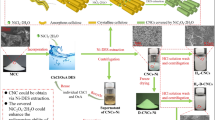
Abstract
Cellulose nanocrystals (CNCs) have been widely used as renewable materials and tough nano-composites due to its excellent properties. Currently, the preparation methods of CNCs require substantial energy and time consumption and the use of toxic chemicals. Herein, cotton and other biomass feedstocks were treated with deep eutectic solvents (DESs) and subsequent high-pressure homogenization (HPH), which was a simple preparation procedure for CNCs. The morphological, spectroscopic, and stability properties of the as-prepared rod-shaped CNCs were characterized using zeta potential, X-ray diffraction, transmission electron microscopy, scanning electron microscopy, fourier transform infrared spectroscopy and thermogravimetric techniques. CNCs with a diameter range of 50–100 nm and a length range of 500–800 nm were successfully assembled. The resulting CNC suspension was stable even after one month of storage. The solvent could be recycled successfully and reused for at least three additional pretreatment cycles while maintaining its pretreatment capability. The use of a DES as both the catalyst and solvent introduces a green chemical process that does not produce any hazardous waste and is an economical process because of the high recyclability (> 85%). The molecular structure changes of cellulose after HPH and DES treatment were also discussed. This is the first report on the recycling and reuse of a DES after preparing CNCs.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, a series of new applications has been developed for the value-added utilization of cotton because of its high concentration of cellulose (approximately 87–90%) (Abdel-Halim 2012; Morais et al. 2013). Cellulose nanocrystals (CNCs) have received much attention for their excellent functionalities, such as high strength, high hydrophilicity, high tensile strength, high dispersion, high rheology and large specific surface area compared to that of other cellulose forms (Li et al. 2017; Park et al. 2013). CNCs are regarded as a high value-added material that can be applied in electronic devices, environmental protection, tissue engineering, cosmetics, drug carriers and other fields, with excellent performance and broad application prospects (Alomayri and Low 2018; Kose et al. 2019; Lu et al. 2016).
CNCs are usually fabricated by direct mechanical treatment, mineral acid hydrolysis or biological treatments (Lee et al. 2018; Li et al. 2012; Wang et al. 2015, 2017). However, these traditional methods have always showed some disadvantages in CNC production. For example, inorganic acid hydrolysis and biological treatment were limited by the harsh reaction conditions, high cost, and long reaction period during the pretreatment process. On the other hand, the mechanical method has been widely studied in the preparation of CNCs due to its simplicity and high efficiency (Ma et al. 2019; Wang et al. 2017). In this regard, it is found that liquid homogeneous nanotechnology is simple and efficient to overcome the hydrogen bonding interactions of cellulose (in which cellulose substrate is first dissolved in a homogeneous solution and recombined by high-pressure shear; then, the regenerated CNCs are obtained), and it is considered to be a promising and sustainable alternative (Li et al. 2016; Zuluaga et al. 2009). Therefore, the development of environmentally friendly or energy-saving methods is still a substantial challenge in the preparation of CNCs, and finding an effective cellulose solvent is essential. Deep eutectic solvent, a new kind of solvent consisting of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), is a kind of green ionic liquid synthesized using either anionic, cationic, or nonionic species; it has rapidly emerged in the current literature and has received increasing attention (Hammond et al. 2017a, b; Procentese et al. 2018; Smith et al. 2014; Zhang et al. 2012a, b; Lynam et al. 2017). The hydrogen bonds in the DES form a strong interaction, enabling this solvent to be applicable for various materials, including hydroxyl-rich polymers (Zuo et al. 2017, 2018; Hammond et al. a, 2017b; Ling et al. 2019; Zhang et al. 2012a, b). DES can be generated with low-toxicity natural ingredients such as choline chloride and organic acids so that they always have advantageous antibacterial, catalytic and solubility properties. The cellulose surface can be modified during CNC preparation because it contains a large number of hydroxyl groups (Laitinen et al. 2017a; Xu et al. 2016). The quality of CNCs was normally improved after modification of natural cellulose fibres prior to CNC release (e.g., a more uniform particle size distribution), and the ion exchange capacity was enhanced (Crawford et al. 2016; Willberg-Keyriläinen et al. 2018). In addition, the energy consumption of CNC production was reduced after pretreatment, which was usually dependent on the carboxyl group entering the cellulose via carboxymethylation in most cases (Liu et al. 2017; Sirviö et al. 2015). Ling et al. fabricated CNCs from cotton at 80 and 100 °C using a DES (with choline chloride: oxalic acid dihydrate ratios of 1:1, 1:2 and 1:3) to investigate the side effects of the fabrication and effect of super-molecular modifications of cellulose (Ling et al. 2019). Their results showed that the breakdown of hydrogen bonding was more significant at a higher acid ratio (choline chloride: oxalic acid = 1:3) in the DES treatment, by which CNCs with better dispersion and a higher aspect ratio might be assembled.
