Abstract
Cotton fiber maturity is an important fiber physical and quality property that impacts downstream fiber processing. Fiber maturity refers to the degree of secondary cell wall thickening. The reference method for fiber maturity measurement is to quantify the secondary cell wall area relative to the perimeter of the fiber via cross-sectional image analysis, a tedious and slow process. A number of approaches have been developed which attempt to measure fiber maturity rapidly. The approach employed in this work is based on the use of attenuated total reflection Fourier transform infrared spectroscopy, and simple algorithms were developed from the spectra to estimate fiber maturity directly. To validate the efficacy of this approach against cross-sectional image analysis, two distinct fiber sets were examined that included a set of developing fibers and a diverse set of developed fibers. Comparison of image analysis and infrared maturity results imply a consistency and equivalency between the two maturity measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is one of the most important and widely grown crops in the world, primarily for its naturally produced textile fiber (Robertson and Roberts 2010). Fiber maturity is often represented by the term circularity (θ), defined as the cross-sectional area of the secondary cell wall (SCW) relative to the area of a circle having the same perimeter (Hequet et al. 2006; Thibodeaux and Evans 1986; Xu and Pourdeyhimi 1994). Cotton fiber maturity is a major yield component and also an essential fiber physical and quality attribute, which is directly linked to fiber breakage and entanglement (neps) during mechanical processing as well as dye uptake in dyed yarn and fabric products (Anthony et al. 1988; Gordon 2007).
Cotton micronaire (MIC), which is a combination of fiber maturity and fineness (Lord 1956), is measured using high volume instrument (HVI) on the basis of measuring air-flow resistance through a plug of cotton fibers of a given weight (ASTM 2012a) and is used in lieu of a specific measure of fiber maturity. Although cotton fibers with high HVI MIC values are generally considered as mature, fibers with low HVI MIC values can be mature but very fine (Kim et al. 2014). Thus, a MIC value alone is not sensitive to compare maturity of cotton fibers with variant fineness values that are often observed in different cotton varieties and species.
Current-in-use cotton fiber maturity measurements include direct methods that involve traditional cross-sectional image analysis (IA) (Hequet et al. 2006; Thibodeaux and Evans 1986; Xu and Pourdeyhimi 1994) and recently proposed Fourier transform infrared (FT-IR) spectroscopy (Liu et al. 2011). The IA maturity readings have been utilized as fiber maturity references for indirect measurement system developments, for example, advanced fiber information system (AFIS) (Bradow et al. 1996; Kim et al. 2014; Paudel et al. 2013), Fineness and Maturity Tester (FMT) (Montalvo et al. 2007), polarized light microscopy (Long et al. 2010), Cottonscope (Kim et al. 2014; Paudel et al. 2013; Rodgers et al. 2012, 2013), as well as near infrared (NIR) and FT-IR spectroscopic approaches (Abidi et al. 2011; Rodgers et al. 2010). Complimentary to above techniques, recent maturity measurement developments include the machine vision systems by various approaches, such as using transfer learning methods (Turner et al. 2017; Shahriar et al. 2013), designing a laser diffraction instrument (Adedoyin et al. 2010), and combining confocal microscopy with image analysis (Turner et al. 2015).
Cross-sectional IA test was originally developed by Thibodeaux and colleagues in the 1980s (Thibodeaux and Evans 1986) and refined through the 1990s to today (Boylston et al. 1993; Guo et al. 2014; Matic-Leigh and Cauthen 1994; Thibodeaux and Rajasekaran 1999; Xu and Huang 2004; Xu and Pourdeyhimi 1994). The cross-sectional IA procedure requires a bundle of fibers to be selected and prepared, embedded in a medium, cut into thin sections and mounted on a microscope slide. Images of the cross-sections are captured using a microscope equipped with a digital camera and then analyzed with a software to estimate the parameters including the fiber cell wall area (Aw, cross-sectional area minus lumen area) and the perimeter of the outside of the fiber (P2). Fiber characteristics such as circularity (θ = 4πAw/(P2)2) and maturity ratio (M = θ/0.577) can be calculated from the Aw and P2 values (Hequet et al. 2006). The divisor of 0.577 was determined by Pierce and Lord for a standard level of maturity (Pierce and Lord 1939). In general, IA determination of fiber maturity is destructive, labor intensive, time consuming and impractical for a large number of fiber samples.
