Abstract
Polypyrrole (PPy) has received extensive attention in supercapacitor electrodes due to its promising electrochemical activity, while the poor cycling stability and densely packed structure limited its electrochemical performance. Herein, we demonstrate the fabrication of flexible and freestanding PPy@bacterial cellulose (BC)/MXene composite film with significantly enhanced electrochemical performance. PPy nanoparticles were uniformly deposited on BC nanofibers via in situ polymerization, and assembled with highly conductive MXene (Ti3C2Tx) nanoflakes through strong interfacial interactions. BC as a biological template can effectively disperse PPy nanoparticles. The intercalation of PPy@BC nanofibers into Ti3C2Tx layers constructs hierarchically packed nanofibrous structure, which provides extensive accessible electrochemical active sites. Freestanding PPy@BC/Ti3C2Tx (PBM) electrode exhibits superior specific capacitance (gravimetric and areal capacitance of up to 550 F g−1 and 879 mF cm−2, respectively) and excellent capacitance retention of 83.5% after 10,000 cycles. In addition, the symmetric supercapacitor assembled by PBM papers present a high energy density of 33.1 W h kg−1 (power density of 243 W kg−1) and excellent capacitance retention. The elaborately designed nanostructure and PPy-Ti3C2Tx hybridization make great contribution to the enhanced electrochemical performance, which provide a feasible method for the fabrication of conductive polymer-based high-performance flexible supercapacitor electrodes.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid widespread of portable electronic devices requires the development of flexible energy storage systems (Simon and Gogotsi 2008). Supercapacitors (SCs) have been widely explored for energy storage application owing to their higher power density, cycle efficiency and rapid charge–discharge rates (Zhang and Zhao 2009; Choi et al. 2012; El-Kady et al. 2016). To date, pseudocapacitive materials such as metal oxides/hydroxides (e.g., RuO2 (Jiang et al. 2018), MnO2 (Zhu et al. 2018) and Ni(OH)2 (Ke et al. 2017)) or conductive polymers (e.g., polypyrrole (PPy) (Song et al. 2015; Kashani et al. 2016) and polyaniline (Ge et al. 2015)) have been widely used as electrode materials to promote electrochemical performance. Specifically, conductive polymers have become increasingly popular as pseudocapacitive electrode material for SCs in virtue of their low cost, good environmental stability (Wang et al. 2012; Huang et al. 2016; Wan et al. 2017), high conductivity in doped state and high specific capacitance (theoretical of PPy is 620 F g−1 (Lota et al. 2004)). However, pristine conductive polymers tend to agglomerate during polymerization, which impede the diffusion and transportation of electrolyte ions. Furthermore, conductive polymers suffer from poor cycling performance owing to the swelling-shrinking behavior during the ion intercalating/deintercalating process.
Recently, transition metal carbides and nitrides, known as MXenes, have arisen as a new family of two-dimensional (2D) functional materials (Lei et al. 2015; Liu et al. 2020; Jin et al. 2020). Among them, titanium carbide (Ti3C2Tx, where Tx stands for the surface termination groups) is the most extensively reported material due to its great conductivity (Geng et al. 2019), good hydrophilicity (Ghidiu et al. 2014), and excellent ion intercalation behavior (Lukatskaya et al. 2013; Mu et al. 2019). It has been demonstrated as promising supercapacitor electrode materials with outstanding electrochemical performance (specific capacitance up to 1500 F cm−3) (Anasori et al. 2017; Lukatskaya et al. 2017). Due to its unique advantages, Ti3C2Tx is expected to serve as desirable supporting materials for the conductive polymer-based electrode. Zhi et.al intercalated PPy into the Ti3C2Tx sheet via electrophoretic deposition, which increased the capacitance from 150 to 203 mF cm−2 (Zhu et al. 2016). Beidaghi et al. reported the oxidant-free polymerization of aniline on the Ti3C2Tx nanoflakes, which intercalated between the Ti3C2Tx layers expanding the interlayer space to facilitate charge transport (VahidMohammadi et al. 2018). Gogotsi et.al prepared hybrid electrodes by in-situ synthesis of pyrrole on Ti3C2Tx, in which the capacitance of the 13 μm thick electrode was as high as 416 F g−1 (Boota et al. 2016). The incorporation of MXene is a feasible strategy to improve the electrochemical performance of intrinsic conductive polymers.
Herein, we report the fabrication of flexible and freestanding PPy@bacterial cellulose (BC)/Ti3C2Tx film (PBM) with well-enhanced electrochemical performance. PBM with hierarchically packed nanofibrous structure was synthesized through BC templated pyrrole polymerization and subsequent self-assembly with Ti3C2Tx nanoflakes. Nanocellulose, such as cellulose nanofibrils (CNFs) and bacterial cellulose (BC), has proven to be a promising candidate for green novel energy device due to its excellent mechanical properties, large specific surface area, light weight and flexibility (Du et al. 2017; Wang et al. 2018; Li et al. 2018). For example, Edberg et al. combined cellulose nanofibrils, sulfonated lignin and conductive polymer to produce supercapacitors with excellent rate performance (Edberg et al. 2018); Zhang et al. used wood cellulose as the raw material and manipulated the carbonization temperature to control the nanofiber structure to obtain high-performance supercapacitors (Zhang et al. 2019). Compared with CNFs prepared by physical method and TEMPO-CNFs prepared by chemical method, BC has very high cellulose content (higher than 99%) and can be easily obtained through simple and non-toxic processing steps (Wu et al. 2016). Meanwhile, the crystallinity of BC comprised of 2–4 nm nanofibrils exceeds 70%, resulting in excellent mechanical robustness (Zhao et al. 2020). Based on the green chemistry and recycling economy, these 1D flexible, low-cost and environmentally friendly BC nanofibers with nanofibrous network are appealing substrate, serving not only as template for uniform deposition of PPy nanoparticles, but the resulted PPy@BC nanofibers intercalated into Ti3C2Tx layers can also effectively prevented restacking of Ti3C2Tx and expand their interlayer space. Moreover, the Ti3C2Tx nanoflakes were closely contacted with PPy@BC hybrid nanofibers to construct continuous conductive pathways. The synergistic enhancement effect endows PBM with good flexibility, high conductivity and facilitates charges/ions diffusion, which greatly contributed to the improved capacitance and cyclic stability. This work provides a simple and feasible strategy to design and fabricate MXene/conductive polymer-based SCs electrode with enhanced electrochemical performance.