In this study, the feasibility of using a DES (choline chloride and oxalic acid) as a pretreatment solvent in the context of cotton biomass CNC production was investigated. To the best of our knowledge, the impact of a recyclable organic acid-based DES on cellulose dissolution efficiency has not yet been reported. In this study, the dissolution of cellulose by DESs overcomes the large resistance of cellulose chains in the solid state. Moreover, the fibre dissolution over time was observed by high-power microscopy. The morphology and physicochemical properties of the as-prepared CNCs were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD) and thermogravimetric analysis (TGA). In addition, the degradation and generation mechanisms of CNCs were studied by FT-IR spectroscopy and CP/MAS 13C NMR spectroscopy. The molecular structure changes of cellulose after HPH and DES treatment were also discussed. Finally, the recyclability of the DES was studied.
Experimental section
Materials
Scoured cotton fibre was obtained from Macklin Biochemical Co., Ltd., Shanghai, China. The cotton fibre was dispersed in water, filtered, washed with ethanol and dried in an oven for 24 h at 60 °C. Choline chloride (ChCl) and oxalic acid dehydrate (Oa) were purchased from Aladdin Biotechnology Co., Ltd., Shanghai, China. The reagents were used of analytical grade without further processing. Deionized water was used throughout the experiments.
Hydrolysis of cotton cellulose using a DES
The DES was prepared by mixing 21.02 g of ChCl and 18.98 g of Oa at 90 °C for approximately 45 min (Scheme 1). After a clear solution was obtained, 0.4 g of treated degreased cotton fibre (DC) was added and mixed with the DES for 6 h. The mixture was first quenched by 150 mL of deionized water after filtration and washed with 500 mL of deionized water. The treated cellulose obtained in this step was named DES-DC. Permeate solution (diluted aqueous-DES) was recovered for further DES recycling and reusing.
Separation of CNCs
The DES-treated dissolved cellulose was uniformly dispersed in ultrapure water to form an aqueous phase dispersion, yielding a 1.0 wt% suspension, and the dispersion was placed in a high-pressure cell homogenizer (UH-05/UH-10/UH-24, Union-Biotech Co., Ltd., Shanghai, China) at 100 MPa for 30 min. The cavitation effect and high-speed impact caused by the sudden loss of pressure made the DES-treated dissolved cellulose suspension become even more ultra-fine. To prevent the instrument from being damaged due to overheating during the high-pressure process, the whole process was carried out in a cooling water-circulating machine at 15 °C. After the HPH process, the CNC colloidal solution was obtained and referred to as H-DES-CNCs (Fig. 1).
Recycling of DESs
The permeated solutions were subjected to distillation for 2 h with 80 °C vacuum using a rotary evaporator. The recovered DES was stored in a dry atmosphere for 4 h for future use. The DES was named according to the number of regenerations, and the first, second, third, fourth, and fifth regenerations were named DES-1st, DES-2nd, DES-3rd, DES-4th, and DES-5th, respectively. Each yield result for DES recovery was determined by the DES volume measurement as shown in the following equation.
Scanning electron microscopy (SEM)
Field emission scanning electron microscopy was used to observe the microscopic surface topography of synthetic samples at different processing stages (FESEM, Nova NanoSEM 230, FEI, USA). All samples were plated with gold for 1 min under vacuum and then observed.
Transmission electron microscopy (TEM)
The morphology and structure of H-DES-CNCs was observed by HRTEM (TECNAI G2F20, USA). The H-DES-CNCs suspension was diluted with deionized water and sonicated for 1 h in a 100 W water bath. A droplet dilution of the suspension was placed on a carbon-coated grid and dried at room temperature. The TEM image was taken at an acceleration voltage of 75 kV.
