Abstract
1,2,3,4-Butanetetracarboxylic acid (BTCA) can cause significant strength loss to cotton fabrics, but it has been proven that a flexible molecular structure could benefit the strength in our previous study. In this research, more flexible copolymers [P(IA–AA)] were synthesized with itaconic acid (IA) and acrylic acid (AA) and creatively used as a formaldehyde-free crosslinking reagent for cotton fabrics to replace BTCA. The optimal synthetic conditions of P(IA–AA) were recommended as that the molar ratio of IA:AA and reaction time were 1:1 and 3 h, respectively. The structure of P(IA–AA) was characterized with proton nuclear magnetic resonance spectroscopy, mass spectroscopy and Fourier transform infrared spectroscopy. Results provided evidence that the P(IA–AA) was synthesized with two IA and two AA molecules (molecular weight was 406). Through careful selection, the optimal anti-wrinkle finishing conditions were P(IA–AA) concentration of 160 g/L, pH value of 2.1, curing temperature of 180 °C, and curing time of 2 min. By comparing with IA, BTCA, citric acid (CA), and copolymers of maleic acid (MA) and AA [P(MA–AA)] in the similar conditions, the P(IA–AA)-treated fabrics showed the highest tearing strength retention of 59.30% and whiteness index of 77.60, and the wrinkle recovery angle of the P(IA–AA)-treated fabrics (245.1° ± 0.85°) was comparable with that of the CA-treated ones (244.7° ± 4.30°). Additionally, by separating the strength loss caused by degradation (TSLD) from that caused by crosslinking (TSLC), the P(IA–AA)-treated fabrics presented about 10% less TSLD than P(MA–AA)-treated ones with a comparable TSLC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dimethyloldihydroxyethyleneurea (DMDHEU) has been used as an anti-wrinkle crosslinking reagent for years due to its high efficiency and low cost (Qi et al. 2016a; Schramm et al. 2014). However, the DMDHEU-treated cotton fabrics and garments could release formaldehyde continuously during their commercial applications (Yang et al. 1998). Formaldehyde had been confirmed to cause cancer and skin allergy to human body (Harifi and Montazer 2012). In order to avoid the release of formaldehyde, formaldehyde-free anti-wrinkle crosslinking reagents have always gained worldwide attention, such as 1,2,3,4-butanetetracarboxylic acid (BTCA) and citric acid (CA) (Ji et al. 2019; Yang et al. 1997). BTCA has shown a prospective effect with sodium hypophosphite (SHP) as a catalyst (Ji et al. 2015). However, BTCA results in a significant strength loss to the treated fabrics, and it is very expensive for commercial use compared with DMDHEU (Ji et al. 2016a, 2018). CA is an eco-friendly crosslinking reagent, but it causes yellowing to the treated cotton fabrics (Tang et al. 2016), which is a main disadvantage for its application.
In our previous research, we investigated the low-molecular-weight copolymers [P(MA–AA)] of maleic acid (MA) and acrylic acid (AA) for anti-wrinkle finishing of cotton fabrics in detail (Liang et al. 2020). Under the similar conditions, results indicated that the wrinkle recovery angle (WRA) of the P(MA–AA)-treated cotton fabrics showed higher than that of the CA-treated ones. More importantly, the whiteness index (WI) of the P(MA–AA)-treated fabrics almost remained unchanged. The tearing strength retention (TSR) of the P(MA–AA)-treated fabrics was 16% higher than that of the BTCA-treated ones, which could be attributed to the more flexible molecular structure of P(MA–AA) (Ji et al. 2016b, c).
However, it is still a main defect for the practical application of P(MA–AA) that it caused about 50% tearing strength loss (TSL) to the treated fabrics. The TSL of crosslinked fabrics is attributed to the acid degradation of cellulose (TSLD) and crosslinking of cellulose chains (TSLC) by polycarboxylic acid (Ji et al. 2016b, c; Qi et al. 2016b), and a more flexible molecular structure may bring more uniform crosslinking inside the cellulose fiber, which will result in less TSLC to the treated fabrics.
