Abstract
The decontamination of excess nutrients by polysaccharide-graphene oxide composites has gained much research attention. However, the usage of synthetic polymers and toxic crosslinking agents affects the environment. This investigation is, therefore, aimed at achieving a simple, effective and nontoxic technique of fabricating nanocomposites by ionotropic gelation of chitosan and tripolyphosphate crosslinking agent. The nanochitosan-graphene oxide composite (NCS@GO) was synthesized and investigated for its potential to remove phosphate (P) and nitrate (N) from aqueous solutions. High and low amounts of zirconium (Zr) were loaded in NCT@GO composite to make it selective for the adsorbate anions. The developed nanocomposites were comparatively explored by N2 isotherms, FTIR, XRD, TGA, DTA, FESEM, EDS with mapping analysis and water regain property. Experimental design was conducted by the five-factorial central composite design-as a branch of response surface methodology (RSM). According to the design of RSM, NCS@GO/H-Zr demonstrated an excellent P and N uptake of 172.41 mgP/g and 138.88 mgN/g, reasonable pH-compatibility from 3 to 11, suitable selectivity for both adsorbates among competitor anions, desired recyclability and desorption efficiency for P and N, and retained 76% and 85% for P and N adsorption ability after ten recycles. The removal capacity of P and N anions were also assessed in bi-component systems. Thermodynamic data were considered, in which it was found that the adsorptive removal of the both anions was endothermic and spontaneous in nature. The adsorption isotherm of P and N on the surface of the NCS@GO/H-Zr was suitable for the Freundlich isotherm model, suggesting the multilayer adsorption. On the basis of kinetic studies, specific rate constants involved in the processes were calculated and the obtained result indicates that the pseudo second order kinetics was found to be a better fit. Real samples analysis indicated that the NCS@GO/H-Zr works well for removal of P and N from contaminated waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution is a major global environmental problem, which have great threats to human beings, plants, and animals in the environment (Ali and Gupta 2006; Pooja et al. 2020). The contaminated waters consist of several classes of organic chemicals, nutrients like P and N, and other elements (Ali 2014; Luo et al. 2020). P and N are the fundamental macronutrients for all the living species. High concentration of P and N anions in water is known to cause eutrophication or algal bloom which this issue is resulting to depletion of dissolved oxygen and death of aquatic organisms (Boeykens et al. 2017; Pan et al. 2020). This is important to note that the eutrophication through the food chain affects humans and creates different diseases as well as natural and genetic disorders. The excessive intake of N from water resources by humans can causes infant methemoglobinemia and teratogenic effects (Shrock and Krasowski 2020). On the other side, P is responsible for different human health problems, including cardiovascular diseases (Rathod et al. 2014). Therefore, the water quality improvement needed decreases in both P and N inputs to water bodies.
Among available conventional methods for the removal of nutrient anions from the aqueous media, adsorption by various nano materials as adsorbent has become a hot topic in the recent decades (Yin et al. 2018). Compared to micro-sized adsorbents utilized in water treatment, nano-sized adsorbents have further efficiency which can be related to their smaller size and high specific surface area (Anbia and Salehi 2016; Salehi et al. 2018). These properties can accelerate the adsorption process shortening the time needed for adsorption equilibrium (Salehi et al. 2020). Although nano adsorbents are effective in removing nutrient, they generally are expensive that this feature restricted their widespread use in practice. Over the past decades, numerous studies have been done to find affordable and applicable components (Ali et al. 2012).
Polysaccharides are recommended as one of the most promising class of bioadsorbents because of good chelation behavior, excellent reactivity and high tendency toward contaminants (Negm et al. 2020). Chitosan (CT) is an abundantly available inexpensive polysaccharide for nutrient adsorption that can be achieved from natural resources (Cui et al. 2019). Functionalization of CT is better than that of other polysaccharides due to its abundant reactive groups such as hydroxyl and amino groups. One of the popular processes for the preparation of CT nanoparticles (NCT) is the ionic gelation of tripolyphosphate (TPP) cross-linking agent and CT (Yazdi et al. 2019). Ionic gelation is well-known due to ease and simplicity of nanoparticle preparation. NCT particles of mean sizes between 30 and 100 nm have been synthesized by this process. However, the poor mechanical properties and inherent water sensitivity of CT have limited it applications especially in water treatment. Nanocomposite technology using nano fillers including layered double hydroxides (Mallakpour and Hatami 2019), zeolites (Yan et al. 2018), silicas (Shan et al. 2019), fly ash (Agarwal et al. 2018), and carbonaceous martials (Rahmi and Nurfatimah 2018) at low adding has formerly been indicated to be an efficient strategy to solve these problems.
Different research and published papers in this field show that the loading of graphene oxide (GO) as a carbonaceous layered material can enhance the strength of polymer composites (Tang et al. 2020; Wang et al. 2019). Thus, it will be interesting to synthesize composite of CS materials with loading of GO. In addition, GO possesses appropriate chemical/mechanical stabilities, high specific surface area, an abundance of oxygen atoms, and good interfacial contact with other materials along with cost effective production on a large scale (Ali et al. 2018). NCT and GO can be composited via bridging mechanisms and interactions of positively charged functional groups on the CT surface and negatively charged functional groups on the GO surface.
To widen the application of NCT@GO nanocomposite for water treatment, functionalization should be made to enhance anion removal efficiency. To improve the affinity of CS-based samples to inorganic anions, metals including zirconium (Zr), aluminum (Al), lanthanum (La), iron (Fe), magnesium (Mg) are widely considered to functionalize the CT surface. As a result, different metal-functionalized CT such as Zr-loaded cross-linked chitosan (Qiang and Yewei 2013), Al-impregnated chitosan (Swain et al. 2009), La-encapsulated chitosan-montmorillonite composite (Banu et al. 2018), Fe-impregnated chitosan (Zhang et al. 2015) and Mg-alginate/chitosan modified biochar (Cui et al. 2016) have also been investigated to remove inorganic anions.
Recent investigations show that Zr-loaded materials were extensively accepted as effective P and N adsorbents due to their high binding selectivity towards P and N anions. Zr is a biologically inert material, inexpensive, non-toxic and insoluble in water that possess excellent biocompatibility with CT-based samples. Even if Zr-functionalized CT was inefficiently recovered after being used as adsorbent, the good biocompatibility will significantly decrease these condcontamination to environment. Zr-chitosan/biochar composite beads (Banu et al. 2019), Zr-Crosslinked Quaternized Chitosan/Bentonite Composite, and Fe3O4/ZrO2/chitosan nanocomposite (Jiang et al. 2013) were the various Zr-based adsorbents reported for the P and N removal.