Experimental section
Materials
All starting materials and reagents were obtained from commercial sources and used without further purification unless otherwise noted. BC aqueous dispersion was purchased from Qihong Co., Ltd, Guilin, China. Ferric chloride hexahydrate (FeCl3·6H2O, AR) was purchased from Kermel Chemical Reagent Co., Ltd. Lithium fluoride (LiF, AR) and Pyrrole (Py, AR) was purchased from Aladdin Inc. (China). Ti3AlC2 powder (400 mesh) was purchased from 11 Technology Co., Ltd. (China).
Preparation of delaminated MXene (Ti3C2Tx) nanoflakes
Ti3C2Tx was prepared by selectively etching off aluminum atoms in MAX powder. In a typical experiment, Ti3AlC2 powder was added to a 9 M hydrochloric acid solution (HCl) in which LiF salt was dissolved (Ti3AlC2:LiF = 1:1 wt./wt.). The etching solution was continuously stirred at 35 °C for 36 h. Subsequently, the etched Ti3C2Tx solid residue was washed with deionized water and centrifuged at 3500 rpm until the supernatant pH exceed 6. Then the sediment was re-dispersed in deionized water by hand-shaking, followed by centrifugation at 3500 rpm for 5 min to obtain the Ti3C2Tx nanoflakes aqueous dispersion. The concentration of the Ti3C2Tx solution was determined by vacuum filtration of a known weight of the supernatant into a film.
Preparation of PPy@BC conductive nanocomposites
The PPy@BC conductive nanocomposites are prepared by in-situ oxidative polymerization of pyrrole self-assembled onto BC. A typical synthesis was as follows. Freshly prepared BC aqueous dispersion (50 g, 0.2 wt%) was added to dimethyl formamide (DMF) solution (2:1, v/v) and stirred vigorously to fully disperse BC nanofibers at 25 °C. Pyrrole (6 mmol, 1 g) was added to the BC suspension and stirred vigorously for 0.5 h to fully self-assemble onto the surface of the BC nanofibers. Then, a mixed solution of ferric chloride hexahydrate (FeCl3·6H2O, 7 mmol, 1.89 g) and 1 M HCl (18 mmol) were added, in which FeCl3·6H2O was used as oxidant catalyst and HCl was used as dopant. The reaction temperature was controlled at 0–4 °C and the reaction time is 6–8 h. After reaction was completed, the resulting precipitate was sequentially washed with ethanol, deionized water and 1 M HCl to remove monomers and oxidants. Finally, the washed product was re-dissolved in 1 M HCl to make PPy@BC black suspension, and the PPy@BC concentration was determined by measuring the residual mass after drying a certain mass of the suspension. For reference, pristine PPy was also synthesized by oxidation polymerization of pyrrole with FeCl3·6H2O (7 mmol, 1.89 g) as oxidant and 1 M HCl (18 mmol) as dopant in H2O-DMF (2:1, v/v) at 4 °C.
Preparation of PBM conductive paper
Delaminated Ti3C2Tx nanoflakes and PPy@BC nanofibers were respectively dispersed in deionized water with a concentration of 0.2 wt% and 0.1 wt%. Different amount of Ti3C2Tx dispersion was added into the PPy@BC dispersion with stirring. The mixture of Ti3C2Tx and PPy@BC was continuously stirred for 4 h. Then the suspension was filtered through vacuum-assisted filtration to obtain PBM composite paper. The mass ratio of PPy@BC to Ti3C2Tx in the composite film were 7:1 5:1, 3:1, 1:1, 1:3 and 1:5, which were denoted as PBM 7:1 5:1, 3:1, 1:1, 1:3 and 1:5, respectively. Depending on the proportion of PPy@BC and Ti3C2Tx, the thickness of the prepared PBM conductive films were between 45 µm and 67 µm (details are shown in Table S1). PPy@BC and BC/Ti3C2Tx papers were also fabricated under the same conditions for comparison.
Characterizations and electrochemical measurements
The morphology and microstructure of BC, PPy@BC, BC/Ti3C2Tx and PBM composite paper were studied by scanning electron microscopy (SEM) on a microscope (JSM-5600, JEOL, Japan). Transmission electron microscopy (TEM) was performed using a transmission electron microscope (JEOL JEM-100CX, Japan) to intuitively observe the morphology of Ti3C2Tx nanoflakes, BC nanofibers, PPy@BC nanofibers and PBM composite. A Nicolet 6700 spectrophotometer (USA) with attenuated total reflectance (ATR) mode was used to collect Fourier transform infrared spectroscopy (FTIR) at ambient temperature. X-ray diffraction (XRD) patterns of Ti3C2Tx film, BC film, PPy@BC film and PBM composite film were recorded with a Philip X pert PRO MPD (Philips Co., Netherlands) X-ray diffractometer. Cu Kα radiation (λ = 1.54 Å) at a generator current of 50 mA was used as the radiation source and the generator voltage was set as 40 kV with a scanning speed of 4°/min. Tensile tests of samples were measured using a universal tensile testing machine (Instron-5560, USA) with an extension rate of 100 mm/min at room temperature. The electric resistance of all samples was measured by a four-point probe measurement with an M-3 mini type four-probe tester (Suzhou Jingge Electronic Co., Ltd., China).