On the basis of attenuated total reflection (ATR) FT-IR spectral differences between immature and mature seed cotton fibers, Liu et al. (2011) proposed simple algorithms to assess cotton fiber infrared maturity (MIR) index. In this concept, the R1 algorithm utilized three IR bands at 1500, 1032, and 956 cm−1, then the R1 value was converted into fiber MIR by assigning the respective MIR values of 0.0 and 1.0 to the most immature and mature fibers in the dataset, and next the MIR values were compared with maturity readings determined from IA test on independent fibers including the 104 reference cotton set (Liu et al. 2011). This work yielded two equations that formed an algorithm to rapidly and non-destructively provide an estimation of MIR. Here, R1 = (I956 − I1500)/(I1032 − I1500) representing the intensity ratio of the intensity at the three IR bands, and MIR = (R1 − R1,sm)/(R1,lr − R1,sm) representing the maturity from FT-IR measurements. R1 was the value for the unknown sample, R1,lr and R1,sm were experimentally determined to be 0.59 and 0.14. In practice, the algorithm is reduced to be MIR = (R1 − 0.14)/0.45. Caution should be taken when analyzing the ATR FT-IR spectra for MIR index in very early developing fibers that exhibit glue-like stickiness, because the dominant presence of physiological sugars (sucrose, glucose, fructose, and galacturonic acid) in younger than 17 days of post-anthesis (DPAs) fibers can contribute significantly to the two bands (1032 and 956 cm−1) used in algorithm calculations (Liu and Kim 2015).
The use of ATR FT-IR method to evaluate the cotton fiber MIR has the following advantages: (1) a simple ATR FT-IR protocol allows direct measurement of cotton fibers and avoids the need to perform any preparation or pretreatment of cotton samples, (2) it can be applied to small amounts of fibers (as little as 0.5 mg) that are sometimes common during cotton breeding and genetic approaches, and (3) it requires only a short time (less than 2 min) for sample loading, spectral acquisition, and subsequent result reporting. Additional value such as fiber infrared crystallinity index (CIIR) could be assessed by different algorithms from the same ATR FT-IR spectra (Liu et al. 2011, 2012; Kim et al. 2018). Although the ATR FT-IR approach is a non-invasive method requiring less fibers for measuring maturity as compared with other conventional methods, this method has not previously been validated with breeder samples and has rarely been used in cotton genetic studies or breeding programs for analyzing fiber maturity. This test neither consumes high energy nor needs high energy equipment.
The objective of this study was to further validate the consistency and agreement between reference IA maturity (MIR) and ATR FT-IR maturity (MIR) measurements on two diverse sets of cotton samples from breeder. The first set was composed of developing fibers at 24, 28, 32, 36 and 40 DPA that vary in fiber maturity from a standard upland cotton variety, Texas Marker-1 (TM-1). The second set consisted of fully developed upland cotton fibers with a broad range of fiber maturity values that have been recently constructed by a genetic approach (Kim et al. 2019). The MIA and MIR values on the two sets were compared to those on the 104 reference fibers that have been used as standard cotton materials for cotton fiber maturity measurement (Hequet et al. 2006; Liu et al. 2011; Paudel et al. 2013).
Experimental
Cotton fibers
First fiber set: developing upland TM-1 (PI 607172) cotton fibers. The Gossypium (G.) hirsutum TM-1 is the upland cotton genetic and genomic standard variety producing average fiber properties (Kohel et al. 1970). They were grown in a field of USDA-ARS in New Orleans, LA during the 2009 crop year. Cotton flowers were tagged on the day of flowering (0 DPA). Two biological replicates of cotton bolls from different TM-1 plants were harvested at 24, 28, 32, 36 and 40 DPA. This fiber set represents normal fiber development as SCW develops.
Second fiber set: developed upland SG-747 × im (SI) and DP-90 × im (DI) fibers. Sure-Grow 747 (SG-747, PVP 9800118) or Deltapine Acala 90 (DP-90, PI 564767) cotton plant was crossed as pollen donor parent in a greenhouse in 2010 with an immature fiber (im) mutant plant to construct a broad range of fiber maturity. The SG-747 is a commercial variety producing coarse fibers (Fang et al. 2013), and DP-90 is an elite commercial cultivar generating fine fibers (Campbell et al. 2013), while im mutant has low fiber maturity due to its thin fiber wall (Kim et al. 2013, 2017). The F1 plants were self-pollinated to obtain F2 seeds. The F2 plants along with parents were grown in the same field as the developing upland TM-1 cotton fibers in 2011.The soil type was Aquent dredged over alluvium in an elevated location to provide adequate drainage. Standard conventional field practices were applied during the growing season.