In order to further reduce the TSL of treated fabrics, low-molecular-weight copolymers [P(IA–AA)] from itaconic acid (IA) and AA were synthesized in this study. Macromolecular copolymers of IA and AA have been used in the synthesis of resins and scale inhibitors (Wan 2014). The low-molecular-weight P(IA–AA) used as a crosslinking reagent for cotton fabrics has not been reported as far as we know. In this research, the influence of molar ratio of IA:AA and reaction time on the anti-wrinkle effect of P(IA–AA)-treated fabrics was systematically investigated. The proton nuclear magnetic resonance spectroscopy (1H NMR), mass spectroscopy (MS), and attenuated total reflection (ATR) infrared were all employed to characterize the obtained low-molecular-weight P(IA–AA). The effects of the concentration of P(IA–AA), curing temperature, curing time, and pH value of the finishing bath on the anti-wrinkle properties of the treated fabrics were studied. Under the similar conditions, the anti-wrinkle effect of P(IA–AA) was compared with that of BTCA, P(MA–AA), CA, and IA. Moreover, the TSL of treated fabrics attributed to TSLD and TSLC was separated from each other, which was correlated with the molecular structure of crosslinking reagents.
Experiment
Materials and chemicals
Fabrics consisting of 100% cotton were purchased from Warren Printing and Dyeing Co., Ltd. (Shanghai, China), which were desized, scoured, bleached, and mercerized. The fabric was 40 s × 40 s plain-woven with weighing 117 g/m2, and the warp and weft density was 133 × 72 threads/inch. Acrylic acid (AA) and itaconic acid (IA) were purchased from TCI Chemical Industry Development Co., Ltd. (Shanghai, China). Analytical reagents including citric acid (CA), 1,2,3,4-butanetetracarboxylic acid (BTCA), potassium persulfate (K2S2O8), and sodium hypophosphite monohydrate (SHP) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were used as received.
Methods
Synthesis of P(IA-AA)
IA and SHP were mixed and transferred into a 150 mL dry three-neck round-bottom flask, and distilled water was charged into the flask as a solvent. And then a reflux condenser, a magnetic stirrer, and a dropping funnel were assembled with the flask. Thereafter, the mixture of IA and SHP was kept stirring at 70–75 °C till dissolved completely. Then AA was added dropwise to the reaction system with a dropping funnel, and within 30 min, K2S2O8 as an initiator was added in batches. The SHP and K2S2O8 were 71% and 12% (w/w) of IA respectively, the molar ratio of distilled water to IA was 18:1, and the molar ratio of IA to AA was 0.83:1, 1:1, 1.2:1, 1.5:1, and 1.8:1, respectively. The synthetic reaction was completed under the temperature range of 70–75 °C after 2–6 h by maintaining reflux throughout the process. And then the flask was charged with three-fold volume of absolute ethanol, and the macromolecular products and residual K2S2O8 were precipitated and removed by filtration. Finally, the filtrate was evaporated to remove the ethanol, and the low-molecular-weight P(IA–AA) was obtained, which was a colorless syrupy liquid. The yield of the obtained P(IA–AA) was calculated, and furthermore it was characterized with 1H NMR, MS, and ATR infrared, respectively.
Characterization of synthesized compounds
1H NMR analysis
The P(IA–AA) was measured with 1H NMR on a 400 MHz NMR spectrometer (Bruker Biospin AG, Karlsruhe, Germany). The deuterium oxide (D2O) was used as the solvent and 50.0 mg obtained P(IA–AA) samples were dissolved in 0.5 ml D2O for measurement.
Infrared spectroscopy
Attenuated total reflection (ATR) infrared spectroscopy was employed to detect P(IA–AA), IA, and AA, and the spectra were recorded on a Fourier transform infrared (FTIR) spectrometer (Thermo Fisher Scientific Co., Ltd., Shanghai, China). Before measurement, the water molecules in chemical samples were removed by drying at 50 °C for 1 h, and then 25.0 mg chemical samples were tested.
The FTIR of fabrics were performed in an absorbance mode to examine the ester bonds of the treated fabrics. Before test, the unreacted carboxyl groups in the treated samples were eliminated by ringing fabrics in a 0.1 mol/L NaOH solution for 4 min and then dried. Firstly, we took the warp yarns apart from the weft yarns, and then mixed them together evenly. Thereafter, we used a scissors to cut the yarns into fine powders carefully. Finally, 1.0 mg sample powders and 100.0 mg dried potassium bromide (KBr) were mixed well and compressed to a transparent tablet. All the spectra were recorded in the range of 4000 to 400 cm–1 with a resolution of 4 cm–1 and scan times of 32.
Pyrolysis LC–MS analysis
The P(IA–AA) was measured with a CBM-20A LC–MS (Shimadzu, Tokyo, Japan) to analyze the molecular weight. In detail, 25.0 mg P(IA–AA) were dissolved in the distilled water, the cracking temperature was 300–900 °C, and the molecular weight was determined as 406.