VersaSTAT3 electrochemical workstation was employed to measure the electrochemical performance of the as-prepared electrodes. A plastic Swagelok cells was used to perform the electrochemical tests. The fabricated freestanding films, over capacitive activated carbon, and Ag/AgCl served as the working electrode, counter electrode, and reference electrode, respectively. A glassy carbon electrode was used as current collectors for the working electrodes. The electrochemical impedance spectroscopy (EIS) was conducted within a frequency range from 200 kHz to 0.01 Hz at an amplitude of 10 mV.
Results and discussion
Morphologies and structures
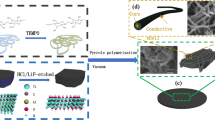
Figure 1 shows the strategy for the preparation of flexible and freestanding PBM composite film. The PPy@BC nanofibers were fabricated by in situ oxidative polymerization of pyrrole with BC as the biotemplate (Fig. 1a). A mixture of ferric chloride hexahydrate (FeCl3·6H2O) and hydrochloric acid (HCl) were added into the BC/pyrrole suspension as oxidant catalyst and dopant, respectively. Pyrrole monomers were deposited on the surface of BC through hydrogen bonding between pyrrole ring and BC. As reaction proceeded, the BC/pyrrole suspension was observed to change from semi-translucent ivory dispersion to suspended dark agglomerates, demonstrating that the surface of BC nanofibers was uniformly coated with the obtained PPy, which blocked their hydrophilic hydroxyl groups. Figure 1b schematically shows the synthesis process of Ti3C2Tx. The delaminated Ti3C2Tx dispersion was prepared by eliminating Al atomic layer in the Ti3AlC2 (MAX) phase structure through etching by LiF and HCl mixed solution and subsequent hand shaking delamination. TEM image (Fig. S1) shows that the delaminated Ti3C2Tx nanoflakes present flat and almost transparent lamellar morphology with average transverse size of several hundred nanometers. The delaminated Ti3C2Tx nanoflakes can form stable and uniform dispersion in aqueous solution. Then PPy@BC and Ti3C2Tx dispersions were mixed to obtain PBM dispersions (Fig. 1c), and freestanding flexible PBM films were obtained through vacuum-assisted filtration (Fig. 1d). The Ti3C2Tx nanoflakes closely contacted with PPy@BC nanofibers through hydrogen bonding. As a result, the PBM suspension exhibits black suspended agglomerates similar to the PPy@BC suspension.
Schematic illustration of the synthesis of PBM composite paper. a Schematic illustration of the synthesis of PPy@BC by in situ oxidative polymerization of pyrrole with BC as the template. b Schematic diagram of Ti3C2Tx obtained by selective etching of aluminum atomic layer from layered Ti3AlC2 phase. c Illustration and photograph of PPy@BC and Ti3C2Tx mixture. PPy@BC nanofibers rely on hydrogen bonding to make close contact with Ti3C2Tx nanoflakes. d Image of the freestanding PBM paper and demonstration of the flexibility of the freestanding PBM film
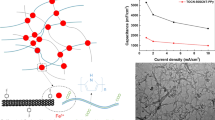
Figure 2a and d exhibit scanning electron microscope (SEM) and transmission electron microscope (TEM) images of pristine BC. BC nanofibers with a diameter between 10 and 40 nm exhibit smooth surface and nanofibrous network, which is similar to the morphology of previously reported BC nanofibers (Qiu et al. 2016). Their 1D structure and high aspect ratio makes an excellent template for loading active materials. Meanwhile, the existence of hydrogen bonding that acts as traction force between pyrrole monomer and BC hydroxyl group is a necessary guarantee for the uniform deposition of PPy on the BC surface (Ma et al. 2016a). After in situ polymerization, PPy nanoparticles with a diameter of 50–60 nm were uniformly grown on the surface of BC nanofibers to form string-bead-like structure (Fig. 2b, e), and the interconnected nanofibrous structure of BC was well-reserved after polymerization (the mass ratio of PPy and BC nanofibers in the synthesized PPy@BC is 7:1). SEM of the PBM (Fig. 2c) presents that Ti3C2Tx nanoflakes are embedded in interconnected PPy@BC nanofibers network without regional agglomeration. The uniform distribution of Ti3C2Tx in PBM is further demonstrated by elemental mapping images (Fig. S2). The nanofibrous structure in composite film is expected to provide accessible active sites and ion diffusion channels. As shown in Fig. 2f, intercalation of PPy@BC nanofibers into Ti3C2Tx nanoflakes to form intimate contact and prevent restacking, which are attributed to the hydrogen bonding forces between N–H and hydroxyl groups on PPy@BC and the oxygen containing groups on the surface of Ti3C2Tx. Their close contact and uniformly distribution of Ti3C2Tx nanoflakes facilitate the construction of continuous conductive pathway. The addition of Ti3C2Tx greatly improved electrical conductivity of the composite films (Fig. S3). The conductivity of PPy@BC is only 136 S m−1, while it significantly increased to 14,245 S m−1 when the mass ratio of PPy@BC to Ti3C2Tx is 1:5 (PBM 1:5).