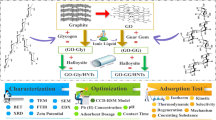
In present work, removal efficiency of Zr-functionalized NCT@GO composite was optimized by RSM for adsorbing P and N anions. The prepared nanocomposites have been identified by N2 isotherms, FTIR, XRD, TGA, DTA, FESEM-EDS with mapping analysis and water regain property. The proposed mechanism of P and N adsorption by the as-synthesized nanocomposites was reviewed. Several variables such as temperature, solution pH, contact time, adsorbate concentration and adsorbent dose were carried out to get the optimal adsorption conditions. Thermodynamic parameters were assessed to find out the nature of adsorption. Different equilibrium and kinetic models are used to obtained data in order to attain insight into anion adsorption characteristics. The P and N uptake in presence of coexisting anions was studied. There usability of Zr-functionalized NCT@GO after ten adsorption-desorption cycles were executed. The applicability of sample at field situations was checked with real water samples.
Materials and methods
Synthesis of NCT@GO and Zr-functionalized NCT@GO
Graphite powder was used to synthesis GO via modified Hummers method. NCT particles was synthesized based on the method by the ionic gelation of CT with TPP as ionic cross linking agent. The method for making NCT particles (Salehi and Anbia 2019) and GO (Mirhosseinian et al. 2020) has been described by our previous works.
The nanochitosan/graphene oxide nanocomposite (NCT@GO) was synthesized as follows: 0.45 g CT (medium molecular weight 75–85%, Aldrich) was dissolved to diluted acetic acid (99%, Merck) and mixed until the CT dissolved completely. After that, 1.85 g TPP (Merck) was dissolved in 50 mL of DI water and the obtained solution was slowly added to the CT solution and mixed for 1 h (NCT solution). 0.15 g GO was completely dispersed in deionized (DI) water under ultrasonication to obtain GO suspension. Then, the GO suspension was dropped at to the NCT solution with vigorous stirring and formed homogeneous NCT-GO mixture after 2 h. Finally, the resulting solid was isolated by centrifugation, washed several times with DI water and oven-dried at 60 °C overnight, and then utilized for P and N adsorption studies.
Preparation of Zr-nanochitosan/graphene oxide nanocomposite (NCT@GO/Zr) was synthesized as follows: required content of zirconium (IV) chloride anhydrous (ZrCl4, Aldrich) was added to the obtained NCT-GO mixture of last section and was mixed for 2 h. When the reaction was completed, the precipitate was separated by centrifugation, rinsed with DI water to remove excess of free ions and dried at 75 °C inside an oven for 5 h (Scheme 1). The synthesized samples are donated as NCT@GO/L-Zr and NCT@GO/H-Zr for 2 M and 5 M of ZrCl4, respectively.
Instrumental techniques
Fourier transform infrared (FTIR) spectra were recorded with a DIGILAB FTS 7000 spectrometer, resolution 4 cm−1, in the range of 4000–400 cm−1 by KBr pellet technique, the amount of the sample being 3 mg in each pellet. Thermogravimetric analysis (TGA) and differential thermal gravimetry (DTG) (BAHR STA 503 instrument) of Zr-functionalized and unfunctionalized adsorbents was carried out in N2 at a flow rate of 20 mL/min. The prepared adsorbents were heated from 20 to 800 °C at a constant rate of 10 °C/min. The X-ray powder diffraction (XRD) data was detected using PHILIPS PW 3710 X-ray diffractometer with Cu and Kα radiation (40 kV, 25 mA) in the 2θ range of 5° to 80°. The crystallite size and characterize the morphology of synthesized materials were checked using Field-emission scanning electron microscopy (FESEM, MIRA3 TESCAN). The surface element compositions of prepared samples were recorded by energy-dispersive X-ray spectroscopy (EDS, VEGA3 TESCAN). The textural properties were measured by a N2 adsorption analysis on a BELSORP-mini II at − 196 °C, and the pore size distribution (PSD) and the surface area were computed by the Barrett, Johner and Halenda (BJH) and Brunauer, Emmett and Teller (BET) methods, respectively.
Water regain property
In order to determine the water adsorption property, precisely weighed amount of adsorbent was immersed in aqueous media for 2 h. After this time, the adsorbents were taken out from aqueous phase. The swelling percentage of the adsorbent can be expressed according to Eq. (1).
where W1 and W2 are the weight of dry and swollen adsorbent, respectively.
Adsorption experiments
For the equilibrium experiments, 20 mL of adsorbate solution in different initial concentrations was added to 50 mL conical flasks containing appropriate mass of synthesized samples. P and N solutions were prepared by dissolving a known amount of potassium phosphate monobasic (KH2PO4) and sodium nitrate (NaNO3) in DI water, respectively. To investigate the impact of various competitive anions on adsorption of P and N species, 20 mL of 150 mg/L analyte solutions with 0.01 g of prepared sample were added into 50 mL conical flasks, the competitive anions were set at 50 mg/L. The pH of P and N solutions was carefully adjusted between 3.0 and 11.0 by adding 0.1 M H2SO4 solution for decreasing the pH and NaOH for increasing the pH. Isotherm, kinetic and thermodynamic evaluations were also carried out in this portion of the study. Adsorption isotherms were performed by adding 0.5 mg/L adsorbent into P and N solution with concentrations of 30–150 mg/L. For kinetic studies, a typical batch adsorption experiment was conducted under 150 mg/L analyte with 0.5 mg/L adsorbent added with the initial pH of 5.0, and the residual P and N concentration at different time intervals was determined. Five different temperatures (293, 298, 303, 308 and 313 K) were used to investigate the adsorption thermodynamics. The adsorbate ion-adsorbent solution was shaken at a thermostatic shaker with certain speed to achieve the equilibrium condition. After pre-defined contact time, the solutions were filtered and analyzed by the ultraviolet spectrophotometric method at a wavelength of 202 nm for N ions. P ions were analyzed at a wavelength of 400 nm by vanadomolybdophosphoric acid method (Federation and Association 2005) using UV/Vis spectrophotometer. The removal percentage (R%), equilibrium adsorption amount (qe, mg/g) and the adsorption amount at different time t (qt, mg/g) are expressed by the following equations, respectively.
where C0 (mg/L), Ce (mg/L), and Ct (mg/L) are the initial liquid-phase concentration, the liquid-phase concentration at equilibrium, and the liquid-phase concentration at time t, respectively; m (g) is the dose of synthesized adsorbents; and V (L) is the volume of the aqueous phase.
Experimental design and central composite design (CCD)
The CCD experiments were established to achieve the optimized removal of P and N anions on Zr-functionalized nanocomposite using the Design-Expert 11.0.3.0 software. Five independent variables including initial concentration, adsorbent dose, adsorption time, pH and solution temperature at five levels of small-factorial CCD combined with RSM was employed which gave a total number of 25 experiments (Table 1).
Four statistical metrics, namely, larger regression coefficient (R2), smaller lack of fit F-value, lower p value, higher sum of squares, have been used in determining the most optimal model. In order to assess the importance of each independent variable, it is needed to obtain the quadratic model significance developed by the analysis of variance (ANOVA) from statistical metrics. The quadratic model equation is represented as follows:
where Y is the response (qe of both anions); \( {\text{X}}_{\text{i}}^{2} \), \( {\text{X}}_{\text{i}} \) and \( {\text{X}}_{\text{j}} \) are level of independent variables; α0 is model constant; αi, αii and αij are the R2 for the linear, quadratic and interaction terms, respectively; and ε is the model residual error.