X-ray diffraction (XRD) analysis
To determine the crystal structure and crystallinity of cellulose, samples after different treatment stages were analysed by a Rigaku D/Max2550VB/PC XRD system. The crystallinity of the samples was concentrated at 2θ between 5° and 45°. The crystallinity index (CrI) was determined by the Segal method as shown below.
where I200 was the counter reading at heights of the 200 peak (2θ = 22.6), representing the crystalline region. Iam was the counter reading at the minimum intensity near 2θ values of 18°, representing the amorphous region of cellulose.
thermogravimetric (TG) analysis
The degradation and dehydration of samples was characterized by a thermogravimetric analyser (TGA, STA449C/6/G, Germany) in a nitrogen atmosphere. Analysis of the sample was carried out at a flow rate of 20 mL min−1 in a nitrogen atmosphere simultaneously with a heating rate of 10 °C min−1 and a set temperature range of 50–600 °C.
Molecular analysis
The effects of the DES and HPH on the structure of CNCs were evaluated by FT-IR and NMR analysis. Functional groups in the fibre samples were identified by FT-IR spectroscopy. The FT-IR spectra were collected using the KBr-disc method on a Vector 33 spectrometer (Nicolet AVATAR 360 FT-IR, USA) over the 400–4000 cm−1 spectral range at a resolution of 2 cm−1.
The Bruker AMX-300 MHz instrument was operated at 7.05 T, and the solid-state 13C NMR spectra at 75.47 MHz were recorded using cross-polarization and magic angle rotation (CP/MAS) techniques. The spinning rate and contact time were 5.0 kHz and 5.0 ms, respectively. The spectral width was 5000 kHz with an acquisition time of 20.48 ms, and the cumulative spectrum included 2000 scans.
Zeta potential and nanometre particle size analysis (ZNA)
The zeta potential of CNCs was detected by a zeta potential analyser (NanoPlus 3, USA).The DES-CNC suspension was ultrasonically treated for 5 min before analysis, and the solid concentration of all samples was 0.1 wt%.
Characterization of recycled DESs
The recovered DES was tested under a high-power microscope. The glass slide holder of the polarizing microscope was heated to 100 °C. A drop of DES was then applied to the glass slide, and a small amount of cotton fibre was placed completely within the DES drop. The dissolution process was observed in real time, and the film was periodically tracked with a camera on a polarizing microscope to record the dissolution process.
According to the ISO 5351 standard, the effect of recycled DES on the degree of polymerization was determined by the intrinsic viscosity method in cuprous ethylenediamine solution.
Results and discussion
Preparation of CNCs
As shown in Scheme 1, the DES system with ChCl as the hydrogen bond donor and Oa as the hydrogen bond acceptor was employed as the green medium for CNC preparation (Yu et al. 2013; Zhang et al. 2012a, b). In the study, an improved cellulose yield was obtained with increasing reaction time in the ChCl/Oa formed DES mixture (Laitinen et al. 2017a, b). In particular, the formed diester could prevent the dissolution of cellulose hydrolysate and thereby increase the yield (Bafti and Khabazzadeh 2014).
Typically, the cotton fibre was first dissolved in a homogeneous DES solution followed by high-pressure homogenization to prepare the CNCs. The HPH transferred energy to the homogeneous cellulose solution, resulting in a decrease in the average particle size of the treated cellulose. The homogenization pressure and homogenization cycle were the dominant factors affecting the CNC properties (Leung et al. 2011; Wang et al. 2017). The average particle size of CNCs decreased with increasing pressure and number of cycles. At pressures below 100 MPa, the average particle size of CNCs decreased from a starting value of approximately 700 nm to less than 350 nm. On the other hand, HPH-treated CNCs had a relatively small average diameter (300 nm) after a 30 min cycle at a fixed pressure of 100 MPa. However, as the pressure or cycle times further increased, the particle size also increased, which might be attributed to the polymerization or re-polymerization of the droplets. It was shown that under 100 MPa, the optimal HPH condition of CNCs was cycled for 30 min (Li et al. 2014; Zhang and Liu 2018).
As shown in Fig. 1a, the nano-structure of CNCs was tested by TEM. The structures of cellulose at different stages (including DC (Fig. 2a), DES-DC (Fig. 2b) and H-DES-CNCs (Fig. 2c)) were examined by SEM. After pretreatment with the DES solution, the morphology and surface of DES-DC changed obviously. In particular, the removal of the low-depolymerized cotton cell wall resulted in a smooth surface and fine fibre structure in the microfibrils (Fig. 2a, b). With the subsequent HPH process performed on the DES-DC, a homogeneous and stable suspension was obtained. It was shown that those CNCs had short rod shapes with diameters of 50–100 nm, lengths of 500–800 nm and smooth surfaces (Fig. 2c), which were very different from the original DC (Sirviö et al. 2016). The changes in fibre size and structure could be attributed to the hydrolytic cleavage of glycosidic bonds of DC during the DES treatment. TEM images (Fig. 1a) indicated that CNCs were nano-sized and had a high aspect ratio, with a diameter range of 40–80 nm and a length range of 300–700 nm. In general, the diameter and length distribution of CNCs, as shown in the TEM image (Fig. 1a) were similar to those from the SEM characterization. Therefore, the DES process has confirmed to be a fast and effective way for CNC preparation from DC with an HPH process (Shakeri and Radmanesh 2013).