For comparison, PPy/Ti3C2Tx suspension was prepared by directly mixing Ti3C2Tx with PPy nanoparticles dispersion (Fig. S4). The SEM image of PPy/Ti3C2Tx hybrid paper (Fig. S4a) clearly exhibits that large particles of PPy are distributed on Ti3C2Tx, forming severe dense packing. The simple mixed PPy/Ti3C2Tx dispersion is unable to form a complete film via natural drying or filtration as shown in Fig. S4b, indicating poor processability. In sharp contrast to PPy/Ti3C2Tx, the PBM composite films are highly flexible, bendable (Fig. 1d) and present appropriate mechanical strength (Fig. S5), indicating that the introduction of BC not only effectively regulates the morphological structure of PPy to facilitate dispersion, but also greatly improves the processability of nanocomposites.
FTIR spectroscopy and XRD analysis
PBM hybrid films were further characterized by Fourier transform infrared (FT-IR) spectroscopy and X-ray diffraction (XRD). As shown in Fig. 3a, pristine PPy exhibits a band at 1644 cm−1 due to the N–H in plane bending vibration, band at 1540 cm−1 corresponding to the C = C stretching vibration in the pyrrole ring, broad band at 1440 cm−1 attributed to the C–N stretching vibration, and band at 1160, 1045 cm−1 related to C–H in-plane ring stretching vibration (Liu et al. 2015; Ma et al. 2016a). For PPy@BC, the C–O stretching vibration peaks of BC (1157, 1049 cm−1) are redshifted to 1118, 1018 cm−1 respectively, indicating the hydrogen bonding between BC and PPy. The obvious peaks at 1648, 1533, 1437 and 1284 cm−1 are attributed to the N–H in plane bending vibration, C=C, C–N ring-stretching vibration and C–N in-plane deformation modes of PPy respectively (Wang et al. 2013; Xu et al. 2013), which demonstrates that BC nanofibers are wrapped with PPy. While for PBM composite film, the peaks belonging to PPy@BC are still retained (1645, 1533, 1435, 1284, 1120 and 1018 cm−1), but the characteristic peak of N–H wagging shifts from 862 to 840 cm−1 and became wider and stronger, indicating the formation of hydrogen bonding between Ti3C2Tx and PPy.
XRD patterns were shown in Fig. 3b, the pattern of pristine BC displays three sharp peaks at 14.6°, 16.8° and 22.7°, corresponding to the typical \((1\;\overline{1}\;0)\), (1 1 0) and (2 0 0) diffraction planes of cellulose I, respectively (Ma et al. 2016b). Pure PPy is known to be amorphous and has a broad diffraction peak at ≈ 25°, corresponding to the scattering of PPy chains (He et al. 2019). After polymerization of PPy onto the BC, the pattern of PPy@BC exhibits significantly weakened characteristic peaks of BC and PPy. While in the XRD pattern of PBM, the characteristic peaks of BC and PPy are retained, and a new sharp strong peak appears near 6° attributing to the incorporation of Ti3C2Tx (Naguib et al. 2014). The downshift from ≈ 5.96° to 5.78° in the Ti3C2Tx (002) peak of PBM indicates an increased interlayer spacing between Ti3C2Tx layers, which is attributed to the intercalation of PPy@BC nanofibers (Cao et al. 2018).
XPS spectroscopy
To further explore the interaction between PPy and Ti3C2Tx, the chemical state of N in the PPy backbone was revealed by X-ray photoelectron spectroscopy (XPS). Figure 3c shows the N1s spectra of PPy@BC. The core N1s spectrum has been deconvoluted into neutral nitrogen (–NH–) at 399.7 eV and protonation benzenoid amine (–N+H–) at 400.8 eV (Ge et al. 1994). After addition of Ti3C2Tx, a new Gaussian peak appeared at high energy tail (Fig. 3d), corresponding to the protonation quinonoid imine (–N+=). The polaron ratio (N+/N), defined as the area ratio between polaron peak (–N+H– and –N+=) and the total nitrogen peak (–NH–, –N+H– and –N+=), increased from 31 to 37%. This may be attributed to the coactions between PPy and Ti3C2Tx, such as hydrogen bonding between the –OH groups of Ti3C2Tx flakes and the nitrogen lone pairs of PPy. Polarons are generally considered to provide electrochemical activity (Song et al. 2014). The increase in the polaron indicates that there may be more ions and electrons to contribute to increase the electrochemical activity.
