Abstract
Wet-spun fibers were prepared from plant-based cellulose nanofibers (CNFs) via a novel approach proposed in a previous report. This method is based on the use of a simple NaOH treatment and the minimization of the use of harmful reagents. The CNF gels prepared using an 8% NaOH solution exhibited good preservation of the cellulose I crystal structure, contributing to the high tensile properties of the spun fibers. This study further explored this approach, and a CNF suspension with 8% NaOH was spun into a 2% sulfuric acid coagulation bath at different spinning rates (1–100 m/min). Straight long fibers with a circular cross-section were obtained at all spinning rates. The orientation index of the CNFs and the tensile properties of the CNF spun fibers increased slightly with increasing spinning rate. However, these values were lower than the values expected based on the previous reports. The aggregation of CNFs in the NaOH solution likely affected these properties. Nevertheless, the advantages of this method, namely the lack of use of toxic solvents and the minimal use of organic solvents, were demonstrated. Furthermore, a high wet strength can be expected due to the formation of interdigitated linkages between the CNFs. These CNF filaments are promising for applications in smart textiles, biosensors, and structural reinforcement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is widely used in the fiber and textile industries due to its high mechanical strength, low toxicity, and good biocompatibility. In addition to being produced from cotton fibers, cellulosic fibers now can also be derived from regenerated cellulose and cellulose derivatives. Currently, wood pulp and cotton are the main sources of cellulose fibers and contain natural cellulose as crystalline microfibrils with a diameter of ~ 3 nm and length of several micrometers. Microfibrils from wood and other plant sources have desirable physical and morphological properties and are isolated as cellulose nanofibers (CNFs). Such CNFs have become popular as an emerging bio-based nanomaterial with potential for use in a wide range of applications in the biomedical, pharmaceutical, and tissue engineering fields.
Recent studies have explored the spinning of CNFs. Regenerated cellulosic fibers generally exhibit deteriorated mechanical properties due to the crystal conversion from cellulose I to cellulose II during processing. Indeed, Nishino et al. (1995) estimated the longitudinal elastic moduli of the crystalline regions of natural cellulose I and cellulose II to be 138 and 88 GPa, respectively, representing a decrease of 36%. (Nishino et al. 1995). Furthermore, the use of toxic chemicals (e.g., carbon disulfide) also contributes to this damage. Since the spinning of CNFs does not require this dissolution process, the stable crystalline structure of natural cellulose is maintained during spinning, leading to the superior mechanical properties of spun CNFs.
Iwamoto et al. (2011) first reported wet spinning using only CNFs where CNFs were prepared from wood pulp and tunicate via an oxidation reaction with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) as a catalyst, followed by wet spinning in an acetone coagulation bath. The use of higher spinning rates led to an improvement in the degree of orientation of the CNFs, with wood CNF-based spun fibers prepared at 100 m/min exhibiting a Young’s modulus and tensile strength of 23.6 GPa and 321 MPa, respectively. TEMPO-mediated CNFs are preferable for wet spinning because of their characteristic uniform narrow diameter and high-viscosity suspensions (Iwamoto et al. 2011; Walther et al. 2011; Torres-Rendon et al. 2014; Lundahl et al. 2016; Shen et al. 2016; Kafy et al. 2017; Vuoriluoto et al. 2017; Yao et al. 2017; Kim et al. 2019). Furthermore, most applications carried out coagulation via dehydration in an organic solvent bath. Alternatively, CNF filaments were prepared via a surface-charge-controlled gel transition (Håkansson et al. 2014). Several studies also explored the dry spinning of CNFs, where CNFs in dried filaments are typically fixed by surface adhesion via hydrogen bonding (Hooshmand et al. 2015; Shen et al. 2016; Ghasemi et al. 2017).