Fabric treatment and test
Fabric treatment
A specific polycarboxylic acid of P(IA–AA), BTCA, CA, P(MA–AA), or IA was dissolved in the distilled water, and then SHP was added as the catalyst. Cotton fabrics were firstly impregnated in a finishing solution and padded through two dips and two nips with a wet pickup about 100%. After being dried at 85 °C for 5 min, the fabrics were cured at 150–190 °C for 1.5–5 min in a curing oven (Werner Mathis, Switzerland). The unreacted polycarboxylic acids as well as the catalyst SHP on the treated fabrics were washed away with a tap water for 5 min. After washing, the treated fabrics were dried at 85 °C for 5 min. Before test, the samples were conditioned for 4 h at a 65 ± 2% relative humidity and 20 ± 1 °C condition.
Wrinkle recovery angle
The measurement of wrinkle recovery angle (WRA) of the fabrics was performed with a M003A wrinkle recovery tester (SDL-Atlas, Rock Hill, South Carolina, USA) according to the method of AATCC 66-2008 “Fabric Wrinkle Recovery: Recover Angle Method”.
Tearing strength retention
Tearing strength of the fabric was evaluated with tearing strength tester (Wenzhou Darong Textile Instrument Co., Ltd., Wenzhou, China) on the basis of the ASTM Testing Method D-1424-1996, “Tear Strength of Fabrics by Impact Pendulum Method”. The calculation of tearing strength retention (TSR) referred to formula (1).
where TS1 indicates the tearing strength of the fabrics after treating, and TS2 indicates the tearing strength of the fabrics without treating. TSR of the cotton fabrics without treating is 100%.
Whiteness index
Whiteness index (WI) of the fabric was measured according to the method of AATCC 110-2005 “Whiteness Determination of Textiles”. And a Datacolor D650 instrument (Datacolor Inc., Lawrenceville, New Jersey, USA) equipped with a color matching system was used to examine the WI of the cotton fabrics.
Washing durability
The washing durability was tested with a soaping machine according to the test method of “AATCC 61-2009 Washing Color Fastness: 2A of Fast Method”.
Tearing strength loss
A 0.5 mol/L NaOH solution was used to hydrolyze the ester crosslinking in the cotton fabrics treated with P(IA–AA) or P(MA–AA) (Yang et al. 2003). After 50 h hydrolysis at room temperature, WRA of the treated fabrics decreased to 140°, which was close to that of the control, indicating the ester crosslinking on the fabrics were completely removed. The total tearing strength loss (TSL) of treated fabrics was calculated by the formula (2).
where TSR was calculated by formula (1). The tearing strength loss due to crosslinking (TSLC) was calculated by the formula (3).
where TS3 is the tearing strength of the treated sample after alkaline hydrolysis, TS4 is the tearing strength of the treated sample before alkaline hydrolysis, and TS2 is the tearing strength of the control. The tearing strength loss due to cellulose degradation (TSLD) was calculated by the formula (4).
Calculation of Connolly solvent excluded volume (CSEV) and number of rotatable bonds (NRB)
The CSEV and NRB of the chemicals were calculated with a ChemBio3D Ultra software as follow: Calculation → MM2 → Minimize Energy → Compute Properties → Chem ProStd → Connolly Solvent Excluded Volume (CSEV), or → Compute Properties → Molecular Topology → Number of Rotatable Bonds (NRB).
Results and discussion
Effects of synthetic factors
In our previous study (Liang et al. 2020), the synthetic conditions, including the molar ratio of IA:AA and the synthetic time, of the copolymers were found to show an impact on the anti-wrinkle properties of treated fabrics. The influence of molar ratio of IA:AA (0.83:1, 1:1, 1.2:1, 1.5:1, and 1.8:1, respectively) on the anti-wrinkle performance of treated cotton fabrics were investigated. After the reaction was finished at 3 h, the low-molecular-weight P(IA–AA) was obtained and further applied as a new crosslinking reagent to treat cotton fabrics.