Electrochemical performances of PBM films in three-electrode system
The electrochemical performances of the fabricated freestanding films were evaluated in a three-electrode system with Ag/AgCl electrode as reference electrode. 3 M H2SO4 was used as electrolyte due to its high conductivity, which facilitates the surface redox reactions of PPy (Boota et al. 2015). Figure 4a compares the CV curves of PBM films with different PPy and Ti3C2Tx contents, all of which are quasi-rectangle, indicating excellent electrochemical reversibility. As PPy content increases, the area of the curve gradually increases. PBM 5:1 exhibits highest capacitance (550 F g−1, 879 mF cm−2, the active material loading is 3.21 mg cm−2), which is among the best values of previous reported similar electrode materials (Table S2). This may be attributed to the uniform regulation of morphology of PPy nanoparticles via BC biotemplate polymerization and the intercalation of nanofibers into Ti3C2Tx layers, resulting in more accessible active sites and high electrochemical activity. PBM 5:1 in the GCD curve (Fig. 4b) has longest discharge time, typical quasi-triangular shape and low IR drop, indicating optimized charge transfer and charge storage capability. Figure 4c intuitively compares the gravimetric capacitance and area capacitance of different PBM films. The capacitance of PBM 5:1 is 8.2% and 38.7% higher than that of PBM 3:1 (505 F g−1, the active material loading is 2.80 mg cm−2) and PBM 7:1 (337 F g−1, the active material loading is 2.27 mg cm−2), respectively. The possible reason is that the optimum loading of Ti3C2Tx in PBM composite reached a good balance between high charge density and Faraday capacitance provided by p-type doped PPy and excellent electrical conductivity resulted from Ti3C2Tx (Snook et al. 2011; Xiong et al. 2018). It is worth noting that Ti3C2Tx exhibits higher redox capacitance at negative potentials (Boota and Gogotsi 2019), so the PBM 1:1 (the active material loading is 3.32 mg cm−2) hybrid electrode with more Ti3C2Tx has the lowest capacitance in our voltage window. Therefore, PBM 5:1 was chosen for the comparative study of electrochemical performance (Other PBM films are shown in Fig. S6). The CV curve (Fig. 4d) exhibits a typical EDLC rectangular shape below 100 mV s−1, indicating good capacitive behavior. As scan rate increases, the rectangular shape of CV curve gradually deviated, which may be caused by the polarization of electrode and increase of the overpotential of ion transport between electrolyte and electrode material (Zhang et al. 2014). The quasi-triangular galvanostatic charging-discharging curves (GCD) at different current densities of 1, 2, 4, 6, 8 and 10 mA cm−2 (Fig. 4e) further demonstrate the high reversibility of charging-discharging process.
Characterization of electrochemical properties of PBM composites: a Comparison of cyclic voltammograms (CV) curves of PBM 1:1 3:1 5:1 and 7:1 at scan rate of 5 mV s−1. b Galvanostatic charge–discharge (GCD) curves of PBM 1:1 3:1 5:1 and 7:1 at 1 mA cm−2. c Effect of different PPy@BC and Ti3C2Tx contents on gravimetric capacitance and areal capacitance measured at 5 mV s−1. The dotted line between the points is for visual viewing. d CV curves of PBM 5:1 film at various scan rates. e GCD curves of PBM 5:1 film at various current densities. f Nyquist impedance plots of PBM 1:1 3:1 5:1 and 7:1. The inset magnifies the high-frequency region
Electrochemical impedance spectroscopy (EIS) measurements were performed to understand the kinetics of electrode process. Nyquist plots (Fig. 4f) of PBM films include two regions, high frequency region of semi-circular arc and low frequency region of straight line, respectively. All curves have similar approximate vertical lines in the low frequency region, indicating rapid diffusion of ions between electrolyte solution and electrode interface. The diameter of semi-circular arc in the high frequency region is usually related to charge transfer resistance. And the smallest semicircle of PBM 5:1 indicates that the proper addition of Ti3C2Tx greatly enhances electrical conductivity of flexible film. As a result, PBM 5:1 presents well-balanced conductivity and charge density, which leads to improved capacitance. Further studies were conducted on sample PBM 5:1.
Comparative study of PBM with PPy@BC and BC/Ti3C2Tx composite electrode films were conducted to further elucidate the electrochemical properties of PBM as shown in Fig. 5. The CV curve of BC/Ti3C2Tx has a quasi-rectangular shape in the potential range of 0–0.5 V (Fig. 5a), while the introduction of PPy leads to extended potential range (from 0 to 0.8 V) and significantly improved gravimetric capacitance (550 F g−1 for PBM 5:1, 53.6% higher than that of BC/Ti3C2Tx 255 F g−1 and the active material loading of BC/Ti3C2Tx is 8.16 mg cm−2). PPy uniformly deposited on the surface of BC nanofibers provides extensive accessible active sites and excellent electrochemical activity, which significantly improve the capacitance. Furthermore, the specific capacitance of PBM 5:1 is 30.9% higher than PPy@BC (380 F g−1, the active material loading is 2.68 mg cm−2), and the areal capacitance of PBM 5:1 (879 mF cm−2) is 42.1% higher than PPy@BC (509 mF cm−2). The improved capacitance is attributed to the uniform distribution of Ti3C2Tx nanoflakes incorporated on the surface of nanofibers, which constructed continuous conductive pathways and increased long-range transport channels of electrons and ions (schematic illustration displayed in Fig. 5f). The GCD curves of PBM 5:1, PPy@BC and BC/Ti3C2Tx films (Fig. 5b) present approximate triangle shapes, indicating high reversibility of redox reactions and good coulombic efficiency. PBM 5:1 has the longest discharge time, indicating its highest capacitance. The electrochemical performance of pristine PPy, PPy@BC, and BC/Ti3C2Tx composites are shown in Fig. S7. Figure 5c compares the Nyquist plots of PBM 5:1, PPy@BC and BC/Ti3C2Tx. The low real-axis intercept value for each sample indicates low series resistance, which makes them attractive for supercapacitor electrodes. The plot of PBM 5:1 is almost vertical in the low frequency region, indicating its excellent capacitive behavior. For comparison, the straight line of BC/Ti3C2Tx is inclined, corresponding to high ion diffusion impedance. In high frequency region, the width of semi-circular arc of PBM 5:1 is between PPy@BC and BC/Ti3C2Tx, which demonstrates that the addition of Ti3C2Tx effectively reduces charge transfer resistance.