All fiber samples were harvested by hand. Developing fibers were manually ginned (i.e., to separate cotton fibers from their seeds), whereas fully developed SI and DI fiber samples were ginned by a laboratory roller gin, prior to drying in a 40 °C incubator. These fibers were stored in a dark storage room with a constant temperature (23 ± 1 °C) and relative humidity (50 ± 10%) prior to the measurements.
Fiber cellulose content determination
Fiber cellulose contents were determined by Updegraff method (Updegraff 1969). This method is generally considered as a reference method for determining SCW development in upland cotton fibers (Meinert and Delmer 1977), but it is a destructive chemical analysis requiring lengthy and laborious processes. Briefly, 10.00 mg of cut fibers were placed into 5 mL of reacti-vials. Non-cellulosic materials in fibers were hydrolyzed with acetic-nitric reagent (a mixture of 73% acetic acid, 9% nitric acid and 18% water). The remaining cellulose was hydrolyzed with 67% sulfuric acid (v/v) and measured by a colorimetric assay with anthrone, in which Avicel PH-101 (FMC, Rockland, ME) was used to establish a calibration curve to be able to determine the concentration of cellulose from spectrophotometric measurement. The average cellulose content for each sample was obtained from two biological and three technical replications.
Fiber maturity ratio determination via image analysis of fiber cross-sections (M IA)
Fiber MIA was determined through the established protocol (Hequet et al. 2006). Individual samples were blended and representative fiber samples were prepared and sectioned. At least 300 fibers were analyzed for each sample. Aw and P2 values were used to calculate circularity (θ). The obtained θ values were converted to MIA using the established equation of M = θ/0.577 (Hequet et al. 2006). M is replaced by MIA to indicate the fiber maturity measurement from the IA test in this work.
Fiber maturity measurement via ATR FT-IR spectroscopy (M IR)
Fiber MIR was estimated by executing simple algorithmic analysis of ATR FT-IR spectra as described previously (Liu and Kim 2015; Liu et al. 2011). To collect the fiber spectra, an FTS 3000MX FTIR spectrometer (Varian Instruments, Randolph, MA) equipped with a ceramic source, KBr beam splitter, deuterated triglycine sulfate (DTGS) detector, and an ATR attachment was used. At least six spectra by re-sampling different locations across each sample were obtained over the range of 4000–600 cm−1 at 4 cm−1 resolution and 16 co-added scans. No baseline correction was applied to these spectra that were recorded in absorbance units, in order to keep consistent with preceding ATR FT-IR studies of cotton fibers (Liu and Kim 2015; Liu et al. 2011).
Fiber MIC measurement via HVI or Fibronaire system
Average of fiber MIC values of developing TM-1 fibers were determined via Fibronaire procedure (on 3.24 g fibers) using Fibronaire system (Motion Control Inc., Dallas, TX), due to their limited quantities (< 5.0 g). MIC attributes from developed fibers were obtained by HVI (USTER Technologies Inc., Knoxville, TN) according to the common-in-use HVI procedure that requires a minimum of 10.0 g fibers. Developing fibers were teased to fluff fibers, whereas developed fibers were measured without pre-treatment for the MIC measurement. Both instruments were properly calibrated according to established procedure (ASTM 2012b).
Results and discussion
First fiber set: upland TM-1 cotton fibers at different stages of development
Table 1 summarizes the mean values and standard deviations (SDs) for the developing TM-1 fibers from IA, ATR FT-IR and Fibronaire measurement as well as cellulose content determination. In order to compare the sensitivity of individual measurement, relative standard deviation (RSD), defined as the ratio of SD/mean, was inserted in Table 1.
During the SCW biosynthesis stage (24–36 DPA), cellulose content of the developing cotton fibers increases dramatically from 58.19% at 24 DPA to 91.21% at 36 DPA (Table 1). The developing fiber at 40 DPA composes of a majority (95.01%) of cellulose, which is common in the fully developed upland cotton fibers (Abidi et al. 2010; Kim 2015; Meinert and Delmer 1977).