Models for equilibrium, kinetics and thermodynamic studies
The Langmuir [Eq. (6)], Harkin-Jura [Eq. (7)], Jovanovic [Eq. (8)], Freundlich [Eq. (9)], Temkin [Eq. (10)], and Dubinin–Kaganer–Radushkevick [DKR, Eq. (11)] isotherm models were used to investigate the adsorption behavior of all the P and N ions on to the Zr-functionalized nanocomposite surface and the equations of these models are as follows:
where KL(L/mg), KJ, KF (L/g), and KDKR (mol2/kJ2), represent the Langmuir bonding term related to interaction energies, the Jovanovic constant, the Freundlich affinity coefficient and the constant related to free energy respectively; qm (mg/g) is the maximum amount of P and N species that could be adsorbed overlaying the entire surface, RL is the equilibrium parameter; AH (g2/L) and BH (mg2/L) are two parameters characterizing the adsorption equilibrium; XT and YT are Temkin constants, bT (kJ/mol) is a constant ascribed to the heat of adsorption, T (K) is the thermodynamic temperature, R (8.314 J/mol K) is the ideal gas constant; n is Freundlich constants; qDKR (mg/g) is the theoretical saturation capacity, ε (kJ/mol) is the Polanyi potential, E (kJ/mol) is mean energy of the adsorption.
To further assess the rate-controlling step in P and N adsorption process, five widely-used adsorption kinetic models namely, the pseudo first order [PFO, Eq. (12)], pseudo second order [PSO, Eq. (13)], Elovich [Eq. (14)], Liquid film diffusion [LFD, Eq. (15)] and intraparticle diffusion [IPD, Eq. (16)] were applied to fit the experimental data. The expression formulas corresponding the above-mentioned kinetic models are given below:
where K1 (L/min), K2 (g/mg min), Kf (min−1) and Kint (g/mg min0.5) are the PFO, PSO, LFD and IPD rate constants; α (mg/g min) and β (g/mg) are adsorption and desorption rate constants.
The adsorption system depends on the reaction temperature and is associated with various thermodynamic parameters. Thermodynamic parameters include Gibbs free energy, entropy and enthalpy for the removal efficiency of N and P anions on prepared sample have been computed by the following relationship:
where ΔG (kJ/mol), ΔS (kJ/mol/K) and ΔH (kJ/mol) are the change in Gibbs free energy, entropy and enthalpy energy, respectively; and Kd is the thermodynamic equilibrium constant.
Results and discussion
Physicochemical characterization of adsorbents
The adsorbents obtained were identified by a variety of conventional methods.
To demonstrate the existence of functional groups on surface of sample, the FTIR spectra of the synthesized samples before and after functionalization were recorded and the results were plotted in Fig. 1. Numerous data in the literature show that there are oxygen atoms in GO as OH, C=O, C–O–C and COOH groups. The presence of bands referring to the C=O, C–O, O–H bond, which suggest the presence of the carboxyl, hydroxyl groups, etc. The different oxygenic groups at the edge or on the surface of the GO sheets have a significant key in the bonded of the GO to the NCT matrix. Spectrum of GO shows absorbance bands of stretching vibrations of O-H bonds at 3390 cm−1, C=O stretching vibrations of the carboxylic and carbonyl groups at 1795 cm−1, skeletal vibrations of C=C bonds at 1650 cm−1 and stretching vibrations of C–O–C bonds in alkoxy or epoxy at 1095 cm−1. All these bonds clearly confirm the present of oxygenic groups in GO structure. The spectrum of NCT exhibited absorption bands at 1690, 1550, and 1095 cm−1, that were related to the vibrations of N–H in NH2, C–N, and C–O–C, respectively. A characteristic strong and broad band around 1150 cm−1 is observed, which can be attributed to the P=O stretching vibration of TPP in the NCT. According to the FTIR spectra of NCT@GO, the NH2 band of NCT at 1690 cm−1 and the COOH band of GO at 1795 cm−1 have reduced and a band observed at 1390 cm−1, that was an absorption band of N=N (Dharupaneedi et al. 2014), it indicates the interaction of GO with NCT. In Zr-functionalized nanocomposites, bands at the below 900 cm−1 are attributed to Zr–O–C and Zr–N bonds (Banu et al. 2019). Unfortunately, the Zr bands cannot be observed at the after 900 cm−1 because of the overlapping of bands and the strong intensity of the NCT@GO structure vibrations.
The thermal properties of as-synthezied nanocomposites was characterized by TGA and DTA from room temperature to 800 °C. Figure 2 illustrates the TGA/DTA curves of GO, NCT, NCT@GO, NCT@GO/L-Zr and NCT@GO/H-Zr. According to TGA/DTA analysis of GO, the thermal gravimetric curve clearly consists of two distinct stages. The first one took place from 100 °C and ascribed to the loss of weakly bound guest water molecules. The second stage is attributed to the elimination of oxygenated functional groups of GO. NCT shows the initial estimated loss of the unbound and physically adsorbed water up to 150 °C. The mass loss after 200 °C is attributed to thermal decomposition of NCT skeleton and also released volatile materials. The NCT@GO display three decomposition steps. The first step, in the range of 30–150 °C, is attributed to endothermic physical water loss from the structure of the nanocomposite. The second step, presented in the ranges of 150–230 °C, is related to pyrolysis of the labile oxygen-containing functional groups of GO. The third step, in the range from 230 to 250 °C, is corresponds to the collapse of CT. The further mass loss exceeds 250 °C is ascribed to the thermal degradation of side groups of GO. Furthermore, the thermal stability of Zr-functionlized NCT@GO is increased slightly, compared with that of pristine NCT@GO. With increasing Zr amount, the decomposition peak temperature shifted to lower temperature.
The particle size and surface morphology of as-synthesized samples are shown in Fig. 3. From the Fig. 3, it is observed that GO presents a multilayer structure. The FESEM image of NCT showed an agglomeration of relatively homogeneous particles, ranging from 36 to 68 nm. The fracture surface of NCT@GO looked differently and refer to two different morphological structures. The presence of two different morphological structures indicates the composite formation. In addition, no significant change is observed in the morphology of the NCT@GO after functionalization. To further study the metal distribution on the surface of Zr-functionalized nanocomposite, the EDS with mapping analysis was carried out. The occurrence of elements like Zr, P, N, C, O and Na are various visible color patterns onto the support of samples. The component of Zr could be seen clearly in the EDS results, which suggested that Zr was loaded successfully into the structure of the functionalized nanocomposite.