Molecular analysis
As shown in Fig. 3, Fig. 4a and Table 1, the structure and properties of cellulose samples were analysed by CP-MAS 13C NMR and FT-IR techniques. The peak changes in the solid-phase CP-MAS 13C NMR spectra at different stages are shown in Table 1 and Fig. 4. The noticeable signals between 60 and 120 ppm were assigned to cellulose. The typical cellulose carbon peaks C1–C6 remained unchanged after the DES pretreatment and the HPH process. In the 13C spectrum, the C4 signal was assigned to the (O(3)H–O(5)) intramolecular hydrogen bond; the C6 signal was assigned to (O(2)H–O(6)) intramolecular hydrogen bonds. The C4 and C6 signals of the cotton fibre treated by DESs were shifted downfield, indicating that the intramolecular hydrogen bonds (O(2)H–O(6)) and (O(3)H–O(5)) of the cellulose were broken. The C4 and C6 signals of CNCs in HPH-treated cotton fibres were the same as those of the DES-treated cotton fibres. HPH had no effect on the intramolecular hydrogen bonds (O(2)HO(6)) and (O(3)HO(5) of cellulose. However, in the 13C spectra, the C2, C3 and C5 signals were shifted downfield, which may be due to the degradation of cellulose at C3 and C5. Cellulose degradation could maintain the chemical integrity of CNCs under HPH conditions (Liu et al. 2017).
Figure 4a shows the FT-IR spectra of DC, DES-DC and H-DES-CNCs. Absorbance peaks of 1425, 1375, 1160, 1115, 1060 and 897 cm−1 were observed, which were related to the typical characteristics groups of cellulose. The presence of the above peaks was consistent with the cotton cellulose structure, indicating that the main structure of the cellulose had not changed during the preparation process (Chen and Wan 2018). A peak at approximately 3100–3500 cm−1 was from the O–H stretching vibration of the hydroxyl group (Tenhunen et al. 2018). This region mainly includes the stretching vibrations of O–H groups in organic matter. It was suggested that there was a negligible effect on hydrogen bonding in the cellulose after DES pretreatment and the HPH process (Hou et al. 2018).
Compared with the FT-IR spectrum of the raw material DC, the spectra of DES-pretreated fibres (DES-DC and H-DES-CNCs) exhibited a new characteristic peak at approximately 1725 cm−1, which was the superposition peak of ester and carboxylic acid vibrations. The overlap of ester and carboxyl vibrations has been observed to be a typical phenomenon of cellulose dicarboxylic acid half esters. This was because the oxalic acid in the DES system led to an increase in the number of carboxyl groups in the cellulose chain, which might be due to the esterification of the cellulose hydroxyl group with oxalic acid. In the FT-IR spectrum of DC, a peak at 1428 cm−1 corresponded to the intramolecular hydrogen bond O(2) H–O (6). The peak at 1420 cm−1 appeared in the spectra of DC, DES-DC and H-DES-CNCs, indicating that the HPH process hardly destroyed the intramolecular hydrogen bonds (O(2) H–O (6)). The preservation of chemical properties was of great significance for further application of cellulose (Wang et al. 2017).
Structural Characterization
XRD patterns of DC, DES-DC and H-DES-CNCs are shown in Fig. 4b. An illustration of the crystallinity indices (CrI) obtained from the XRD patterns of various fibres was interpolated in the XRD spectrum. The diffraction patterns of the three cellulosic materials showed characteristic peaks of cellulose I (with intense peaks at 17.42°, 19.17° and 26.52°), which indicated that no rearrangement into other cellulose isomers occurred. The XRD results show that the crystal morphology of cellulose did not change during the treatment but that the crystallinity was increased (Zhang and Liu 2018). As shown in Fig. 4b, the crystallinity of DC, DES-DC and H-DES-CNCs was 63.5%, 84.25% and 77.6%, respectively. The homogenization process caused a certain damage or peeling of the crystalline region in DES-DC. The principle of the increase in CrI was similar to that of the previously reported hydrolysis treatment with hydrochloric acid (Mascheroni et al. 2016). The result indicated that the DC-CNCs formed amorphous regions after treatment with HPH, and the crystallinity index was lower than that of other samples. Cellulose chains broke during the HPH process, causing damage or spalling of the cellulose crystallization zone (Morais et al. 2013).