The present work further explored a newly proposed approach of the wet spinning of CNFs via alkaline gelation. This simple hydrogel preparation method involves the alkaline treatment and subsequent neutralization of a CNF suspension, and was described in a previous report (Abe and Yano 2011). Unlike traditional polymer hydrogels, CNF hydrogels were prepared without dissolving the CNFs in specific solvents, and this approach tends to lead to the formation of a highly porous crystalline nano-network. CNF gels can adopt two different crystal forms, namely cellulose I or II, depending on the concentration of the alkaline solution. The use of an 8 wt% sodium hydroxide (NaOH) solution led to the formation of a CNF gel comprising mostly cellulose I due to the partial interdigitation between the surface chains of the cellulose nanofibers (Okano and Sarko 1985). The CNFs in the hydrogel were fixed by these strong interdigitated linkages, and the hydrogel exhibited high tensile properties in the wet state because of the cellulose I crystalline network (Abe and Yano 2012). The previously proposed gelation system was adapted in this study to prepare long CNF-based filaments. A CNF suspension was mixed with NaOH and wet-spun into an acidic solution bath. The CNFs coagulated due to stable gelation during neutralization. The main goal of this study was to evaluate whether this method can be applied to spun CNFs. Furthermore, the effect of the spinning rate on the degree of orientation and the tensile properties of the CNF-based filaments was investigated by X-ray diffraction (XRD) and tensile testing.
Experimental
Preparation of cellulose nanofibers
Wood powder from Hinoki cypress (Chamaecyparis obtusa) was sieved using a No. 60 mesh. The procedure described in the previous studies was followed (Abe et al. 2007; Abe and Yano 2009, 2010). The wood powder was purified using a series of chemical treatments. Lignin was removed from the powder using an acidified sodium chlorite solution at 70 °C for 5 h. The powder was transferred to 5 wt% potassium hydroxide at 80 °C for 2 h to remove hemicellulose. The powder was rinsed with distilled water to neutralize the residue. The α-cellulose content of the purified sample was ~ 86%, as determined by extraction with 17.5 wt% NaOH.
The undried purified sample was suspended in distilled water (1 wt%) and passed through a grinder (MKCA6-3; Masuko Sangyo Co., Ltd., Saitama, Japan) at 1500 rpm twice to obtain an aqueous suspension of cellulose nanofibers. The grinding treatment was performed with a clearance gauge of − 4.5 (corresponding to a 0.45 mm shift) from the zero position.
Wet-spinning
NaOH was added to the CNF suspension to a NaOH concentration of 8 wt%. The suspension was stirred vigorously using a magnetic stirrer for 1 h and centrifuged at 18,000 rpm at 25 °C for 20 min. The CNF concentration of the suspension increased to 4.7 wt% (without considering NaOH weight).
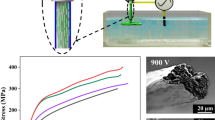
A schematic of the wet-spinning process is shown in Fig. 1. The concentrated CNF suspension was spun using a syringe pump from a nozzle (diameter = 0.4 mm) in a 2% sulfuric acid coagulation bath at 25 °C. Spinning rates of 1, 10, and 100 m/min were investigated to control the alignment of the CNFs in the spun fibers. The coagulation bath was stirred gently using a magnetic stirrer to avoid the deformation of the spun fibers during coagulation. The spun fibers were left in the bath for 10 min to achieve full coagulation and then were immersed in distilled water for 6 h to remove the unwanted salts. The filament ends of the spun fibers were fixed, and the samples were air-dried.
Characterization
The CNF spun fibers were coated with platinum using an ion sputter coater and were observed by field-emission scanning electron microscopy (FE-SEM; JSM-6700F; JEOL Ltd., Tokyo, Japan) at 1.5 kV.
The cellulose crystal form of the spun fibers was evaluated using XRD (Rigaku, Japan) with CuKα radiation (40 kV and 300 mA) in the 2θ range from 5° to 40° in the reflection mode. A bundle of ten spun fibers was used for the XRD measurements and CNF sheets and commercial rayon fibers were prepared and characterized by XRD for comparison. The CNF sheets were prepared by filtration of 0.2 wt% CNF suspension followed by hot-pressing at 120 °C, and a commercial viscose rayon fiber was used as control for the cellulose II sample.