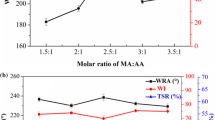
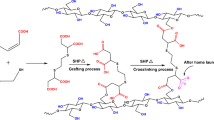
Figure 1a demonstrates that the WRA of the treated fabrics ascended with the molar ratio of IA:AA increasing and then diminished. The maximum WRA of the treated fabrics was around 230° at the molar ratio of IA:AA = 1:1, almost 90° higher than that of the control. And the fabric WI was not affected much after the P(IA–AA) treating (Fig. 1b). As shown in Scheme 1, the P(IA–AA) presents a longer molecular chain, which results in a flexible structure of P(IA–AA). The flexible structure of the crosslinking reagent can bring uniform crosslinking inside the cellulose fibers, which reduces the TSLC. The high TSR (Fig. 1c) proved that the flexible structure of P(IA–AA) reduced the TSL of the treated fabrics. The existence of unsaturated polycarboxylic acids may result in yellowing of the treated fabrics (Tang et al. 2016). However, the high WI of the treated fabrics (Fig. 1b) indicated the inexistence of C=C bonds in the obtained P(IA–AA) and the occurrence of the reaction between IA and AA (Scheme 1). Both IA and AA are unsaturated dicarboxylic acids. The strong electron-withdrawing effect of the carboxylic group in the molecule endues a high reactivity to the unsaturated C=C bond (Peng et al. 2012; Yang and Gu 2001; Bajaj et al. 1993), and therefore it is easy for IA and AA to radically polymerize. The results in Fig. 1a–c showed that the molar ratio of IA:AA = 1:1 benefit the improvement of anti-wrinkle properties of the treated fabrics.
Effect of synthetic conditions a–c molar ratio of itaconic acid: acrylic acid (IA: AA), and d–f reaction time on the anti-wrinkle performance. Notes The concentration of P(IA–AA) was 180 g/L, the curing temperature and time was 180 °C × 2 min. WRA and WI of the control sample are around 140° and 74.00, respectively
By controlling the molar ratio of IA:AA = 1:1, the synthetic reaction time of IA and AA was selected as 2 h, 3 h, 4 h, 5 h, and 6 h, respectively. The fabrics treated with P(IA–AA) obtained at different reaction times are shown in Fig. 1d–f. It was seen in Fig. 1d that the WRA of treated fabrics presented the highest value of 230° at the reaction time of 3 h, and then it showed a downtrend with reaction time further going. Moreover, the WI of treated fabrics always remained constant, and the TSR is constant at a high value of 65% (Fig. 1e, f). It can be explained as that when the reaction time was 3 h, the appropriate molecular size of the obtained P(IA–AA) benefited its diffusion into the fiber and crosslinking with cellulose. If the reaction time was prolonged, macromolecular products could be generated in the synthetic system (Chen et al. 2005). As reported, macromolecular products did not easily diffuse into the amorphous region of cellulose fiber to crosslink with cellulose (Qi 2017), which inhibited the improvement of anti-wrinkle properties of the treated fabrics.
The suitable synthetic conditions were demonstrated as molar ratio of IA:AA = 1:1, temperature of 75 °C, and reaction time of 3 h, the yield of obtained P(IA–AA) was calculated around 60%. The obtained P(IA–AA) was characterized with MS, 1H NMR, and ATR respectively, and results are presented in Fig. 2.
As shown in the MS spectrum of P(IA–AA) (Fig. 2a), the molecular weight of obtained product is deduced as 407 − 1 = 406.
The 1H NMR spectrum of P(IA–AA) was demonstrated in Fig. 2b, the analysis of 1H NMR (400 MHz, D2O) are as follow, (δ): 1.6–1.9 (6H, CH3); 2.0–2.4 (4H, CH2); 2.5–2.9 (6H, CH, CH2), H of –COOH does not signal in D2O.
Figure 2c presented the disappearance of transmittance peaks at 1645–1620 cm−1 and 860–820 cm−1, which corresponded to the vibration of C=C bonds of IA and AA, in the P(IA–AA) spectrum, which indicated the successful polymerization of IA and AA. The strong transmittance peak observed around 1700 cm−1 is the typical of carboxylic carbonyl groups, and the transmittance peak located near 3300 cm−1 was attributed to hydroxyl groups (Luo et al. 2018; Vadakkekara et al. 2020). According to the results of 1H NMR, MS, and ATR infrared spectra, it can be concluded that the maximum molecular weight of the synthesized P(IA–AA) is 406, which is the product of the polymerization of two IA and two AA molecules.
Effects of different treating factors
As known, the anti-wrinkle properties of treated fabrics are strongly influenced by the treating conditions. Firstly, cotton fabrics were treated with five different concentrations (100 g/L, 120 g/L, 140 g/L, 160 g/L, and 180 g/L, respectively) of P(IA–AA) aqueous solution, and the anti-wrinkle properties of the P(IA–AA)-treated fabrics are shown in Fig. 3a–c.