The TGA and differential thermogravimetric (DTG) curves for DC, DES-DC and H-DES-CNCs are shown in Fig. 4c, d. Small mass losses were observed in the 50–130 °C region due to evaporation of water and low molecular weight compounds. DC began to degrade at approximately 314.68 °C, and the DTG curve (Fig. 4d) shows a major decomposition peak at 350.01 °C. These peaks were caused by thermal decomposition of hemicellulose and α-cellulose (Sirviö et al. 2018). The thermal decomposition start point (Ton) indicated the initial degradation temperature, and the maximum decomposition temperature (Tmax) indicated the maximum degradation rate. DTG analysis of the DES-DC fibres indicated that the Ton and Tmax shifted to 315.88 °C and 352.11 °C, respectively (weight loss 58.81%). The Ton and Tmax of the H-DES-CNCs shifted to 304.3 °C and 343.14 °C, respectively (weight loss 62.10%). The CNCs had lower thermal stability owing to the HPH treatment, which destroyed part of the crystalline area (Tenhunen et al. 2018).
Physical stability
The surface charge of the fresh CNCs and the mature CNCs for 1 day, 3 days, 7 days, 15 days and 30 days storage was approximately − 45 to − 40 mV (Fig. 5a). The stability of the zeta potential indicated that there was a strong electrostatic repulsion between the CNC fibres (Zhang and Liu 2018). This finding was also confirmed by the Tyndall phenomenon of the CNC suspensions (Fig. 5c). The oxalic acid group in the DES system played an important role in the esterification of hydroxyl groups at C6 positions on the hydrophilic (1–10) lattice plane, thus breaking the hydrogen bonding between microfibrils and causing more fibrillation (Ling et al. 2019; Ma et al. 2019). After the hydrolysis of cellulose in the DES, hydrophilic carbonyl groups were formed on the surface, and these carbonyl (C=O) groups were detected by FT-IR measurements (Fig. 4a). Dynamic light scattering (DLS) analysis showed that the size of CNCs was 50–200 nm and that they could be well dispersed in aqueous solution (Fig. 5b) (Li et al. 2014; Niu et al. 2017; Sirviö et al. 2016).
Solubility and mechanism
Photomicrographs of the DES dissolving cellulose under polarized microscopy at different times are shown in Fig. 6. The cellulose before dissolution was a brightly coloured rod-shaped crystal. The brightness and length of the cellulose were significantly reduced when the dissolution time was 0.5 h. A large number of dissolved black lumps appeared and indicated significant cellulose dissolution. The undissolved portion of the cellulose was uniformly dispersed in the DES. After 2.0 h of dissolution, only a small number of bright spots appeared, and the surface texture of the heating table was clearly observed, indicating that the DES substantially dissolved the cellulose with increasing time. When the dissolution time reached 2.5 h, the cellulose was completely dissolved (Liu et al. 2017). Subsequently, DES-DC was an intermediate obtained after DES treatment, with a recovery of 73.8 ± 0.3%.
In this study, the DES, as both the catalyst and solvent for the reaction can be easily recycled. This approach provides a green alternative to the traditional strong acid method. The DES hydrolysis mechanism (presented in the middle of Fig. 6) has a certain similarity with that of acid hydrolysis. The hydroxyl groups of cellulose in the interaction of chlorine (Cl−) in DES played an important role. When the DES was heated and cellulose was dissolved, the ion pairs of ChCl were dissociated into [Ch]+ cations and [Cl]− anions. Simultaneously, the formation of charged ChCl electron donor-electron acceptor (EDA) complexes occurred between the hydrogen and oxygen atoms of the cellulose molecules.