The degree of the nanofiber orientation was measured in the spun fibers prepared at 1 and 100 m/min using single-crystal XRD with an imaging plate (Rigaku, Japan). The orientation index (fc) of the cellulose crystals in the spun fibers was calculated according to:
where HW is the half-width of the azimuthal direction of the equatorial (200) reflection.
Tensile testing of the spun fibers was conducted using a universal material testing machine (model 3365; Instron Corp., Canton, MA) at a crosshead speed of 1 mm/min with a gauge length of 25 mm. The cross-section of the spun fibers was circular (based on FE-SEM observations), and therefore, the Young’s modulus and tensile strength were calculated based on the mean value of the diameter measured at five points on each spun fiber. The mean values of the Young’s modulus, tensile strength, and fracture strain were obtained using the results obtained for at least five samples.
Results and discussion
A previous study demonstrated that fine nanofibers were successfully obtained from wood powder with diameters in the range of 12–20 nm (Abe et al. 2007). The uniform CNFs isolated in this study corresponded to cellulose microfibril aggregates (Donaldson 2007). The high viscosity of the CNF suspension was attributed to the homogeneous dispersion of hydrophilic CNFs with a high surface-to-volume ratio. The addition of NaOH to the CNF suspension led to a slight decrease in the viscosity due to the aggregation of the CNFs. Therefore, the CNF suspension became more concentrated during centrifugation, and increased from 0.8 to 4.7 wt%.
Although the CNFs aggregated in the NaOH solution, the suspension was easily disrupted by brief agitation. When the CNF suspensions with NaOH were spun into the acidic coagulation bath, the CNFs were fixed in a stable fibrous hydrogel upon neutralization (Fig. 2). Regardless of the spinning rate, straight long fibers were obtained due to the gentle stirring of the bath using a magnetic stirrer.
The morphology and diameter (ca. 90 μm) of the CNF spun fibers were similar regardless of the spinning rate. The cross-section and side view FE-SEM images of the CNF spun fibers prepared at a spinning rate of 10 m/min are shown in Fig. 3. The cross-section was approximately circular, and no micro-voids or large deformations were observed (Fig. 3a). The side of the fiber had a longitudinal grooved surface (Fig. 3b, c). However, alignment of the CNFs could not be observed in the spun fibers prepared at any spinning rate.
Native cellulose can be converted from cellulose I to cellulose II by soaking in a strong alkaline solution, washing with water, and drying. The wood CNF exhibited a typical cellulose I crystal form, while the commercial rayon fiber (regenerated cellulose) exhibited a cellulose II crystal form (Fig. 4). The XRD pattern of the CNF spun fibers prepared with 8 wt% NaOH indicated that cellulose I was prevalent. However, small peaks attributed to cellulose II at the 2θ angles of ~ 12.3° and 20° were also observed. This crystal conversion was caused by the partial interdigitation between the surface chains of the CNFs.
The orientation index and tensile properties of the CNF spun fibers prepared at the rates of 1, 10, and 100 m/min are given in Table 1, where the orientation index was estimated based on the half-width of the equatorial (200) reflection in the azimuthal direction. An increase in the spinning rate from 1 to 100 m/min led to a slight increase in the orientation index. The values of the Young’s modulus, tensile strength, and strain at break also increased slightly. However, when the spinning rate was increased from 10 m/min to 100 m/min, the tensile properties remained almost unchanged. Previous studies of CNF spinning found that an increased spinning rate led to an improvement in the orientation index of the CNFs as well as in the mechanical properties of the CNF spun fibers (Iwamoto et al. 2011; Torres-Rendon et al. 2014; Hooshmand et al. 2015; Kafy et al. 2017). Iwamoto et al. (2011) reported that the orientation index of TEMPO-mediated CNFs in spun fibers increased from 0.67 to 0.72 for an increase in the spinning rate from 1 to 100 m/min, and Young’s modulus increased from 11.6 to 23.6 GPa. However, the findings of this study indicated that increased spinning rate had little effect on the orientation index of the CNFs. Consequently, the tensile properties of the spun fibers were relatively low compared to those reported in the previous studies. The CNFs exhibited mild aggregation in the NaOH solution that perturbed their alignment, as was expected to occur at the higher spinning rates. Furthermore, the aggregation that occurred prior to the coagulation likely led to an inhomogeneous CNF spun fiber structure, thereby resulting in a lower tensile strength. This problem may be overcome via mechanical agitation of the CNF suspension with NaOH, or by using a stretching method to improve the orientation index.