Figure 4 displayed the XRD patterns of GO, NCT, NCT@GO, NCT@GO/L-Zr and NCT@GO/H-Zr. GO pattern exhibits peaks at the 2ϴ of 15.1° and 49.5° which is ascribed to the crystalline structure of GO (Saxena et al. 2011). The characteristic peaks of NCT occurred at 2θ = 9.8° and 22.9° that suggests the amorphous nature of the NCT (Bagheri et al. 2015, Zhang et al. 2014). The NCT@GO displayed a broad peak at 2θ = 22.9° because of the amorphous state of the NCT. The XRD spectra of NCT@GO was more similar to that of NCT, which may be because that NCT@GO is mainly composed of NCT. The absence of the crystalline peak in the NCT@GO reveals that GO was dispersed into the NCT matrix which this results was also obtained by other researchers (Yadav and Ahmad 2015). Compared with the XRD patterns between unfunctionalized and Zr-functionalized nanocomposite, the the intensity and position of the peak changed. The position of peak at 2θ = 22.9° in nanocomposite was shifted to 29.5° after loading with Zr ions with an extraordinary broad peak.
Figure 5 illustrated the N2 isotherm and corresponding PDS of the GO, NCT, NCT@GO, NCT@GO/L-Zr and NCT@GO/H-Zr. The obtained results shown in Fig. 5a, b demonstrate the existence of mesopores in the synthesized materials, classified as type IV as defined by IUPAC. The principal textural parameters of the samples are provided in Table 2. After Zr loading, the textural characterizations of the NCT@GO decreased as the Zr loading amount increased, revealing that Zr was introduced into the structure of the nanocomposite.
The N2 isotherm and PDS results indicate that the preparation of Zr-functionalized nanocomposite was effective material and corroborate with the FTIR, TGA, DTA, XRD, FESEM and EDS with mapping analysis.
P and N removal from aqueous solution by synthesized adsorbents
The P and N adsorption capacities of synthesized adsorbents are plotted in Fig. 6a, b, respectively. The NCT@GO nanocomposite demonstrated a better adsorptive removal ability for P and N ions than the NCT particles. The key reason is the presence of GO, which has a large BET surface area that leads to higher adsorption performance. The large BET surface area of GO contains a number of surface functional groups, including epoxy, carboxyl and hydroxyl, which these oxygenous groups make GO hydrophilic and easily dispersible in water as well as in organic medium. The results depicted in scheme 1 show that the long chains and abundant functional groups in CT is attached on the GO surface by self-assembled method.
The adsorption mechanism of P and N on NCT@GO nanocomposite was supported by coordination and electrostatic interactions. The prepared nanocomposite possesses NCS and GO which includes many amine, hydroxyl, and carboxyl groups gets protonated in strongly acidic conditions and thus could bind with the both anions by means of electrostatic attraction as well as complexation. As cleared in Fig. 6, the adsorption capacity of anions was observed to be improved with increasing amounts of Zr ion which implies that P and N adsorption occurred significantly through uptake on Zr-active sites in the nanocomposites. ZrCl4 reacted with the functional groups of NCT@GO nanocomposite and also Zr4+ metal ions into the structure of functionalized sample which increased the adsorption sites on the nanocomposite. The increase in the Zr amount embedded per unit mass of NCT@GO provides more binding sites for P and N removal.
Based on the selectivity principle of the HSAB model, the positive charge on NCS@GO/Zr surface because of \( {\text{OH}}_{2}^{ + } \), \( {\text{NH}}_{3}^{ + } \) and Zr4+ metal ions as hard acids which adsorb efficiently by a hard base both adsorbates via hard acid-hard base electrostatic interaction.
Optimization of CCD by desirability function for P and N adsorption
NCS@GO/H-Zr is used for further studies because of its high adsorption capacity toward P and N removal. RSM involves a data set of proper statistical and mathematical skills which are used for designing the experiments to attain the mutual and main influences between the efficient variables of the process. The coefficients are analyzed by the ANOVA and the corresponding statistical metrics are listed in Table 3. Based on the ANOVA table, an empirical relation between important variables and adsorption of P and N can be expressed by the following polynomial:
where A (initial concentration), B (adsorbent dose), C (contact time), D (pH), and E (temperature) are in coded factors.
Quadratic models for responses of P and N adsorption have displayed small lack of fit F-value (0.0531 and 0.0343), low p-value (< 0.0001), high sum of squares, revealing the adequacy for demonstrating both the responses. The values of R2 for the models were 0.9999 and 0.9998 for P and N removal efficiency, respectively. These values close to 1 indicate validity of the model, which shows acceptable agreement with the actual and predicted data. The Adj-R2 and Pred-R2 for P ion was 0.9995 and 0.9945, respectively and for N ion was 0.9994 and 0.9952, respectively. The R2 values Adj and Pred were close to each other and their difference less 0.02 demonstrated the experimental data very well-fitted with quadratic model. Signal-to-noise ratio was estimated by Adequate Precision (AP). The models developed for P and N adsorption have shown AP of 176.62, and 152.63, respectively which are an adequate signals for the model (AP > 4).
Influence of variables as response surface
The influence of interaction between five independent input and output variables and their interactions on the dependent variables can be considered by response surface 3-dimensional (3D) plots. The 3D response surfaces plots, as the functions of two variables at the center level of other variables, are shown in Figs. 7 and 8 and reflect the presence of interactions among variables. The goal of the optimization process was to get maximum adsorption performance at appropriate parameter conditions. The optimal level of five variables for maximum both anions adsorption were achieved as initial concentration of P and N anions = 150 mg/L, adsorbent dose = 0.01 g, contact time = 20 min, initial pH of solution = 5 and temperature = 313 K.
Influence of adsorbent dose
The correlation between the P and N adsorption capacity with respect to varying the NCT@GO/H-Zr nanocomposite amount is depicted in Figs. 7a and 8a, respectively. The removal efficiency was found to decline gradually when the adsorbent amount was increased up to 0.025 g. The reduce of the adsorption performance can be ascribed to unsaturated available binding sites during the adsorption process. The high removal efficiency of NCT@GO/H-Zr for both adsorbates meant large numbers of pollutants could be sufficiently adsorbed under relatively low adsorbent usage.
Influence of adsorption pH
The pH of the aqueous solution is one of the most critical variables that greatly impacts the adsorptive removal of species at the adsorbent-solution interfaces, as it may alter the basic and acidic property of adsorbent surface and the existing form of species in solution, as well as H+ and OH− ions of solution may compete for the available surface sites.
All solutions were adjusted to pH values equal to 3, 5, 7, 9, and 11 by utilizing pH regulator solution. Figures 7c and 8c showed that the amount of P and N adsorbed changed with increase in pH from 3 to 11 due to the protonation and/or deprotonation of synthesized nanocomposite. At acid condition, a large number of functional groups on the NCT@GO/H-Zr surface were protonated, thus the positive charged nanocomposite surface attracts more adsorbate anions via electrostatic interaction. Increasing the pH could lead to the change of the positively-charged surface gradually into negative as a result of the deprotonation effect, therefore, electrostatic attraction between the adsorbate anions and the negative charges on the adsorbent surface became negligible and the amount of adsorption is consequently dropped. In addition to this, when the pH of the solution increases, the increase of OH− concentration will make the strong competition with P and N anions become further, and the coexistence of OH− would considerably restrict the uptake of the both anions on NCT@GO/H-Zr nanocomposite. A similar tendency was indicated in the research regarding removal of P using metal ions cross-linked alginate assisted biocomposite (Kumar and Viswanathan 2017) and in the study concerning removal of N using chitosan-polystyrene–Zn nanocomposite (Keshvardoostchokami et al. 2017). Experimental data show that the removal efficiency of NCT@GO/H-Zr is pH-dependent, but it still demonstrates excellent P and N adsorption over a wide pH range.
