fine powder was also tested under the standard reaction conditions. The same process was followed as earlier for monitoring the progress of the reaction, extraction and drying of products to result 35 mol% and 25 wt% (UPLC) yield of 5-HMF and 49 mol% and 31 wt% (UPLC) yield of furfural as per contents of cellulose and hemicellulose present in it (Table 3, entry 3). This variation in respective yields of final products clearly reflected that the unwashed/raw sugarcane bagasse might be containing free sugar units which led to enhance the yield of 5-HMF.

After getting influenced by such results, we further decided to apply the standard reaction conditions on corncob biomass. Therefore, under this study, the corncob powder (1 g) was also treated under the same optimized condition and after extraction and drying it was resulted with 40 mol% and 28 wt% (UPLC) yield of 5-HMF and 78 mol% and 50 wt% (UPLC) yield of furfural (Table 3, entry 4). The mixture of 5-HMF and furfural was also separated by solvent extraction process using hexane.
Further, in our curiosity we have decided to use waste potato biomass for this conversion. Therefore, under this study first time the oven dried fine powder of raw potato (1 g) was applied under the optimized condition and following the same approaches as described for other biomass. Interestingly, as per our expectations the applied experimental conditions procured 5-HMF in good yield as described in Table 3, entry 5. Further, to avoid drying of potato, the squeezed paste of potato was also checked under the developed process. Fortunately, the applied reaction protocol also provided the similar yield (Table 3, entry 6). These findings explicitly demonstrated that raw potatoes could be a cost effective alternative for 5-HMF production, economy creation and boosting the potato cultivation, and enhancing the economy of farmer. Moreover, to check the vast applicability of the process, various carbohydrates like corn-powder, starch, glucose and fructose have also been tested under our established protocol as summarized in Table 3. The corn-powder under experimental conditions resulted 5-HMF in 47 mol% and 33 wt% yield (Table 3, entry 7). The same process also found to be effective for fructose and yielded with 72 mol% and 50 wt% 5-HMF (Table 3, entry 10). Interestingly, this could be the first single process applicable for complex lignocellulosic bio-mass, polymeric carbohydrates and carbohydrate monomers under similar conditions with considerably good yields (Table 3, entry 8, 9). Yet this type of single process, which could be applicable for vast substrate scopes are rarely reported.
500-g scale reaction for 5-HMF synthesis from biomass
The commercial production of 5-HMF from non-edible lignocellulosic biomass as feedstock is a highly challenging task to achieve till today. From a long time, the industrial production of 5-HMF has been targeted from fructose, an edible source. Hence, sustainable development of scalable process for conversion of lignocellulosic biomass, cellulose and hemi-cellulose mixture to specific 5-HMF and furfural as by-product is strictly needed.
After fascinated by this background and successful conversion of different lignocellulosic biomass and carbohydrates to respective products in one-gram scale, the optimized methodology was further checked up to 500-g scale reaction from cellulose as feedstock for 5-HMF synthesis. Under this investigation, initially the cellulose powder (500 g) was subjected to react with oxalic acid dihydrate (500 g), AlCl3 (75 g) and 4 N HCl (194 mL), activated charcoal (100 g) in DMSO and MIBK:2-butanol biphasic solvent system in the same manner as already described in experimental description; Cellulose powder to 5-HMF Synthesis (500-gram scale synthesis). Gratifyingly, the employed reaction conditions procured 5-HMF in 47 mol% and 33 wt% yield (Table 4, entry 1).

In the same manner, once the process found to be successful for cellulose feedstock then the process straightforwardly applied on sugarcane-bagasse biomass in 500-g scale under the standard reaction protocol (Table 4, entry 2). The preparation of biomaterial and quantification of cellulose and hemicellulose were achieved following the earlier protocols. In this study, sugarcane bagasse powder (500-g) was directly utilized as initial feedstock under optimized reaction conditions in the same manner and equivalency as explained in experimental “Sugarcane bagasse to 5-HMF and furfural synthesis (500-gram scale synthesis)” section. Interestingly, the employed reaction parameters delivered 5-HMF in 43 mol% and furfural in 82 mol% with respect to cellulose and hemicellulose contents respectively (Table 4, entry 2). The enhanced yield of furfural in the scale-up reaction may be attributed to the more solvent availability in comparison to 1-g scale reaction. Both 5-HMF and furfural were easily separated by solvent extraction with hexane to achieve high purity of both the compounds.