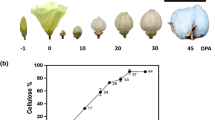
In line with cellulose content elevating from 24 to 40 DPA fibers, the corresponding MIA value increases from 0.40 to 1.02, MIR dimension raises from 0.42 to 0.88, and Fibronaire MIC varies from 2.40 to 4.74 (Table 1). The observation that MIR value does not increase from 32 DPA to 36 DPA might be due to small sample sizes. Typical microscopic images of fiber cross-sections in Fig. 1 highlight the apparent trend of SCW thickening for these developing fibers at 24, 28, 36 and 40 DPA.
Distinctions on these developing TM-1 fibers could also be monitored by ATR FT-IR spectral intensity differences in the 1600–800 cm−1 region (Fig. 2), in which great spectral intensity changes occur. From 24 to 40 DPA, intensities of the bands at 1158, 1104, 1055 and 1028 cm−1 decrease, while those in the 1000–875 cm−1 region increase. Spectral intensity variations of those bands have been characterized well in earlier studies (Abidi et al. 2014; Liu and Kim 2015) and their assignments are summarized in Table 2. A steady intensity increasing or decreasing of these characteristic bands in Fig. 2 echoes well with SCW thickening in Fig. 1.
With fiber developing, all indices (cellulose content, MIA, MIR, and Fibronaire MIC) in Table 1 increase, but their RSDs differ and decrease in a general order of MIA (0.36), MIR (0.26), Fibronaire MIC (0.26), and cellulose content (0.18). The lower RSD of 0.18 could imply the least sensitivity and efficiency of cellulose content values in reflecting fiber maturity of developing fibers among four methods examined in Table 1.
Table 3 summarizes the Pearson correlation (r, or univariate correlation coefficient) between the measurements among the set of developing fibers. Any pair of the four indices had positive and strong correlations with others (r > 0.90). This observation indicates that each of the four indices could be applied to monitor the maturation process in developing cotton fibers, although cellulose content exhibits the lowest sensitivity for these developing TM-1 fibers.
Figure 3 compares the fiber maturity values between reference IA method and ATR FT-IR measurement, and a strong linear relationship (r = 0.95*) is observed between MIA and MIR values with a slope of 0.65, an intercept of 0.22, and a coefficient of determination (R2) of 0.90.
Comparison of MIA against MIR values in first fiber set (red circle) and second fiber set (blue times). MIA and MIR readings (black dotted) on 104 reference fibers from the reference (Liu et al. 2011) were inserted for verification. Statistical significance was shown at the p-level value under 0.05 (*), 0.01 (**), and 0.001 (***). (Color figure online)
Fiber maturity would be variable within a plant, within a boll, and even across a single seed since fiber cell wall developments are greatly affected by growth conditions and micro environments (Bradow et al. 1996; Ayele et al. 2018). Therefore, IA procedure requires vast sample sizes as well as accurate and high resolution detection system. Average MIA value is generally obtained from a minimum of 300 fiber cross-sections (Thibodeaux and Evans 1986; Boylston et al. 1993; Hequet et al. 2006).
A single parameter, such as average maturity indices in Table 1, might not be sufficient to describe the maturity of a large quantity of fibers because of its maturity variability. In this regard, Xu and his colleagues (Guo et al. 2014) introduced the skewness of a maturity distribution to be an essential parameter for classifying the maturity distribution pattern in a sample, aiming to improve the consistency of fiber maturity detections and to effectively reduce the bias on immature fibers. As highlighted in this manuscript, average MIR value from six IR spectral measurements is significantly correlated with mean MIA value from at least 300 cross-sections in a sample, implying that MIR represents fiber maturity despite the variation within a fiber sample. Meanwhile, MIA distribution in a sample is acquired from 300 MIA values of individual cross-sections because of MIA determination at single fiber level, whereas MIR distribution is unavailable due to IR spectral measurement at bundle fiber level.
Turner et al. (2015) reported the combination of confocal microscopy and image analysis as an independent measuring method to validate a large variation in maturity within a single fiber. The finding implies that, to get accurate and desired maturity value in a sample, the measurement devices must be high resolution and sample sizes must be vast. This need makes high throughput one of the most important features in developing next-generation maturity measurement tools. The results reported here demonstrate ATR FT-IR spectroscopy as one of rapid and high throughput methods for fiber maturity measurements. To meet the challenge for either of the instruments to measure the same property but by different methodologies, and to copy with the problem that samples presented to one instrument are not available to the other, Turner et al. (2017) proposed a novel transfer learning regression method that utilizes data from an older instrument to train a new instrument for assessing the same property. Results indicated the effectiveness of the proposed regression model on both synthetic and real world data sets, and also demonstrated how this technique can be applied to train a new instrument for determining cotton fiber maturity.