Isothermal, kinetic and thermodynamic studies
The equilibrium isotherm is essential to express the adsorption behavior between liquid phase and the solid phase. It exhibits the uptake performance of an adsorbent in terms of concerning the equilibrium concentration of P and N ions. The results depicted in Figs. 7 and 8 show that the P and N removal performances of the NCT@GO/H-Zr increased when increasing their initial aqueous concentrations. This is because a further initial concentration of both anions can provide an additional driving force to overcome the resistance of the mass transfer in the interphase of the bulk solution and adsorbent surface.
In this study, Langmuir (Langmuir 1918), Freundlich (Freundlich 1907), Harkins-Jura (Iyer and Kunju 1992), Temkin (Temkin 1940), Jovanovic (Jovanovic 1969) and DKR (Hu and Zhang 2019) models were applied to describe adsorption isotherm data and the equation constant and given in Table 4. The linear plot of isotherm models for the adsorption of P and N ions were presented in Figs. S1 and S2 (Supplementary material), respectively.
The adsorptive removal of P and N ions on NCT@GO/H-Zr is fitted better by Freundlich isotherm model (R2 = 0.9976 and 0.9973, respectively) demonstrating that the synthesized nanacomposite surface might include of binding sites with different affinities, i.e., heterogeneous adsorption surface and multiple layer uptake takes place on the active sites of the adsorbent surface.
In the Freundlich model, n is the heterogeneity factor which suggests the favorability degree of adsorption system. The values of n are lower than 10 for both analytes indicative of favourable P and N ion adsorption by the NCT@GO/H-Zr. Another key parameter for measuring the favorability of the adsorption process is a dimensionless constant called the (RL). From the data listed in Table 4, it is seen that NCT@GO/H-Zr was favornature for adsorption of P and N ions from aqueous media by reason of the RL (0.12 and 0.26) values in the range of 0 < RL < 1.
The qm predicted by Langmuir model is beneficial for evaluating removal efficiency of the adsorbent. It can be seen from the values of qm in Table 5 that the qm achieved for both adsorbates on NCT@GO/H-Zr was relatively higher than that other adsorbents.
Besides, the values of bT in Temkin model and E in DKR model could infer the type of adsorption that is chemical adsorption or physical adsorption. The bT and E results listed in Table 4 indicate that chemical adsorption is the mechanism responsible for the P and N adsorption on NCT@GO/H-Zr.
Adsorption kinetics are mainly examined to attain the contact time that is needed to achieve the maximum removal efficiency of a material with the equilibrium stage. The influence of contact time on P and N uptake was evaluated, with the results showing that the adsorptive removal of anions increased fairly fast before 20 min and after this adsorption rate was approximately constant (Figs. 7b, 8b). The removal rate of NCT@GO/H-Zr was faster than that of other composites made from chitosan cited in previous literatures (Cui et al. 2019). Such fast adsorption rate is indicative of easily accessible active sites on the NCT@GO/H-Zr surface. To elucidate the mechanism of adsorption, like chemical reaction, diffusion in the liquid film, intraparticle diffusion and mass transfer, different adsorption kinetic models were applied in optimum conditions. The PFO, PSO, Elovich, LFD and IPD models were employed to analyze the P and N removal rate and find out the adsorption kinetics. The plots of linear form of the kinetic models for the adsorption of P and N ions were depicted in Figs S3 and S4, respectively. Table 4 summaries the calculated kinetic parameters of each model. A comparison of the values of correlation coefficients (R2) shows that the PSO model (R2 = 0.9998 for P ions and R2 = 0.9990 for N ions) was the best one in describing the behavior of adsorbent for removal of P and N species. Hence, the results stated that P and N chemisorption step was the rate-limiting step controlling the overall adsorption rate of both analytes by NCT@GO/H-Zr.
Temperature changes kinetic energy of a molecule and thus it impacts chemisorption. It is evident from Figs. 7d, 8d that the adsorption performance improved with increasing temperature. The improve in adsorption ability with temperature indicted that the binding sites, accessible for uptake have raised with temperature. The influences of temperature on the adsorption can be descripted by the assessment of the thermodynamic parameters. The calculated values of thermodynamic study are collected in Table 6, where ΔH and ΔS of P and N uptake onto Zr-functionalized nanocomposite are positive. The former demonstrates an endothermic nature of this process, while the latter implies that degree of randomness increases with temperature at the adsorbent-adsorbate interface.
The ΔG value, for both adsorbates, become more negative with the increase of temperature form 293 to 313 K, indicating the spontaneous character of the process at elevated temperature.
Simultaneous adsorption of P and N ions
The removal efficiency of the NCT@GO/H-Zr toward adsorbates in both single-component and binary (bi)-component systems was performed which is shown in Fig. 9. As can be seen, the P and N removal onto Zr-functionalized nanocomposite in both single-component and bi-component systems improved with raising the initial concentration of P and N solution, and the equilibrium curves exhibited a slow growth trend when the equilibrium concentration is near to 150 mg/L. In addition, the removal efficiency of P ions on NCT@GO/H-Zr was further than that of N ions in bi-component system, in good accordance with the tendency in single-component system. Results of simultaneous adsorption experiments showed that P and N were bound to same adsorption sites on NCT@GO/H-Zr, and the competition influence between them was almost significant during the simultaneous adsorption process. N ions cannot compete with P ions for active sites, and even the initial concentration of N ions was fewer than that of P ions. When binding sites were insufficient, P ions was suppressed by the competition of N ions. This behavior is mainly related to the surface properties of the adsorbent and ionic potential of adsorbates. Jóźwiak et al. (Jóźwiak et al. 2019) were synthesized CT for simultaneous adsorption of P and N ions, and they reported obvious competition between P and N ions.
Influence of competitor anions
The inorganic anions can compete with P and N anions to react with Zr-active sites of the nanocomposite. The competitive anions including bicarbonate (\( {\text{HCO}}_{3}^{ - } ) \), fluoride \( ({\text{F}}^{ - } \)), chloride (\( {\text{Cl}}^{ - } \)) and sulfate (\( {\text{SO}}_{4}^{2 - } \)) were investigated by keeping 150 mg/L of initial P and N concentration and 150 mg/L concentration of other inorganic anions at ambient temperature and were remained all other parameters as constant. The influence of these ions on the removal efficiency of both the adsorbates is presented in Fig. 10. It is important to note that the orders of the influence of different inorganic anions on the P and N removal was correlated with affinity of synthesized samples toward anions. The screen impact attained by divalent \( {\text{SO}}_{4}^{2 - } \) was demonstrated to be more substantial compared to the other univalent \( {\text{HCO}}_{3}^{ - } \), \( {\text{F}}^{ - } \) and \( {\text{Cl}}^{ - } \) ions. \( {\text{SO}}_{4}^{2 - } \) decreased the P and N adsorption performance because of its divalent anion with further charge density and have a tendency to adsorb more readily than monovalent anions. Results of binary solutions showed that P ion uptake was found to be better than N ion because of polyprotic nature of P ions and anions with greater ionic strength possess higher propensity to the NCT@GO/H-Zr surface.