Finally, the raw waste potato was also allowed to undergo standard reaction conditions in 500-g scale. Before utilizing raw potato as initial feed, it was dried and grinded into fine powder (following earlier approach). The same process was utilized for monitoring the reaction, extraction and drying of final product as already explained to result 5-HMF in 141.65 g (40 mol%, 28 wt% UPLC yield) and UPLC purity about 92% (Table 4, entry 3). These results clearly indicated that shifting from 1 g to 500-g scale did not alter the yields of the reaction highly as it usually happens in existing processes. Hence, this study explicitly showed the potential of the process for future commercial production of 5-HMF and furfural. Further, comparing with the existing reports (Chiappe et al. 2017; Siankevich et al. 2016), this is the first scale-up attempt for one-pot conversion of lignocellulosic biomass or carbohydrates to 5-HMF synthesis. Surprisingly, in our process, the yields of 5-HMF and furfural were found to be comparable and higher in some cases with the existing literature reports for lab-scale process (Zhang et al. 2017a; Chareonlimkun et al. 2010a, b).
Additionally, the industrial production of 5-HMF and furfural was estimated to be around 111.8 and 142.2 kg/Ton dry weight of sugarcane bagasse respectively, and around 101.6 and 111.8 kg/Ton for corncob biomass.
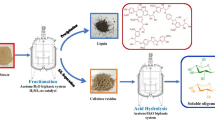
Further, improvements in the process are still ongoing in our lab for scale-up production under flow system and to reduce solvent volume and its recyclability to reduce waste and make the process more economic. Considering our best execution approach for scale-up process development, we have proposed a flow diagram to implement the production of 5-HMF or furfural, recovery of used solvents and final utilization of by-product and bio-char has been shown in Fig. 4. The process described under this study has been protected by patent application (Das et al. 2018).
Conclusion
A scalable process has been developed which could be considered as economical, energy efficient and highly specific for industrial applications. Further, the developed process found to be applicable for vast substrate scopes such as lignocellulose, cellulose, sugarcane bagasse, corncob, waste potato, corn starch, starch, glucose and fructose. This is a first compact report that could be applicable for complex biomass to simplest carbohydrate in one-pot reaction with high specificity. The established protocol offered best yields of 5-HMF and furfural from biomass (mixture of cellulose and hemicellulose) at applied feed loadings. The synergistic effect of organic, mineral and Lewis acids were critically investigated and found to be the main cause of conversion. The explicit study revealed that activated charcoal enhanced the specificity of the reaction significantly and also enhanced in situ stability of 5-HMF under reaction condition. Overall, the process is highly specific and no need of tedious purification required to achieve high purity of the product. The solvents could be recyclable after simple processing. After reaction, the crude solid waste could be useful as bio-char and may be lignin separation for agriculture, polymer and other applications. As a whole, the process found to be compatible for scale-up synthesis and applicable for wide range of cellulosic biomass for 5-HMF production.
References
Asghari FS, Yoshida H (2006) Acid-catalyzed production of 5-hydroxymethyl furfural from D-fructose in subcritical water. Ind Eng Chem Res 45(7):2163
Binder JB, Raines RT (2009) Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc 131(5):1979–1985
Catrinck MN, Barbosa PS, Filho HRO, Monteiro RS, Barbosa MHP, Ribas RM, Teófilo RF (2020) One-step process to produce furfural from sugarcane bagasse over niobium-based solid acid catalysts in a water medium. Fuel Process Technol 207:106482
Chareonlimkun A, Champreda V, Shotipruk A, Laosiripojana N (2010a) Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2–ZrO2 under hot compressed water (HCW) condition. Bioresource Technol 101:4179–4186
Chareonlimkun A, Champreda V, Shotipruk A, Laosiripojana N (2010b) Reactions of C5 and C6-sugars, cellulose, and lignocellulose under hot compressed water (HCW) in the presence of heterogeneous acid catalysts. Fuel 89(10):2873–2880
Chiappe C, Douton MJR, Mezzetta A, Pomelli CS, Assanelli G, de Angelis AR (2017) Recycle and extraction: cornerstones for an efficient conversion of cellulose into 5-hydroxymethylfurfural in ionic liquids. ACS Sustain Chem Eng 5(6):5529–5536
Climent MJ, Corma A, Iborra S (2011) Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem 13(3):520–540
Das P, Kumar A, Shaifali (2018) Process development for 5-hydroxymethylfurfural (5-HMF) synthesis from carbohydrates. IN 201811023331, WO 2019244166
Dutta S, De S, Alam MI, Abu-Omar MM, Saha B (2012) Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J Catal 288:8–15