Comparison of electrochemical performance: a Cyclic voltammograms (CV) at 5 mV s−1. b Galvanostatic charge–discharge cycles (GCD) at current densities of 1 mA cm−2. c Nyquist plots. The inset magnifies the high-frequency region. d Gravimetric capacitance of PBM 5:1, PPy@BC and pristine PPy versus different scan rate. e Cycling stability of PBM 5:1 and PPy@BC electrode measured at 20 mV s−1, confirming the high cycle stability of PPy in the PBM composite. f Schematic illustration of ions and electrons transport in PBM electrode
Figure 5d compares rate-dependent behavior of PBM 5:1, PPy@BC, and pristine PPy. The dense packing structure of PPy leads to reduced active site and hinders in-time ions intercalation/extraction, resulting in capacitance retention of 45% at 100 mV s−1. However, the capacitance retention rates of PPy@BC and PBM 5:1 at 100 mV s−1 are 59% and 65%, respectively. This is attributed to the fact that BC template effectively regulates the aggregation mode of PPy resulting in uniform dispersion and high conductivity of Ti3C2Tx. Figure 5e shows cycling stability of PBM 5:1 and PPy@BC. Generally, pristine PPy exhibits poor cycling stability due to structural polarization and counterion drain effect (Heinze et al. 2010; Liu et al. 2014). The capacitance retention of PPy@BC drops to 67.3% after 10,000 cycles at 20 mV s−1. To contrast, the capacitance retention of PBM 5:1 is as high as 83.5%. The well-improved capacitance retention during cycling may be attributed to close contact between PPy@BC and Ti3C2Tx nanoflakes which effectively inhibit swelling-shrinking during ion intercalating and deintercalating process.
Symmetric supercapacitor
In order to further evaluate the capacitor devices performance of freestanding PBM composite papers, electrochemical characterization was performed by sandwiching two PBM 5:1 films into a symmetric supercapacitor (Fig. 6). The CV curves (Fig. 6a and S8) appear distortion because scan rate increases from 1 to 100 mV s−1 resulting in potential polarization of the PBM electrode. The GCD curves at different current densities (Fig. 6b) present quasi-triangle shapes, revealing low resistance, fast ion and electron transfer kinetics and high coulombic efficiency (Boota and Gogotsi 2019). Gravimetric capacitance of PBM symmetric supercapacitor was calculated from discharge curve of GCD (Fig. 6c). The capacitance at current density of 1 mA cm−2 is 294 F g−1. As the current of charging and discharging process gradually increases, PBM electrode exhibits great capacitance retention. The capacitance is 236 F g−1 when current density is increased to 10 mA cm−2, which is only 19.7% lower than the value of 1 mA cm−2. In addition, the energy density of up to 33.1 W h kg−1 (power density of 243 W kg−1) can be obtained with specific capacitance at 1 mA cm−2, surpassing most previous reported similar symmetric supercapacitor (Fig. 6d and detailed comparison data is given in Table S3). High capacitance, excellent energy density and outstanding capacitance retention of PBM symmetric supercapacitors can be attributed to the well-designed PBM nanostructures and synergies between components. EIS curve (Fig. 6e) shows a small semi-circular arc in the high frequency region and a nearly vertical straight line in the low frequency region, which also reveal that the device has low transfer resistance and fast ion electron transport. Additionally, three PBM symmetric supercapacitors were assembled in series into one energy storage device. After charging to 3 V, it is demonstrated that a red LED light can be easily illuminated (Fig. 6f).
Electrochemical performance of PBM symmetric supercapacitors: a Cyclic voltammetry curves of PBM symmetric supercapacitors at different scan rate. b Galvanostatic charge–discharge curves at various current densities. c Gravimetric capacitance under different current densities. The illustration shows the structure of PBM symmetrical two-electrode supercapacitor. d Ragone plots of PBM symmetric supercapacitor in comparison to other PPy-based symmetric supercapacitors. e Nyquist plots. The inset magnifies the high-frequency region. f Three PBM symmetric supercapacitors in series illuminate a red LED light
Conclusions
In summary, we demonstrate a facile strategy for the fabrication of PPy@BC/Ti3C2Tx composite film through in situ polymerization of PPy on BC nanofibers and their self-assembly with Ti3C2Tx nanoflakes. As a template, BC effectively regulates the morphology of PPy nanoparticles to prevent dense packing, and intimately contacts Ti3C2Tx nanoflakes through hydrogen bonding, resulting a hierarchically packed nanofibrous structure and conductive pathways. The elaborately designed structure and the synergistic effect impart PBM desirable electrical conductivity (up to 14,245 S m−1) and enhanced electrochemical performance. PBM composite film exhibits high specific capacitance (gravimetric capacitance of 550 F g−1, area capacitance of 879 mF cm−2) and excellent cycling stability (capacitance retention rate is 83.5% after 10,000 cycles) as freestanding supercapacitor electrode. Furthermore, the as-prepared symmetrical supercapacitor of sandwiched freestanding PBM papers shows high capacitance of 294 F g−1, desirable energy density (33.1 W h kg−1 at power density of 243 W kg−1) and great capacitance retention at high current density. This facile strategy demonstrates a feasible approach for the fabrication of freestanding high-performance conductive polymer-based SCs electrode.