Recently, the regenerated cellulose fiber has undergone remarkable development, and the fiber produced via the direct dissolution system using an ionic liquid exhibited a maximum initial elastic modulus of 34 GPa (Sixta et al. 2015). Nevertheless, the high crystallinity and modulus of cellulose I enable the spun fibers to exhibit superior mechanical properties to those of the regenerated fiber, particularly for the elastic modulus. A recent study on TEMPO-oxidized CNF spun fibers reported that the highest Young’s modulus (37.5 GPa) and tensile strength (543.1 MPa) were achieved by stretching the spun fibers (Kim et al. 2019).
Conclusion
Spinning of CNFs is expected to widely expand the range of their applications, particularly in the areas of composites, nonwovens, and textiles (Lundahl et al. 2017). This study proposed a new method to produce wet-spun fibers from CNFs based on gelation via a NaOH treatment followed by neutralization. A CNF suspension with 8% NaOH was wet-spun into a 2% sulfuric acid coagulation bath at 25 °C at different spinning rates (1–100 m/min) and straight long filaments with a circular cross-section were obtained at all spinning rates. The spun fibers consisted mostly of cellulose I, and the XRD patterns exhibited only minor characteristic cellulose II peaks due to the partial interdigitation between the cellulose nanofibers. The orientation index of the CNFs and the tensile properties of the CNF spun fibers increased slightly with increasing spinning rate. However, the tensile properties of the spun fibers were lower than those reported in the previous studies. This lower strength was attributed to the aggregation of the CNFs in the NaOH solution that interfered with the alignment of the CNFs during spinning. Furthermore, the obtained spun fibers exhibited an inhomogeneous structure. This is a serious challenge that must be overcome in the subsequent research.
The advantages of this method were successfully demonstrated. The gelation achieved using NaOH treatment did not require the dissolution process, and thus, toxic solvents were not used for dissolution and minimal amounts of organic solvents were used for coagulation. Furthermore, very strong interdigitated linkages were formed between the CNFs. Coagulation of CNFs in organic solvents is typically achieved via hydrogen bonds on the surface of the CNFs. However, hydrogen bonding is not resistant to moisture; thus, the wet strength of such spun fibers is severely compromised. By contrast, the interdigitated linkages between the CNFs in this study involved recrystallization and are expected to maintain the strength of the CNF-based hydrogels in water.
This study did not optimize the various spinning conditions such as the nozzle diameter and coagulation bath, and did not test some stretching treatment. However, the development of CNF spinning is still in its initial stages, and it is expected that further improvements of CNF spinning will possible in the future based on the accumulated knowledge and technology related to the traditional cellulose spinning fibers.