Garcia-Perez M, Chaala A, Roy C (2002) Co-pyrolysis of sugarcane bagasse with petroleum residue. Part II. Product yields and properties. Fuel 81(7):893−907
Haworth WN, Jones WGM (1944) The conversion of sucrose into furan compounds- Part 1: 5-hydroxymethylfurfuraldehyde and some derivatives. J Chem Soc 667–670
Kläusli T (2014) AVA Biochem: Commercialising renewable platform chemical 5-HMF. Green Process Synth 3(3):235–236
Liu X, Min X, Liu H, Cao Y, Liu Y, Han M, Sun Z, Ji S (2020) Efficient conversion of cellulose to 5-hydroxymethylfurfural catalyzed by a cobalt-phosphonate catalyst. Sustain Energy Fuels. https://doi.org/10.1039/D0SE01006E
Liu Y, Li Zh, Yang Y, Hou Y, Wei Z (2014) A novel route towards high yield 5-hydroxymethylfurfural from fructose catalyzed by a mixture of Lewis and Bronsted acids. RSC Adv 4(79):42035–42038
Mika LT, Csefalvay E, Nemeth A (2018) Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem Rev 118:505–613
Nassar MM (1998) Thermal analysis kinetics of bagasse and rice straw. Energy Sources 20(9):831–837
Overton JC, Engelberth AS, Mosier NS (2020) Single-vessel synthesis of 5-hydroxymethylfurfural (HMF) from milled corn. ACS Sustain Chem Eng 8(1):18–21
Peterson AA (2008) Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ Sci 1(1):32–65
Putten R-JV, van der Waal JC, Jong ED, Rasrendra CB, Heeres HJ, Vries JGD (2013) Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem Rev 113(3):1499–1597
Questell-Santiago YM, Zambrano-Varela R, Amiri MT, Luterbacher JS (2018) Carbohydrate stabilization extends the kinetic limits of chemical polysaccharide depolymerization. Nat Chem 10:1222–1228
Ram S, Sharma AK, Chauhan AS, Das P (2020) Palladium-catalyzed ortho-halogen-induced deoxygenative approach of alkyl aryl ketones to 2-vinylbenzoic acids. ChemComm 56:10674–10677
Rapp KM (1988) Process for preparing pure 5-hydroxymethylfurfuraldehyde. US 4740605
Roman-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA (2007) Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 447(7147):982–985
Shil AK, Kumar S, Reddy CB, Dadhwal S, Thakur V, Das P (2015) Supported palladium nanoparticle-catalyzed carboxylation of aryl halides, alkenylsilanes, and organoboronic acids employing oxalic acid as the C1 source. Org Lett 17(21):5352–5355
Siankevich S, Fei Z, Scopelliti R, Jessop PG, Zhang J, Yan N, Dyson PJ (2016) Direct conversion of mono- and polysaccharides into 5-hydroxymethylfurfural using ionic-liquid mixtures. Chemsuschem 9(16):2089–2096
Thakur V, Kumar A, Sharma N, Shil AK, Das P (2018) Supported palladium nanoparticles catalyzed reductive carbonylation of nitroarenes to N-arylformamides. Adv Synth Catal 360(3):432–437
Xu C, Paone E, Rodríguez-Padrón D, Luque R, Mauriello F (2020) Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem Soc Rev 49:4273–4306
Yi G, Teong SP, Zhang Y (2016) Base-free conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over a Ru/C catalyst. Green Chem 18(4):979–983
Yin S, Pan Y, Tan Z (2011) Hydrothermal conversion of cellulose to 5-hydroxymethyl furfural. Int J Green Energy 8(2):234–247
Yu IKM, Tsang DCW (2017) Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresource Technol 238:716–732
Yu IKM, Tsang DCW, Yip ACK, Chen SS, Wang L, Ok YS, Poon CS (2017) Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): controlling relative kinetics for high productivity. Bioresource Technol 237:222–230
Zhang L, Xi G, Zhang J, Yu H, Wang X (2017a) Efficient catalytic system for the direct transformation of lignocellulosic biomass to furfural and 5–hydroxymethylfurfural. Bioresource Technol 224:656–661
Zhang Z, Song J, Han B (2017b) Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem Rev 117(10):6834–6880
Zhao S, Cheng M, Li J, Tian J, Wang X (2011) One pot production of 5-hydroxymethylfurfural with high yield from cellulose by a Brønsted–Lewis–surfactant-combined heteropolyacid catalyst. ChemComm 47(7):2176–2178
Zhou C, Zhao J, Yagoub AEA, Ma H, Yu X, Hu J, Bao X, Liu S (2017) Conversion of glucose into 5-hydroxymethylfurfural in different solvents and catalysts: Reaction kinetics and mechanism. Egypt J Pet 26(2):477–487
Acknowledgments
We are grateful to the Director of CSIR-IHBT for providing the necessary facilities during the course of this work. The authors thank CSIR, New Delhi for financial support as part of the project no. MLP-0203. AK, ASC, Shaifali thank CSIR, DST-INSPIRE and UGC, New Delhi for awarding fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The image of corncob was taken from google website and the link was provided in SI.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, A., Chauhan, A.S., Shaifali et al. Lignocellulosic biomass and carbohydrates as feed-stock for scalable production of 5-hydroxymethylfurfural. Cellulose 28, 3967–3980 (2021). https://doi.org/10.1007/s10570-021-03764-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03764-3