References
Anasori B, Lukatskaya MR, Gogotsi Y (2017) 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater 2:16098. https://doi.org/10.1038/natrevmats.2016.98
Boota M, Gogotsi Y (2019) MXene—conducting polymer asymmetric pseudocapacitors. Adv Energy Mater 9:1802917. https://doi.org/10.1002/aenm.201802917
Boota M, Hatzell KB, Kumbur EC, Gogotsi Y (2015) Towards high-energy-density pseudocapacitive flowable electrodes by the incorporation of hydroquinone. Chemsuschem 8:835–843. https://doi.org/10.1002/cssc.201402985
Boota M, Anasori B, Voigt C et al (2016) Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv Mater 28:1517–1522. https://doi.org/10.1002/adma.201504705
Cao W-T, Chen F-F, Zhu Y-J et al (2018) Binary strengthening and toughening of MXene/cellulose nanofiber composite paper with nacre-inspired structure and superior electromagnetic interference shielding properties. ACS Nano 12:4583–4593. https://doi.org/10.1021/acsnano.8b00997
Choi BG, Yang M, Hong WH et al (2012) 3D macroporous graphene frameworks for supercapacitors with high energy and power densities. ACS Nano 6:4020–4028. https://doi.org/10.1021/nn3003345
Du X, Zhang Z, Liu W, Deng Y (2017) Nanocellulose-based conductive materials and their emerging applications in energy devices—a review. Nano Energy 35:299–320. https://doi.org/10.1016/j.nanoen.2017.04.001
Edberg J, Inganäs O, Engquist I, Berggren M (2018) Boosting the capacity of all-organic paper supercapacitors using wood derivatives. J Mater Chem A 6:145–152. https://doi.org/10.1039/C7TA06810G
El-Kady MF, Shao Y, Kaner RB (2016) Graphene for batteries, supercapacitors and beyond. Nat Rev Mater 1:16033. https://doi.org/10.1038/natrevmats.2016.33
Ge H, Qi G, Kang E-T, Neoh KG (1994) Study of overoxidized polypyrrole using X-ray photoelectron spectroscopy. Polymer 35:504–508. https://doi.org/10.1016/0032-3861(94)90503-7
Ge D, Yang L, Fan L et al (2015) Foldable supercapacitors from triple networks of macroporous cellulose fibers, single-walled carbon nanotubes and polyaniline nanoribbons. Nano Energy 11:568–578. https://doi.org/10.1016/j.nanoen.2014.11.023
Geng L, Zhu P, Wei Y et al (2019) A facile approach for coating Ti3C2Tx on cotton fabric for electromagnetic wave shielding. Cellulose 26:2833–2847. https://doi.org/10.1007/s10570-019-02284-5
Ghidiu M, Lukatskaya MR, Zhao M-Q et al (2014) Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516:78–81. https://doi.org/10.1038/nature13970
He W, Zhao G, Sun P et al (2019) Construction of Longan–like hybrid structures by anchoring nickel hydroxide on yolk–shell polypyrrole for asymmetric supercapacitors. Nano Energy 56:207–215. https://doi.org/10.1016/j.nanoen.2018.11.048
Heinze J, Frontana-Uribe BA, Ludwigs S (2010) Electrochemistry of conducting polymers—persistent models and new concepts. Chem Rev 110:4724–4771. https://doi.org/10.1021/cr900226k
Huang Y, Li H, Wang Z et al (2016) Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 22:422–438. https://doi.org/10.1016/j.nanoen.2016.02.047
Jiang Q, Kurra N, Alhabeb M et al (2018) All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv Energy Mater 8:1703043. https://doi.org/10.1002/aenm.201703043
Jin X, Wang J, Dai L et al (2020) Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem Eng J 380:122475. https://doi.org/10.1016/j.cej.2019.122475
Kashani H, Chen L, Ito Y et al (2016) Bicontinuous nanotubular graphene–polypyrrole hybrid for high performance flexible supercapacitors. Nano Energy 19:391–400. https://doi.org/10.1016/j.nanoen.2015.11.029
Ke Q, Guan C, Zhang X et al (2017) Surface-charge-mediated formation of H-TiO2@Ni(OH)2 heterostructures for high-performance supercapacitors. Adv Mater 29:1604164. https://doi.org/10.1002/adma.201604164
Lei J-C, Zhang X, Zhou Z (2015) Recent advances in MXene: preparation, properties, and applications. Front Phys 10:276–286. https://doi.org/10.1007/s11467-015-0493-x
Li S-C, Hu B-C, Ding Y-W et al (2018) Wood-derived ultrathin carbon nanofiber aerogels. Angew Chem Int Ed 57:7085–7090. https://doi.org/10.1002/anie.201802753
Liu T, Finn L, Yu M et al (2014) Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett 14:2522–2527. https://doi.org/10.1021/nl500255v
Liu Y, Zhou J, Tang J, Tang W (2015) Three-dimensional, chemically bonded polypyrrole/bacterial cellulose/graphene composites for high-performance supercapacitors. Chem Mater 27:7034–7041. https://doi.org/10.1021/acs.chemmater.5b03060
Liu J, Liu Z, Zhang H-B et al (2020) Ultrastrong and highly conductive MXene-based films for high-performance electromagnetic interference shielding. Adv Elect Mater 6:1901094. https://doi.org/10.1002/aelm.201901094
Lota K, Khomenko V, Frackowiak E (2004) Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J Phys Chem Solids 65:295–301. https://doi.org/10.1016/j.jpcs.2003.10.051
Lukatskaya MR, Mashtalir O, Ren CE et al (2013) Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341:1502–1505. https://doi.org/10.1126/science.1241488
Lukatskaya MR, Kota S, Lin Z et al (2017) Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat Energy 2:17105. https://doi.org/10.1038/nenergy.2017.105