Effect of treating conditions: a–c the concentration of P(IA-AA), and d–f the curing temperature on the anti-wrinkle finishing performance of the treated fabrics. Notes a–c The curing temperature and time was 180 °C × 2 min; d–f P(IA–AA) concentration was 160 g/L, and curing temperature and time were 150–170 °C × 3 min, or 180 °C, 190 °C × 2 min. WRA and WI of the control sample are 140° and 74.00, respectively
It was observed that the WRA of the treated fabrics increased as the concentration of P(IA–AA) increased to160 g/L, and then it almost stagnated with the concentration further rising (Fig. 3a), while the cotton fabrics showed a high WI after treatment regardless of the concentration of P(IA–AA) (Fig. 3b). The TSR decreased greatly but always kept higher than 60% as the concentration of P(IA–AA) increased to 160 g/L (Fig. 3c). This could be explained as that when the concentration of P(IA–AA) increased, the crosslinking reaction between cellulose chains and P(IA–AA) were promoted, which resulted in a significant increase of WRA. However, after the concentration of P(IA–AA) reached to 160 g/L, the crosslinking reaction between P(IA–AA) and cellulose reached saturation with no significant WRA increase as the P(IA–AA) concentration continued to increase. At the same time, the acidic degradation of the treated fabrics from the excessive P(IA–AA) led to an abrupt decrease of the tearing strength. Therefore, the suitable concentration of P(IA–AA) was 160 g/L.
The crosslinking reaction between P(IA–AA) and cellulose occurs in the curing stage. At a high temperature, the adjacent carboxylic groups of P(IA–AA) form cyclic anhydride first, and then esterification of the cyclic anhydride and cellulose occurs (Yang 1991; Yang and Wang 1996). With the suitable concentration of P(IA–AA) being 160 g/L, the curing temperatures were changed to 150 °C, 160 °C, 170 °C, 180 °C, and 190 °C, respectively. The performance of the P(IA–AA)-treated fabrics are described in Fig. 3d–f. The WRA of the treated fabrics increased as the curing temperature increased, and at 180 °C, the maximum WRA of the treated fabrics almost reached to 243° (Fig. 3d). In the case of TSR (Fig. 3e), it always decreased with rising the curing temperature from 150 °C to 190 °C, while the WI of the treated fabrics remained constant (Fig. 3f). This could be explained as that when the curing temperature increased, the intermolecular collision between the P(IA–AA) molecules and cellulose chains was improved, thus enhancing the reactivity of P(IA–AA) as well as the WRA of the treated fabrics. However, at a higher curing temperature, the acidic degradation of cellulose chains was also accelerated, which resulted in a sharp decrease of TSR. The suitable curing temperature was selected as 180 °C to balance the WRA and the TSR.
In order to investigate the effect of curing time on the anti-wrinkle performance of the P(IA–AA)-treated fabrics, the curing temperatures were set at 170 °C and 180 °C, respectively. As shown in Table 1, at 170 °C with a longer curing time, the P(IA–AA)-treated fabrics indicated a higher WRA. At the curing conditions of 170 °C × 4 min or 170 °C × 5 min, the WRA of the treated fabrics almost showed the same. However, the TSR of the treated fabrics decreased greatly with a longer curing time. With the curing time increasing from 1.5 to 2.5 min, the WRA of the treated fabrics cured at 180 °C was higher than that cured at 170 °C. At the curing conditions of 180 °C × 2 min or 180 °C × 2.5 min, the WRA values of treated fabrics were comparable, but TSR decreased with the curing time being prolonged. The WI of treated fabrics almost showed the same as that of the control at 170 °C and 180 °C. The data in Table 1 indicated that 180 °C × 2 min is the optimal curing condition.
Besides the concentration of P(IA–AA), curing temperature, and curing time, the pH value of finishing bath also shows an influence on the anti-wrinkle properties of treated fabrics. To investigate the influence of pH value of finishing bath on the anti-wrinkle performance of the treated fabrics, 0.1 mol/L NaOH solution was applied to adjust the pH value of the finishing bath to 2.1, 2.5, 3.0, and 3.5 respectively before padding the fabrics.