Desorption and recyclability studies
Meanwhile the regeneration and reuse ability of the adsorbent is paramount essential for environment and economic point of view; thus, adsorption–desorption cycle studies were carried out to evaluate the performance of regenerating the NCT@GO/H-Zr.
In order to determine the regeneration ability of the NCT@GO/H-Zr, Na2CO3, NaCl, KCl and DI water, was tried as the desorption eluents for the desorption experiment. The saturated adsorbent was shaken with 20 mL of desorption solutions and the amount of both P and N desorbed in 2 h was detected by means of ultraviolet spectrophotometer. The regeneration outcomes are depicted in Fig. 11a.
Among these eluents, Na2CO3 possesses high percentages of desorption and was would be the best one. Therefore, a regeneration experiment for ten cycles was performed using 0.5 M Na2CO3 as a preferable eluting agent to understand the reuse potential of NCT@GO/H-Zr nanocomposite. Desorption and regeneration studies indicate that the amounts of recovery were negligible changed up to 10 cycles and the desorption percentage of P and N remained over 76% and 85%, respectively (Fig. 11b). This reduce can be due to the fact that the both adsorbates are adsorbed to the available surface sites of prepared sample via electrostatic attractions and are not completely reversible in successive adsorption-desoprtion cycles. In comparison, NCT@GO/H-Zr had a better affinity for P ions and illustrated a higher reusability for N ion removal during the regeneration cycles. The reusability of NCT@GO/H-Zr significantly recuperates its overall cost for practical application.
Water regain property
The stability of the adsorbents in aqueous medium was checked by the swelling ratio of the adsorbents. The swelling behavior of the synthesized samples was conducted at DI water. The swelling values of NCT, NCT@GO and NCT@GO/H-Zr were 20.2, 14.6 and 8.3%, respectively. This result confirms that the swelling percentage of NCT@GO/H-Zr was decreased when compared to NCT@GO and NCT.
Real samples analysis
The adsorption potential of any adsorbent from real samples is an important issue that must be consider. To evaluate the applicability of the synthesized nanocomposite for treating polluted water, real sample was collected from the nearby rural area of Alborz district, Iran. The real sample was filtered through 0.45 µm membrane filter before adsorption experiment to remove the suspended solid. Then 0.01 g NCT@GO/H-Zr was added into 20 ml water sample and the mixed solution was shaken in a thermostat shaker for 20 min at 313 K. Thereafter, the composite was filtered off, and the residual concentration of the P and N remained in liquid phase was determined by spectrophotometry at the maximum wavelength for each adsorbate. Results of the NCT@GO/H-Zr application for water sample are represented in Table 7. The values obtained confirm the good ability of the utilization of Zr-functionalized nanocomposite for the recovery of both anions. The methodology is effective, convenient and economical, therefore, it can be employed as a remediation technique.
Conclusions
The outcome of this study demonstrates the removal efficiency of synthesized NCS@GO, NCS@GO/L-Zr and NCS@GO/H-Zr nanocomposites towards P and N adsorption from aqueous media. Loading high and low amounts of Zr on the surface of NCT@GO could prepare a high-efficient P and N adsorbents. Compared to that of NCS@GO/L-Zr nanocomposite, the adsorption ability of NCS@GO/H-Zr was improved due to the further active sites on NCS@GO/H-Zr surface. The synthesized samples were characterized by N2 isotherms, FTIR, XRD, TGA, DTA, FESEM, EDS with mapping analysis and water regain property. Optimal values for adsorption of the both adsorbates by NCS@GO/H-Zr were achieved from the quadratic model obtained from CCD. The optimum results of temperature (313 K), pH (5), contact time (20 min), adsorbent dose (0.01 g) and initial concentration (150 mg/L) were recorded using the desirability function. Based on the negative values of ΔG at different temperatures and the positive values of ΔH and ΔS, it can be said that the adsorption of both the anions on NCS@GO/H-Zr is spontaneous and endothermic. In addition, there is a randomness increase in liquid-solid boundary of adsorbate and adsorbent. The equilibrium adsorption data were analyzed using Langmuir, Harkin-Jura, Jovanovic, Freundlich, Temkin, and DKR isotherm models, and the Freundlich model was found to be adequate in describing the removal of adsorbates onto the NCS@GO/H-Zr. The results revealed that the maximum adsorption performance of P and N with using this adsorbent were 172.41 mgP/g and 138.88 mgN/g, much further than that of most the reported values of other adsorbents. Employing the PFO, PSO, Elovich, LFD, and IPD models to examine the adsorption kinetics revealed that the PSO model best fits the experimental data. The simultaneous P and N adsorption performance indicate that adsorption ability of P was greater than N. The competitor anions study showed that P and N removal were slightly inhibited by \( {\text{HCO}}_{3}^{ - } \), \( {\text{F}}^{ - } \) and \( {\text{Cl}}^{ - } \), but, the adsorption of the both adsorbates was inhibited remarkably by \( {\text{SO}}_{4}^{2 - } \). Desorption and regeneration studies indicate that P and N anions could be recovered appropriately by 0.5 M Na2CO3 and the maximum removal efficiency was not affected up to the tenth cycle. Results of real samples experiments confirmed that the synthesized novel nanocomposite has promising applications in in the remediation of water eutrophication.