On the one hand, the dissociated [Ch]+ cations and [Cl]− anions diffused into the cellulose molecular chain space. Thereafter, the free [Cl]− combined with the protons of the hydroxyl group H–O… H, while the free [Ch]+ was more likely to attack the oxygen atom of the hydroxyl group. On the other hand, within a single cellulose chain, the carbon atom of the β-1,4-glycosidic bond attracted [Cl]−, and then, the oxygen atom in the glycosidic bond interacted with [Ch]+. These interactions resulted in the cleavage of extensive hydrogen bonding networks and β-1,4-glycosidic bonds in the cellulose chain, thereby separating the fibres. Therefore, the hydrolysis of the glycosidic bond resulted in a large decrease in the molecular size and degree of polymerization of the cellulose. The hydrolysis of cellulose with the Oa system DES also involved esterification of the hydroxyl group, except for chain scission. The carboxyl group was introduced along the surface of the crystal that had lost the fibre cell, eventually causing the CNC surface to become negatively charged. This negative ion stabilization by the electrostatic force of the electric double layer could effectively prevent aggregation of the CNCs caused by hydrogen bonding, which was a significant reason for the stability of the colloidal suspensions (Laitinen et al. 2017b).
Recycling and reusing of DES
In terms of the ability to dissolve fibres, the recovery capacities for the fresh DES and the fifth recycled DES were different. The solubility was continuously decreased, and the dissolution tendency of the cellulose in the polarizing microscope field of view became less pronounced. For the DES-3rd solvent, the cellulose particles in the field of view were decreased in size, and the undissolved cellulose was evenly distributed in the DES. Ultimately, DES-5th only facilitated partial dissolution of cellulose. The recovery yields of fresh and recycled DES as well as the viscosity test results are shown in Fig. 7b. After the separation of the DES-DC, 91.34%, 90.2%, 88%, 86% and 84% of the DES-1st, DES-2nd, DES-3rd, DES-4th, and DES-5th could be recycled through a distillation process. The recovery rate of the DES recovered by vacuum distillation was high, and the recovery rate of all five DES cycles was above 85%. The viscosity of the DES recovered by recycling was slightly lower than that of fresh DES, possibly due to residual moisture present during the recovery process (Hou et al. 2018). In general, the viscosity of the DES was related to its purity and ability to dissolve cellulose, indicating that the DES after recovery had a poorer ability to dissolve cellulose than the fresh DES. This result was consistent with the results observed by polarized light microscopy (Fig. 7a). A possible reason for this outcome was that Oa was consumed in the process of dissolving cellulose in the DES. Thus, the content of DES gradually decreased in the recovered solution, resulting in a decrease in cellulose solubility. (Hou et al. 2018; Laitinen et al. 2017a, b).
Applicability and comparison
The feasibility of DES pretreatment combined with HPH for different cellulosic biomass materials was studied by comparing cotton, wood pulp and bamboo pulp CNCs. Moreover, the CNCs obtained in this study were compared with the different raw materials and different methods reported in the literature, including raw materials, preparation methods, diameter width, length, CrI and Tmax of CNCs. As listed in Table 2, the prepared CNCs (Entries 1–3) have higher crystallinity and Tmax than do those obtained by other methods, indicating that the thermal stability and crystallization characteristics of CNCs were improved after DES pretreatment. CNCs prepared by hydrolysis, including strong acid (sulfuric acid, phosphoric acid or mixed acid), ionic liquid hydrolysis, and TEMPO oxidation techniques, were compared to the DES-CNCs. The morphology, size, crystallinity and thermal stability of the DES-CNCs were comparable to those from these other methods, but this process was greener and more efficient.
CNCs are promising green nanomaterials that have broad application prospects in the emerging bio-economy. CNCs are inherently less toxic in dilute solutions and are biodegradable. Therefore, considering the full chain for their production, it is important to produce CNCs through environmentally sustainable methods with minimal operational hazard. The method of DES pretreatment combined with the HPH process to treat cellulose for preparing CNCs could be regarded as one of the most feasible methods. However, there is still much room for further improvement.
Conclusion
In conclusion, CNC preparation from cellulose by DES and HPH treatment is an efficient, environmentally friendly and economical methodology. The DES has a good effect on the cleavage of strong hydrogen bonds and the expansion of undissolved fragments in cotton fibres. The CNCs exhibited short rod shapes with diameters of 50–100 nm, lengths of 500–800 nm, smooth surfaces and high crystallinity (77.6%), high thermal stability (> 315.8 °C) and high potential (up to − 40 mV). Moreover, the DES can be recycled for at least three pretreatment cycles while maintaining its pretreatment capability. Therefore, this method is conducive to the rapid green processing of biomass (cotton) cellulose without affecting the yield and performance of CNCs. The high aspect ratio, high crystallinity and high potential of CNCs make them promising optical materials for chiral nematic liquid crystals, pharmaceutical carriers and membrane additives.