The WRA of the treated fabrics gradually decreased with the pH value increasing, but the TSR showed an opposite trend as the pH value increased from 2.1 to 3.5 (Table 2). The WI of the fabrics treated at different pH conditions were not decreased compared with that of the control. The TSL of the treated fabrics was attributed to the acid degradation of cellulose (TSLD) and the crosslinking (TSLC) of the fabrics. With the pH value of the finishing baths increasing, TSLD was declined. At the same time, less crosslinking occurred on the treated fabrics and consequently increase the TSR of the treated fabrics. Results showed that when the pH value of the finishing baths was about 2.1, WRA, TSR, and WI were appropriate for the treated fabrics.
For the treated fabrics, the durability to washing is very important for its practical applications. With the suitable concentration of P(IA–AA) being 160 g/L, curing temperature at 180 °C, curing time for 2 min, and pH value at 2.1, the fabrics were treated and then the durability of treated fabrics was performed according to the test methods. The change of WRA was presented in Fig. 4. It indicated that after washing with 20 times, the WRA of the treated fabrics was decreased by 15.3°. It is well known that the crosslinking between cellulose chains and P(IA–AA) molecules is ester bond (Yang 1991; Yang and Wang 1996). The pH of the washing solution was measured as 9.3, the decrease of WRA might be attributed to the hydrolysis of ester bonds between cellulose chains and P(IA–AA).
Comparison of different polycarboxylic acids
To investigate the difference among P(IA–AA), P(MA–AA), BTCA, CA, and IA in anti-wrinkle treatment of cotton fabrics and the profound reason of the high TSR of the P(IA–AA)-treated fabrics, a comparison research with equimolar concentration was designed, and results are shown in Table 3.
Under the similar conditions, Table 3 showed that the ester bond absorbance of treated fabrics is positively correlated with the WRA. The polycarboxylic acids crosslinks with the cellulose chains by esterification (Yang 1991; Yang and Wang 1996), and therefore the rising of crosslinking extent, accompanied by an increase in the ester bond absorbance, results in the increasing WRA of the treated fabrics. Moreover, the P(IA–AA)-treated fabrics showed a comparable WRA value about 245° to the CA-treated ones, but higher than the IA-treated ones. The fabrics treated with P(IA–AA) showed the highest WI of 77.30, about 26 higher than that treated with CA (51.66). Among the mentioned polycarboxylic acids in this study, the P(IA–AA)-treated fabrics showed the highest TSR of 59.30%, which was about 24% higher than the BTCA-treated ones. As presented in Table 3, the tearing strength loss of cotton fabrics treated with P(MA–AA) was almost 8% lower than that treated with P(IA–AA). In a word, the synthesized P(IA–AA) can be used for the anti-wrinkle finishing of cotton fabrics instead of P(MA–AA), IA, CA, or BTCA.
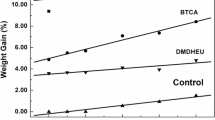
As reported (Qi 2017), the size of crosslinking reagents had an impact on the diffusion of crosslinking reagents into the fibers. Moreover, the flexibility of crosslinking reagents showed an influence on the crosslinking efficiency, as well as the TSR (Qi 2017). To further clarify the effects of the size and flexibility of different crosslinking reagents on the anti-wrinkle properties of treated fabrics, the connolly solvent excluded volume (CSEV) and the number of rotatable bonds (NRB) of crosslinking reagents were calculated according to the test methods. As the NRB value of crosslinking reagents increased, the molecular structure of crosslinking reagents presented more flexible. As presented in Fig. 5, the crosslinking reagents with flexible structure will enhance the strength (Qi 2017).
As presented in Table 4, among the selected crosslinking reagents, CA shows the smallest CSEV value of 137.12, indicating the best diffusibility into fibers to esterify with cellulose. BTCA, with the CSEV value of 174.44, also shows a suitable molecular size for diffusion into fibers to crosslink with cellulose. However, as seen from Table 3, the anti-wrinkle performance of the CA-treated fabrics cannot compete with the BTCA-treated ones. It is possible that the higher crosslinking efficiency is related to the higher NRB of crosslinking reagents (Qi 2017; Hu et al. 2018), consequently BTCA showed a better anti-wrinkle finishing effect than CA. P(MA–AA) and P(IA–AA) showed a larger molecular size than both CA and BTCA, with a value of CSEV being 301–304 and 335–339, respectively. It would be more difficult to diffuse into fibers to cause crosslinking reaction. Owing to the larger molecular size of P(IA–AA), the P(MA–AA) showed a promising anti-wrinkle effect (Table 3), although the P(IA–AA) showed higher NRB value. The results indicated that only the suitable CSEV and NRB can improve the anti-wrinkle performance effectively.