References
Abe K, Yano H (2009) Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose 16:1017–1023
Abe K, Yano H (2010) Comparison of the characteristics of cellulose microfibril aggregates isolated from fiber and parenchyma cells of Moso bamboo (Phyllostachys pubescens). Cellulose 17:271–277
Abe K, Yano H (2011) Formation of hydrogels from cellulose nanofibers. Carbohyd Polym 85:733–737
Abe K, Yano H (2012) Cellulose nanofiber-based hydrogels with high mechanical strength. Cellulose 19:1907–1912
Abe K, Iwamoto S, Yano H (2007) Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromol 8:3276–3278
Donaldson L (2007) Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci Technol 41:443–460
Ghasemi S, Tajvidi M, Bousfield DW, Gardner DJ, Gramlich WM (2017) Dry-spun neat cellulose nanofibril filaments: influence of drying temperature and nanofibril structure on filament properties. Polymers 9:392
Håkansson KMO, Fall AB, Lundell F, Yu S, Krywka C, Roth SV, Santoro G, Kvick M, Wittberg LP, Wågberg L, Söderberg LD (2014) Hydrodynamic alignment and assembly of nanofibrils resulting in strong cellulose filaments. Nat Commun 5(1):4018. https://doi.org/10.1038/ncomms5018
Hooshmand S, Aitomäki Y, Norberg N, Mathew AP, Oksman K (2015) Dry-spun single-filament fibers comprising solely cellulose nanofibers from bioresidue. ACS Appl Mater Interfaces 7:13022–13028
Iwamoto S, Isogai A, Iwata T (2011) Structure and mechanical properties of wet-spun fibers made from natural cellulose nanofibers. Biomacromol 12:831–836
Kafy A, Kim HC, Zhai L, Kim JW, Hai LV, Kang TJ, Kim J (2017) Cellulose long fibers fabricated from cellulose nanofibers and its strong and tough characteristics. Sci Rep 7:17683
Kim HC, Kim D, Lee JY, Zhai L, Kim J (2019) Effect of wet spinning and stretching to enhance mechanical properties of cellulose nanofiber filament. Int J Precis Eng Manuf Green Technol 6:567–575
Lundahl MJ, Cunha AG, Rojo E, Papageorgiou AC, Rautkari L, Arboleda JC, Rojas OJ (2016) Strength and water interactions of cellulose I filaments wet-spun from cellulose nanofibril hydrogels. Sci Rep 6:30695
Lundahl MJ, Klar V, Wang L, Ago M, Rojas OJ (2017) Spinning of cellulose nanofibrils into filaments: a review. Ind Eng Chem Res 56:8–19
Nishino T, Takano K, Nakamae K (1995) Elastic modulus of the crystalline regions of cellulose polymorphs. J Polym Sci, Part A: Polym Chem 33:1647–1651
Okano T, Sarko A (1985) Mercerization of cellulose II. Alkali-cellulose intermediates and a possible mercerization mechanism. J Appl Polym Sci 30:325–332
Shen Y, Orelma H, Sneck A, Kataja K, Salmela J, Qvintus P, Suurnäkki A, Harlin A (2016) High velocity dry spinning of nanofibrillated cellulose (CNF) filaments on an adhesion controlled surface with low friction. Cellulose 23:3393–3398
Sixta H, Michud A, Hauru L, Asaadi S, Ma Y, King AWT, Kilpeläinen I, Hummel M (2015) Ioncell-F: a high-strength regenerated cellulose fibre. Nord Pulp Pap Res J 30:43–57
Torres-Rendon JG, Schacher FH, Ifuku S, Walther A (2014) Mechanical performance of macrofibers of cellulose and chitin nanofibrils aligned by wet-stretching: a critical comparison. Biomacromol 15:2709–2717
Vuoriluoto M, Orelma H, Lundahl M, Borghei M, Rojas OJ (2017) Filaments with affinity binding and wet strength can Be achieved by spinning bifunctional cellulose nanofibrils. Biomacromol 18:1803–1813
Walther A, Timonen JV, Díez I, Laukkanen A, Ikkala O (2011) Multifunctional high-performance biofibers based on wet-extrusion of renewable native cellulose nanofibrils. Adv Mater 23:2924–2928
Yao J, Chen S, Chen Y, Wang B, Pei Q, Wang H (2017) Macrofibers with high mechanical performance based on aligned bacterial cellulose nanofibers. ACS Appl Mater Interfaces 9:20330–20339
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abe, K., Utsumi, M. Wet spinning of cellulose nanofibers via gelation by alkaline treatment. Cellulose 27, 10441–10446 (2020). https://doi.org/10.1007/s10570-020-03462-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03462-6