References
Abdel-Halim ES (2012) An effective redox system for bleaching cotton cellulose. Carbohyd Polym 90:316–321
Alomayri T, Low IM (2018) Synthesis and characterization of mechanical properties in cotton fiber-reinforced geopolymer composites. J Asian Ceram Soc 1:30–34
Bafti B, Khabazzadeh H (2014) Dimethylurea/citric acid as a highly efficient deep eutectic solvent for the multi-component reactions. J Chem Sci 126:881–887
Besbes I, Alila S, Boufi S (2011) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohyd Polym 84:975–983
Chen Z, Wan C (2018) Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour Technol 250:532–537
Crawford DE, Wright LA, James SL, Abbott AP (2016) Efficient continuous synthesis of high purity deep eutectic solvents by twin screw extrusion. Chem Commun 52:4215–4218
De Morais TE, Corrêa AC, Manzoli A, de Lima LF, de Oliveira CR, Mattoso LHC (2010) Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 17:595–606
Hammond OS, Bowron DT, Edler KJ (2017a) The effect of water upon deep eutectic solvent nanostructure: an unusual transition from ionic mixture to aqueous solution. Angew Chem Int Ed 56:9782–9785
Hammond OS, Edler KJ, Bowron DT, Torrente-Murciano L (2017b) Deep eutectic-solvothermal synthesis of nanostructured ceria. Nat Commun 8:14150
Hou X, Li A, Lin K, Wang Y, Kuang Z, Cao S (2018) Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment. Bioresour Technol 249:261–267
Kose O, Tran A, Lewis L, Hamad WY, MacLachlan MJ (2019) Unwinding a spiral of cellulose nanocrystals for stimuli-responsive stretchable optics. Nat Commun 10(1):510
Laitinen O, Ojala J, Sirviö JA, Liimatainen H (2017a) Sustainable stabilization of oil in water emulsions by cellulose nanocrystals synthesized from deep eutectic solvents. Cellulose 24:1679–1689
Laitinen O, Suopajärvi T, Österberg M, Liimatainen H (2017b) Hydrophobic superabsorbing aerogels from choline chloride-based deep eutectic solvent pretreated and silylated cellulose nanofibrils for selective oil removal. ACS Appl Mater Inter 9:25029–25037
Lee M, Heo MH, Lee H, Lee H, Jeong H, Kim Y, Shin J (2018) Facile and eco-friendly extraction of cellulose nanocrystalsvia electron beam irradiation followed by high-pressure homogenization. Green Chem 20:2596–2610
Leung ACW, Hrapovic S, Lam E, Liu Y, Male KB, Mahmoud KA, Luong JHT (2011) Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 7:302–305
Li J, Wei X, Wang Q, Chen J, Chang G, Kong L, Su J, Liu Y (2012) Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohyd Polym 90:1609–1613
Li J, Wang Y, Wei X, Wang F, Han D, Wang Q, Kong L (2014) Homogeneous isolation of nanocelluloses by controlling the shearing force and pressure in microenvironment. Carbohyd Polym 113:388–393
Li Y, Liu Y, Chen W, Wang Q, Liu Y, Li J, Yu H (2016) Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green Chem 18:869–1160
Li D, Henschen J, Ek M (2017) Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem 19:5564–5567
Ling Z, Edwards J, Guo Z, Prevost N, Nam S, Wu Q, French A, Xu F (2019) Structural variations of cotton cellulose nanocrystals from deep eutectic solvent treatment: micro and nano scale. Cellulose 26:861–876
Liu Y, Guo B, Xia Q, Meng J, Chen W, Liu S, Wang Q, Liu Y, Li J, Yu H (2017) Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustain Chem Eng 5:7623–7631
Lu Q, Lin W, Tang L, Wang S, Chen X, Huang B (2015) A mechanochemical approach to manufacturing bamboo cellulose nanocrystals. J Mater Sci 50:611–619
Lu Q, Cai Z, Lin F, Tang L, Wang S, Huang B (2016) Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain Chem Eng 4:2165–2172
Lynam JG, Kumar N, Wong MJ (2017) Deep eutectic solvents ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour Technol 238:684–689
Ma Y, Xia Q, Liu Y, Chen W, Liu S, Wang Q, Liu Y, Li J, Yu H (2019) Production of nanocellulose using hydrated deep eutectic solvent combined with ultrasonic treatment. ACS Omega 4:8539–8547
Mascheroni E, Rampazzo R, Ortenzi MA, Piva G, Bonetti S, Piergiovanni L (2016) Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 23:779–793