References
Agarwal S, Rajoria P, Rani A (2018) Adsorption of tannic acid from aqueous solution onto chitosan/NaOH/fly ash composites: Equilibrium, kinetics, thermodynamics and modeling. J Environ Chem Eng 6:1486–1499
Ali I (2014) Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev 43:175–205
Ali I, Gupta V (2006) Advances in water treatment by adsorption technology. Nat Protoc 1:2661
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183
Ali I, Alharbi OM, Tkachev A, Galunin E, Burakov A, Grachev VA (2018) Water treatment by new-generation graphene materials: hope for bright future. nviron. Sci Pollut Res 25:7315–7329
Anbia M, Salehi S (2016) Synthesis of polyaniline/mesoporous carbon nanocomposites and their application for CO2 sorption. J Polym Res 23:124
Antunes E, Jacob MV, Brodie G, Schneider P (2018) Isotherms, kinetics and mechanism analysis of phosphorus recovery from aqueous solution by calcium-rich biochar produced from biosolids via microwave pyrolysis. J Environ Chem Eng 6:395–403
Bagheri M, Younesi H, Hajati S, Borghei SM (2015) Application of chitosan-citric acid nanoparticles for removal of chromium (VI). Int J Biol Macromol 80:431–444
Banu HT, Meenakshi S (2017) One pot synthesis of chitosan grafted quaternized resin for the removal of nitrate and phosphate from aqueous solution. Int J Biol Macromol 104:1517–1527
Banu HT, Karthikeyan P, Meenakshi S (2018) Lanthanum (III) encapsulated chitosan-montmorillonite composite for the adsorptive removal of phosphate ions from aqueous solution. Int J Biol Macromol 112:284–293
Banu HT, Karthikeyan P, Meenakshi S (2019) Zr4+ ions embedded chitosan-soya bean husk activated bio-char composite beads for the recovery of nitrate and phosphate ions from aqueous solution. Int J Biol Macromol 130:573–583
Boeykens SP, Piol MN, Samudio Legal L, Saralegui AB, Vázquez C (2017) Eutrophication decrease: phosphate adsorption processes in presence of nitrates. J Environ Manag 203:888–895
Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164:1012–1018
Chatterjee S, Lee DS, Lee MW, Woo SH (2009) Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate. J Hazard Mater 166:508–513
Cui X, Dai X, Khan KY, Li T, Yang X, He Z (2016) Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour Technol 218:1123–1132
Cui X, Li H, Yao Z, Shen Y, He Z, Yang X, Ng HY, Wang C-H (2019) Removal of nitrate and phosphate by chitosan composited beads derived from crude oil refinery waste: sorption and cost-benefit analysis. J Clean Prod 207:846–856
Dewage NB, Liyanage AS, Pittman CU Jr, Mohan D, Mlsna T (2018) Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour Technol 263:258–265
Dharupaneedi SP, Anjanapura RV, Han JM, Aminabhavi TM (2014) Functionalized graphene sheets embedded in chitosan nanocomposite membranes for ethanol and isopropanol dehydration via pervaporation. Ind Eng Chem Res 53:14474–14484
Fan Y, Li Y, Wu D, Li C, Kong H (2017) Application of zeolite/hydrous zirconia composite as a novel sediment capping material to immobilize phosphorus. Water Res 123:1–11
Federation WE, Association APH (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Hamoudi S, Belkacemi K (2013) Adsorption of nitrate and phosphate ions from aqueous solutions using organically-functionalized silica materials: kinetic modeling. Fuel 110:107–113
Hu Q, Zhang Z (2019) Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: a theoretical analysis. J Mol Liq 277:646–648
Huang X, Liao X, Shi B (2009) Adsorption removal of phosphate in industrial wastewater by using metal-loaded skin split waste. J Hazard Mater 166:1261–1265
Huang W-Y, Li D, Liu Z-Q, Tao Q, Zhu Y, Yang J, Zhang Y-M (2014) Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La (OH) 3-modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem Eng J 236:191–201
Islam M, Patel R (2010) Synthesis and physicochemical characterization of Zn/Al chloride layered double hydroxide and evaluation of its nitrate removal efficiency. Desalination 256:120–128
Iyer K, Kunju A (1992) Extension of Harkins—Jura adsorption isotherm to solute adsorption. Colloids Surf 63:235–240
Jiang H, Chen P, Luo S, Tu X, Cao Q, Shu M (2013) Synthesis of novel nanocomposite Fe3O4/ZrO2/chitosan and its application for removal of nitrate and phosphate. Appl Surf Sci 284:942–949
Jovanovic D (1969) Physical adsorption of gases, I, Isotherms for monolayer and multilayer adsorption. Kolloid Z Z Polym 235:1203
Jóźwiak T, Filipkowska U, Szymczyk P, Kuczajowska-Zadrożna M, Mielcarek A (2017) The use of cross-linked chitosan beads for nutrients (nitrate and orthophosphate) removal from a mixture of P-PO4, N-NO2 and N-NO3. Int J Biol Macromol 104:1280–1293
Jóźwiak T, Filipkowska U, Szymczyk P, Mielcarek A (2019) Sorption of nutrients (orthophosphate, nitrate III and V) in an equimolar mixture of P-PO4, N–NO2 and N–NO3 using chitosan. Arab J Chem 12:4104–4117
Karthikeyan P, Banu HAT, Meenakshi S (2019) Synthesis and characterization of metal loaded chitosan-alginate biopolymeric hybrid beads for the efficient removal of phosphate and nitrate ions from aqueous solution. Int J Biol Macromol 130:407–418
Keshvardoostchokami M, Babaei S, Piri F, Zamani A (2017) Nitrate removal from aqueous solutions by ZnO nanoparticles and chitosan-polystyrene–Zn nanocomposite: kinetic, isotherm, batch and fixed-bed studies. Int J Biol Macromol 101:922–930
Kumar IA, Viswanathan N (2017) Fabrication of metal ions cross-linked alginate assisted biocomposite beads for selective phosphate removal. J Environ Chem Eng 5:1438–1446
Kumar IA, Viswanathan N (2019) Micro-encapsulation and hydrothermal tuning of amine decorated magnetic alginate hybrid beads for nitrate and phosphate remediation. J Taiwan Inst Chem Eng 102:283–296
Kumar IA, Jeyaprabha C, Meenakshi S, Viswanathan N (2019) Hydrothermal encapsulation of lanthanum oxide derived Aegle marmelos admixed chitosan bead system for nitrate and phosphate retention. Int J Biol Macromol 130:527–535
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li R, Wang JJ, Zhou B, Awasthi MK, Ali A, Zhang Z, Gaston LA, Lahori AH, Mahar A (2016) Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci Total Environ 559:121–129
Lin J, He S, Wang X, Zhang H, Zhan Y (2019) Removal of phosphate from aqueous solution by a novel Mg(OH)2/ZrO2 composite: adsorption behavior and mechanism. Colloids Surf A 561:301–314
Luo W, Huang Q, Zhang X, Antwi P, Mu Y, Zhang M, Xing J, Chen H, Ren S (2020) Lanthanum/Gemini surfactant-modified montmorillonite for simultaneous removal of phosphate and nitrate from aqueous solution. J Water Process Eng 33:101036
Mallakpour S, Hatami M (2019) Fabrication and characterization of pH-sensitive bio-nanocomposite beads havening folic acid intercalated LDH and chitosan: drug release and mechanism evaluation. Int J Biol Macromol 122:157–167