In order to further study the strength loss of cotton fabrics treated with P(IA–AA) or P(MA–AA), the treated fabrics were alkaline treated according to the test methods section. Consequently, TSLD and TSLC of the treated fabrics can be separated from each other. Results are summarized in Table 5. Table 5 showed that the TSL of the cotton fabrics treated with P(MA–AA) was 48.80%, almost 8% higher than that treated with P(IA–AA). The TSLD of the fabrics treated with P(IA–AA) was about 10% lower than that treated with P(MA–AA). And the TSLC of the fabrics treated with P(IA–AA) was almost equivalent to that treated with P(MA–AA). This suggested that the size and the flexibility of the crosslinking reagent affected the strength loss of the treated fabrics (Ji et al. 2016b).
P(MA–AA) (Scheme 2), which indicated a smaller molecular size than P(IA–AA) (Table 4), had an advantage to diffuse into the cotton fabrics more rapidly and deeply, thus it established more uniform crosslinking in the fabrics. Consequently, the P(IA–AA)-treated fabrics presented a little higher TSLC than the P(MA–AA)-treated ones. However, it was much easier for the small-size P(MA–AA) to diffuse into fibers to cause the irreversible acidic degradation of cellulose. At the same time, pH values of the P(MA–AA) and the P(IA–AA) aqueous solutions were about 1.8 and 2.1, respectively, therefore P(MA–AA) solution could cause more acid degradation of cellulose than P(IA–AA) solution. As a result, the TSL of the P(IA–AA)-treated fabrics was lower than that of the P(MA–AA)-treated ones.
Conclusions
Low-molecular-weight copolymers of P(IA–AA) were successfully synthesized with the optimal conditions that the molar ratio of IA:AA was selected as 1:1, and reaction temperature and time were 75 °C and 3 h, respectively. The characterization results showed that the maximum molecular weight of the synthesized P(IA–AA) was 406, which was composed of two IA and two AA molecules. For the anti-wrinkle finishing of fabrics, the recommended P(IA–AA) concentration was 160 g/L, and the recommended curing temperature and time were 180 °C and 2 min, respectively. By comparing the anti-wrinkle effect of P(IA–AA) with that of BTCA, P(MA–AA), CA, and IA under the equimolar concentrations, the WRA of the P(IA–AA)-treated fabrics was about 245°, which was equivalent to that of the CA-treated ones. TSR (59.3%) and WI (77.30) of the P(IA–AA)-treated fabrics showed the highest values among the selected polycarboxylic acids, and TSR showed about 24% higher than that of the BTCA-treated fabrics. Furthermore, by comparing TSL values, the TSLD of the P(MA–AA)-treated fabrics showed about 10% higher than that of the P(IA–AA)-treated ones, because the pH value of the P(MA–AA) solution was 1.8, lower than that of the P(IA–AA) solution at 2.1. The TSLC of the P(IA–AA)-treated fabrics showed 1.4% higher than that of the P(MA–AA)-treated ones, which may be attributed to the higher molecular size of P(IA–AA). Overall, the obtained P(IA–AA) presented a potential application in anti-wrinkle finishing of cotton fabrics to retain a high tearing strength of the treated fabrics.