Ma L, Liu R, Niu H et al (2016a) Freestanding conductive film based on polypyrrole/bacterial cellulose/graphene paper for flexible supercapacitor: large areal mass exhibits excellent areal capacitance. Electrochim Acta 222:429–437. https://doi.org/10.1016/j.electacta.2016.10.195
Ma L, Liu R, Niu H et al (2016b) Flexible and freestanding supercapacitor electrodes based on nitrogen-doped carbon networks/graphene/bacterial cellulose with ultrahigh areal capacitance. ACS Appl Mater Interfaces 8:33608–33618. https://doi.org/10.1021/acsami.6b11034
Mu X, Wang D, Du F et al (2019) Revealing the pseudo-intercalation charge storage mechanism of mxenes in acidic electrolyte. Adv Funct Mater. https://doi.org/10.1002/adfm.201902953
Naguib M, Mochalin V, Barsoum M et al (2014) 25th Anniversary article: MXenes: a new family of two-dimensional materials. Adv Mater 26:992–1005. https://doi.org/10.1002/adma.201304138
Qiu Y, Qiu L, Cui J, Wei Q (2016) Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater Sci Eng C 59:303–309. https://doi.org/10.1016/j.msec.2015.10.016
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854. https://doi.org/10.1038/nmat2297
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sour 196:1–12. https://doi.org/10.1016/j.jpowsour.2010.06.084
Song Y, Xu J-L, Liu X-X (2014) Electrochemical anchoring of dual doping polypyrrole on graphene sheets partially exfoliated from graphite foil for high-performance supercapacitor electrode. J Power Sour 249:48–58. https://doi.org/10.1016/j.jpowsour.2013.10.102
Song Y, Liu T-Y, Xu X-X et al (2015) Pushing the cycling stability limit of polypyrrole for supercapacitors. Adv Funct Mater 25:4626–4632. https://doi.org/10.1002/adfm.201501709
VahidMohammadi A, Moncada J, Chen H et al (2018) Thick and freestanding MXene/PANI pseudocapacitive electrodes with ultrahigh specific capacitance. J Mater Chem A 6:22123–22133. https://doi.org/10.1039/C8TA05807E
Wan C, Jiao Y, Li J (2017) Flexible, highly conductive, and free-standing reduced graphene oxide/polypyrrole/cellulose hybrid papers for supercapacitor electrodes. J Mater Chem A 5:3819–3831. https://doi.org/10.1039/C6TA04844G
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828. https://doi.org/10.1039/C1CS15060J
Wang H, Bian L, Zhou P et al (2013) Core–sheath structured bacterial cellulose/polypyrrole nanocomposites with excellent conductivity as supercapacitors. J Mater Chem A 1:578–584. https://doi.org/10.1039/C2TA00040G
Wang Y, Lin X, Liu T et al (2018) Wood-derived hierarchically porous electrodes for high-performance all-solid-state supercapacitors. Adv Funct Mater 28:1806207. https://doi.org/10.1002/adfm.201806207
Wu Z-Y, Liang H-W, Chen L-F et al (2016) Bacterial cellulose: a robust platform for design of three dimensional carbon-based functional nanomaterials. Acc Chem Res 49:96–105. https://doi.org/10.1021/acs.accounts.5b00380
Xiong D, Li X, Bai Z, Lu S (2018) Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small 14:1703419. https://doi.org/10.1002/smll.201703419
Xu J, Zhu L, Bai Z et al (2013) Conductive polypyrrole–bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org Electron 14:3331–3338. https://doi.org/10.1016/j.orgel.2013.09.042
Zhang LL, Zhao XS (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38:2520–2531. https://doi.org/10.1039/B813846J
Zhang Z, Xiao F, Qian L et al (2014) Facile synthesis of 3D MnO2–graphene and carbon nanotube–graphene composite networks for high-performance, flexible, all-solid-state asymmetric supercapacitors. Adv Energy Mater 4:1400064. https://doi.org/10.1002/aenm.201400064
Zhang Z, Li L, Qing Y et al (2019) Manipulation of nanoplate structures in carbonized cellulose nanofibril aerogel for high-performance supercapacitor. J Phys Chem C 123:23374–23381. https://doi.org/10.1021/acs.jpcc.9b06058
Zhao D, Zhu Y, Cheng W et al (2020) Cellulose-based flexible functional materials for emerging intelligent electronics. Adv Mater. https://doi.org/10.1002/adma.202000619
Zhu M, Huang Y, Deng Q et al (2016) Highly flexible, freestanding supercapacitor electrode with enhanced performance obtained by hybridizing polypyrrole chains with MXene. Adv Energy Mater 6:1600969. https://doi.org/10.1002/aenm.201600969
Zhu S, Li L, Liu J et al (2018) Structural directed growth of ultrathin parallel birnessite on β-MnO2 for high-performance asymmetric supercapacitors. ACS Nano 12:1033–1042. https://doi.org/10.1021/acsnano.7b03431
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51861165203, 51903166) and China Postdoctoral Science Foundation (2019M653398). We would like to thank the Analytical & Testing Centre of Sichuan University for XRD, SEM, TEM, FTIR and we would be grateful to Guiping Yuan for her help of TEM work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, Q., Zhan, Z., Chen, B. et al. Biotemplate synthesis of polypyrrole@bacterial cellulose/MXene nanocomposites with synergistically enhanced electrochemical performance. Cellulose 27, 7475–7488 (2020). https://doi.org/10.1007/s10570-020-03310-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03310-7