Mirhosseinian NS, Anbia M, Salehi S (2020) Preparation and characterization of superhydrophobic melamine and melamine-derived carbon sponges modified with reduced graphene oxide–TiO2 nanocomposite as oil absorbent materials. J Mater Sci 55:1536–1552
Negm NA, Hefni HHH, Abd-Elaal AAA, Badr EA, Abou Kana MTH (2020) Advancement on modification of chitosan biopolymer and its potential applications. Int J Biol Macromol 152:681–702
Orlando U, Baes A, Nishijima W, Okada M (2002) Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 48:1041–1046
Pan J, Gao B, Song W, Xu X, Yue Q (2020) Modified biogas residues as an eco-friendly and easily-recoverable biosorbent for nitrate and phosphate removals from surface water. J Hazard Mater 382:121073
Pooja D, Kumar P, Singh P, Patil S (2020) Sensors in water pollutants monitoring: role of material. Springer, New York
Qiang GYXJB, Yewei D (2013) Adsorption of sulfate onto Zr (IV) loaded cross-linked chitosan. Chin J Environ Eng 5:2019–2024
Rahmi L, Nurfatimah R (2018) Preparation of polyethylene glycol diglycidyl ether (PEDGE) crosslinked chitosan/activated carbon composite film for Cd2+ removal. Carbohydr Polym 199:499–505
Rajeswari A, Amalraj A, Pius A (2015) Removal of phosphate using chitosan-polymer composites. J. Environ Chem Eng 3:2331–2341
Rajeswari A, Amalraj A, Pius A (2016) Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J Water Process Eng 9:123–134
Rathod M, Mody K, Basha S (2014) Efficient removal of phosphate from aqueous solutions by red seaweed, Kappaphycus alverezii. J Clean Prod 84:484–493
Rodrigues LA, da Silva MLCP (2010) Thermodynamic and kinetic investigations of phosphate adsorption onto hydrous niobium oxide prepared by homogeneous solution method. Desalination 263:29–35
Salehi S, Anbia M (2019) Performance comparison of chitosan–clinoptilolite nanocomposites as adsorbents for vanadium in aqueous media. Cellulose 26:5321–5345
Salehi S, Hosseinifard M (2020) Highly efficient removal of phosphate by lanthanum modified nanochitosan-hierarchical ZSM-5 zeolite nanocomposite: characteristics and mechanism. Cellulose 5:6141–6152
Salehi S, Anbia M, Hosseiny AH, Sepehrian M (2018) Enhancement of CO2 adsorption on polyethylenimine functionalized multiwalled carbon nanotubes/Cd-nanozeolite composites. J Mol Struct 1173:792–800
Salehi S, Mandegarzad S, Anbia M (2020) Preparation and characterization of metal organic framework-derived nanoporous carbons for highly efficient removal of vanadium from aqueous solution. J Alloys Compd 812:152051
Saxena S, Tyson TA, Shukla S, Negusse E, Chen H, Bai J (2011) Investigation of structural and electronic properties of graphene oxide. Appl Phys Lett 99:013104
Shan W, Zhang D, Wang X, Wang D, Xing Z, Xiong Y, Fan Y, Yang Y (2019) One-pot synthesis of mesoporous chitosan-silica composite from sodium silicate for application in Rhenium(VII) adsorption. Microporous Mesoporous Mater 278:44–53
Shen H, Wang Z, Zhou A, Chen J, Hu M, Dong X, Xia Q (2015) Adsorption of phosphate onto amine functionalized nano-sized magnetic polymer adsorbents: mechanism and magnetic effects. RSC Adv 5:22080–22090
Shrock DL, Krasowski MD (2020) Chapter 23.5—Methemoglobinemia due to dietary nitrate. In: Ketha H, Garg U (eds) Toxicology cases for the clinical and forensic laboratory. Academic Press, Boca Raton, pp 469–472
Sowmya A, Meenakshi S (2014) A novel quaternized chitosan–melamine–glutaraldehyde resin for the removal of nitrate and phosphate anions. Int J Biol Macromol 64:224–232
Swain S, Dey R, Islam M, Patel R, Jha U, Patnaik T, Airoldi C (2009) Removal of fluoride from aqueous solution using aluminum-impregnated chitosan biopolymer. Sep Sci Technol 44:2096–2116
Tang T, Cao S, Xi C, Li X, Zhang L, Wang G, Chen Z (2020) Chitosan functionalized magnetic graphene oxide nanocomposite for the sensitive and effective determination of alkaloids in hotpot. Int J Biol Macromol 146:343–352
Teimouri A, Nasab SG, Vahdatpoor N, Habibollahi S, Salavati H, Chermahini AN (2016) Chitosan/Zeolite Y/Nano ZrO2 nanocomposite as an adsorbent for the removal of nitrate from the aqueous solution. Int J Biol Macromol 93:254–266
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Wang Z, Guo H, Shen F, Yang G, Zhang Y, Zeng Y, Wang L, Xiao H, Deng S (2015) Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4 +), nitrate (NO3−), and phosphate (PO43−). Chemosphere 119:646–653
Wang S, Ma X, Zheng P (2019) Sulfo-functional 3D porous cellulose/graphene oxide composites for highly efficient removal of methylene blue and tetracycline from water. Int J Biol Macromol 140:119–128
Wu F-C, Tseng R-L, Juang R-S (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35:613–618
Wu B, Fang L, Fortner JD, Guan X, Lo IM (2017) Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res 126:179–188
Xi Y, Mallavarapu M, Naidu R (2010) Preparation, characterization of surfactants modified clay minerals and nitrate adsorption. Appl Clay Sci 48:92–96
Xiong W, Peng J (2008) Development and characterization of ferrihydrite-modified diatomite as a phosphorus adsorbent. Water Res 42:869–4877
Yadav M, Ahmad S (2015) Montmorillonite/graphene oxide/chitosan composite: synthesis, characterization and properties. Int J Biol Macromol 79:923–933
Yan B, Zeng C, Yu L, Wang C, Zhang L (2018) Preparation of hollow zeolite NaA/chitosan composite microspheres via in situ hydrolysis-gelation-hydrothermal synthesis of TEOS. Microporous Mesoporous Mater 257:262–271
Yang L, Yang M, Xu P, Zhao X, Bai H, Li H (2017) Characteristics of nitrate removal from aqueous solution by modified steel slag. Water 9:757
Yazdi F, Anbia M, Salehi S (2019) Characterization of functionalized chitosan-clinoptilolite nanocomposites for nitrate removal from aqueous media. Int J Biol Macromol 130:545–555
Yin Q, Wang R, Zhao Z (2018) Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J Clean Prod 176:230–240
Zamparas M, Drosos M, Georgiou Y, Deligiannakis Y, Zacharias I (2013) A novel bentonite-humic acid composite material Bephos™ for removal of phosphate and ammonium from eutrophic waters. Chem Eng J 225:43–51
Zhang L, Liu X, Xia W, Zhang W (2014) Preparation and characterization of chitosan-zirconium (IV) composite for adsorption of vanadium (V). Int J Biol Macromol 64:155–161
Zhang J, Chen N, Tang Z, Yu Y, Hu Q, Feng C (2015) A study of the mechanism of fluoride adsorption from aqueous solutions onto Fe-impregnated chitosan. Phys Chem Chem Phys 17:12041–12050
Acknowledgments
The authors would like to thank the Materials and Energy Research Center (Grant No. 9911940) for the financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salehi, S., Hosseinifard, M. Optimized removal of phosphate and nitrate from aqueous media using zirconium functionalized nanochitosan-graphene oxide composite. Cellulose 27, 8859–8883 (2020). https://doi.org/10.1007/s10570-020-03382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03382-5