Morais JPS, Rosa MDF, de Souza FMDSM, Nascimento LD, Do Nascimento DM, Cassales AR (2013) Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohyd Polym 91:229–235
Niu F, Li M, Huang Q, Zhang X, Pan W, Yang J, Li J (2017) The characteristic and dispersion stability of nanocellulose produced by mixed acid hydrolysis and ultrasonic assistance. Carbohyd Polym 165:197–204
Park JH, Oh KW, Choi H (2013) Preparation and characterization of cotton fabrics with antibacterial properties treated by crosslinkable benzophenone derivative in choline chloride-based deep eutectic solvents. Cellulose 20:2101–2114
Procentese A, Raganati F, Olivieri G, Russo ME, Rehmann L, Marzocchella A (2018) Deep eutectic solvents pretreatment of agro-industrial food waste. Biotechnol Biofuels 11(1):37
Shakeri A, Radmanesh S (2013) Preparation of cellulose nanofibrils by high-pressure homogenizer and zein composite films. Adv Mater Res 829:534–538
Sirviö JA, Honkaniemi S, Visanko M, Liimatainen H (2015) Composite films of poly(vinyl alcohol) and bifunctional cross-linking cellulose nanocrystals. ACS Appl Mater Inter 7:19691–19699
Sirviö JA, Visanko M, Liimatainen H (2016) Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules 17:3025–3032
Sirviö JA, Visanko M, Ukkola J, Liimatainen H (2018) Effect of plasticizers on the mechanical and thermomechanical properties of cellulose-based biocomposite films. Ind Crop Prod 122:513–521
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Tenhunen T, Lewandowska A, Orelma H, Johansson L, Virtanen T, Harlin A, Österberg M, Eichhorn S, Tammelin T (2018) Understanding the interactions of cellulose fibres and deep eutectic solvent of choline chloride and urea. Cellulose 25:137–150
Wang Y, Wei X, Li J, Wang F, Wang Q, Chen J, Kong L (2015) Study on nanocellulose by high pressure homogenization in homogeneous isolation. Fiber Polym 16:572–578
Wang Y, Wei X, Li J, Wang F, Wang Q, Zhang Y, Kong L (2017) Homogeneous isolation of nanocellulose from eucalyptus pulp by high pressure homogenization. Ind Crop Prod 104:237–241
Willberg-Keyriläinen P, Hiltunen J, Ropponen J (2018) Production of cellulose carbamate using urea-based deep eutectic solvents. Cellulose 25:195–204
Xu G, Ding J, Han R, Dong J, Ni Y (2016) Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour Technol 203:364–369
Yu H, Qin Z, Liang B, Liu N, Zhou Z, Chen L (2013) Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J Mater Chem A 1:3938
Zhang R, Liu Y (2018) High energy oxidation and organosolv solubilization for high yield isolation of cellulose nanocrystals (CNC) from Eucalyptus hardwood. Sci Rep 8(1):16505
Zhang Q, Benoit M, De Oliveira VK, Barrault J, Jérôme F (2012a) Green and inexpensive choline-derived solvents for cellulose decrystallization. Chem Eur J 18:1043–1046
Zhang Q, De Oliveira VK, Royer S, Jérôme F (2012b) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:718–7146
Zuluaga R, Putaux JL, Cruz J, Vélez J, Mondragon I, Gañán P (2009) Cellulose microfibrils from banana rachis: effect of alkaline treatments on structural and morphological features. Carbohyd Polym 76:51–59
Zuo M, Le K, Li Z, Jiang Y, Zeng X, Tang X, Sun Y, Lin L (2017) Green process for production of 5-hydroxymethylfurfural from carbohydrates with high purity in deep eutectic solvents. Ind Crop Prod 99:1–6
Zuo M, Le K, Feng Y, Xiong C, Zeng X, Tang X, Sun Y, Lin L (2018) An effective pathway for converting carbohydrates to biofuel 5-ethoxymethylfurfural via 5-hydroxymethylfurfural with deep eutectic solvents (DESs). Ind Crop Prod 112:18–23
Acknowledgments
This work was supported by the Natural Science Foundation of China (Nos. 21676223, 21978248), the special fund for Fujian Ocean High-Tech Industry Development (No. FJHJF-L-2018–1), China, and the Energy development Foundation of the College of Energy, Xiamen University (No. 2017NYFZ02).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Li, J., Zeng, X. et al. Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent. Cellulose 27, 1301–1314 (2020). https://doi.org/10.1007/s10570-019-02867-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02867-2