References
Bajaj P, Paliwal DK, Gupta AK (1993) Acrylonitrile-acrylic acids copolymers. 1. Synthesis and characterization. J Appl Polym Sci 49:823–833
Chen D, Yang CQ, Qiu X (2005) Aqueous polymerization of maleic acid and cross-linking of cotton cellulose by poly(maleic acid). Ind Eng Chem Res 44:7921–7927
Harifi T, Montazer M (2012) Past, present and future prospects of cotton cross-linking: new insight into nano particles. Carbohydr Polym 88:1125–1140
Hu H, Dong X, Tang W, Yao J, He J (2018) Effects of molecular structures of poly-carboxylic acids on crosslinking reaction. Dye Finis 01:0001–0005
Ji B, Tang P, Yan K, Sun G (2015) Catalytic actions of alkaline salts in reactions between 1,2,3,4-butanetetracarboxylic acid and cellulose: II. Esterification. Carbohydr Polym 132:228–236
Ji B, Qi H, Yan K, Sun G (2016a) Catalytic actions of alkaline salts in reactions between 1,2,3,4-butanetetracarboxylic acid and cellulose: I. Anhydride formation. Cellulose 23:259–267
Ji B, Yan K, Sun G (2016b) Investigation on functional properties of 1,2,3,4-butanetetracarboxylic acid cross-linked fabrics impacted by molecular structures and chemical affinity of catalysts. Ind Eng Chem Res 55:5216–5222
Ji B, Zhao C, Yan K, Sun G (2016c) Effects of acid diffusibility and affinity to cellulose on strength loss of polycarboxylic acid crosslinked fabrics. Carbohydr Polym 144:282–288
Ji B, Zhao C, Yan K, Sun G (2018) Effects of divalent anionic catalysts on cross-linking of cellulose with 1,2,3,4-butanetetracarboxylic acid. Carbohydr Polym 181:292–299
Ji B, Tang P, Hu C, Yan K (2019) Catalytic and ionic cross-linking actions of L-glutamate salt for the modification of cellulose by 1,2,3,4-butanetetracarboxylic acid. Carbohydr Polym 207:288–296
Liang T, Yan K, Zhao T, Ji B (2020) Synthesis of a low-molecular-weight copolymer by maleic acid and acrylic acid and its application for the functional modification of cellulose. Cellulose 27:5665–5675
Luo M, Li H, Huang C, Zhang H, Xiong L, Chen X, Chen X (2018) Cellulose-based absorbent production from bacterial cellulose and acrylic acid: synthesis and performance. Polymers 10:702–719
Peng H, Yang CQ, Wang X, Wang S (2012) The combination of itaconic acid and sodium hypophosphite as a new cross-linking system for cotton. Ind Eng Chem Res 51:11301–11311
Qi H (2017) Formaldehyde-free anti-wrinkle finishing on cotton fabrics by polycarboxylic acids. Donghua University, Shanghai
Qi H, Huang Y, Sun G, Qing F, Yan K (2016a) Anti-crease finishing of cotton fabrics based on crosslinking of cellulose with acryloyl malic acid. Carbohydr Polym 135:86–93
Qi H, Pan J, Qing F, Yan K, Sun G (2016b) Anti-wrinkle and UV protective performance of cotton fabrics finished with 5-(carbonyloxy succinic)-benzene-1,2,4-tricarboxylic acid. Carbohydr Polym 154:313–319
Schramm C, Rinderer B, Tessadri B (2014) Non-formaldehyde, crease resistant agent for cotton fabrics based on an organic–inorganic hybrid material. Carbohydr Polym 105:81–89
Tang P, Ji B, Sun G (2016) Whiteness improvement of citric acid crosslinked cotton fabrics: H2O2 bleaching under alkaline condition. Carbohydr Polym 147:139–145
Vadakkekara GJ, Thomas S, Nair CPR (2020) Sodium itaconate grafted nanocellulose for facile elimination of lead ion from water. Cellulose 27:3233–3248
Wan Y (2014) Synthesis and properties of low molecular weight poly(acrylic acid-co-itaconic acid). Zhengzhou University, Zhengzhou
Yang CQ (1991) FT-IR spectroscopy study of the ester crosslinking mechanism of cotton cellulose. Text Res J 61:433–440
Yang CQ, Gu X (2001) Polymerization of maleic acid and itaconic acid studied by FT-Raman spectroscopy. J Appl Polym Sci 81:223–228
Yang CQ, Wang X (1996) Formation of cyclic anhydride intermediates and esterification of cotton cellulose by multifunctional carboxylic acids: an infrared spectroscopy study. Text Res J 66:595–603
Yang C, Wang X, Kang I (1997) Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Text Res J 67:334–342
Yang CQ, Xu L, Li S, Jiang Y (1998) Nonformaldehyde durable press finishing of cotton fabrics by combining citric acid with polymers of maleic acid. Text Res J 68:457–464
Yang CQ, Hu C, Lickfield GC (2003) Crosslinking cotton with poly(itaconic acid) and in situ polymerization of itaconic acid: fabric mechanical strength retention. J Appl Polym Sci 87:2023–2030
Acknowledgments
This research was funded by the National Natural Science Foundation of China (51803025), the Fundamental Research Funds for the Central Universities (2232020D-21), and the China Postdoctoral Science Foundation Project (2018M641893).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, T., Yan, K., Zhao, T. et al. High strength retention of cellulose fibers crosslinking with synthesized low-molecular-weight copolymers of itaconic acid and acrylic acid. Cellulose 28, 1167–1178 (2021). https://doi.org/10.1007/s10570-020-03574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03574-z