Second fiber set: the developed upland SG-747 × im (SI) and DP-90 × im (DI) fibers with various maturity values
Fiber maturity and cellulose content in crossed SI and DI fibers are compared in Tables 4 and 5, respectively. Fully developed SI fibers (Table 4) and DI fibers (Table 5) consist of more than 90.00% cotton cellulose. Unlike the pattern of cellulose content, MIA increases from 0.54 to 0.99 for SI fibers and from 0.63 to 1.11 for DI fibers, MIR fluctuates from 0.51 to 0.82 for SI fibers and from 0.52 to 0.85 for DI fibers, and HVI MIC ranges from 2.67 to 5.47 for SI fibers and from 3.13 to 5.73 for DI fibers. Fiber maturity is a continuous variable and fibers with MIA range of > 1.0, 1.0–0.8, 0.8–0.7, and < 0.7 were considered to be very mature, mature, immature, and uncommon/very immature, respectively (Kim et al. 2014).
As seen in Tables 4 and 5, RSDs are nearly equivalent between SI and DI fibers for cellulose content (0.01 vs. 0.02), MIA (0.20 vs. 0.24), MIR (0.19 vs. 0.21), and HVI MIC (0.22 vs. 0.24). In general, RSDs are nearly unchanged among MIA, MIR, and HVI MIC values, which are much larger than that of cellulose content measurement. Traditional microscopic images of fiber cross-sections in Fig. 4 show the SCW thickening of these developed fibers from three SI (SI-16, SI-09, SI-01) and three DI (DI-02, DI-11, DI-34) upland cotton lines.
Figure 5 provides representative ATR FT-IR spectra of the same three DI (DI-02, DI-11, DI-34) fibers as in Fig. 4. Trend of spectral intensity changes in Fig. 5 is similar to that of SCW thickening in Fig. 4, and also resembles those in Fig. 2 in the aspect of increasing MIA values.
Comparison of r in Table 6 indicates that cellulose content did not exhibit any strong correlations with fiber maturity values from IA and ATR FT-IR measurement as well as HVI MIC measurement. Besides cellulose content, any pair of three indices (MIA, MIR and HVI MIC) had positive and strong correlations with each other. In general, MIA had higher correlation with MIR (r = 0.91) than with HVI MIC (r = 0.84), whereas MIR had nearly equal correlation with MIA (r = 0.91) to that with HVI MIC (r = 0.92).
The relationship between MIA and MIR values for the second fiber set was inserted in Fig. 3 for comparison. The slope (slope = 0.66) in MIR versus MIA regression for the developed SI and DI fibers is identical to that for the developing fibers (slope = 0.65), the intercept for the developed fibers (intercept = 0.17) resemble that for the developing fibers (intercept = 0.22), and also the R2 for these developed fibers (R2 = 0.83) is similar to that for the developing fibers (R2 = 0.90).
In initial investigation for proposing the MIR algorithm, Liu et al. (2011) used the 104 reference fibers to compare two maturity readings between IA and ATR FT-IR measurement. For verification, the MIA and MIR values on the 104 reference fibers were inserted in Fig. 3. A combination of three fiber sets shows a significant correlation (p < 0.001, r = 0.85***) between two maturity determinations, and a general relationship between the two is included in Fig. 3. Both the first and second fiber set are unblended fibers as breeder’s samples, while the 104 reference fibers were well-blended fibers representing two main cotton variety species (upland and pima) and different growing locations (Hequet et al. 2006).
Considering the quite differences in the origin of the fiber sets and also in the date of their IA and ATR FT-IR measurements, the observation in Fig. 3 validates the consistency and equivalence in fiber maturity determination between conventional IA protocol and ATR FT-IR procedure. Notably, the MIR values from the ATR FT-IR measurement are not equal to the exact MIA values from the IA test, but these MIR values could be converted into MIA readings by the general equation given in Fig. 3.
Examination of RSD values in Tables 1, 4 and 5 reveals a clear decrease for cellulose content index from developing fibers (Table 1) to developed fibers (Tables 4 and 5), however, this trend is not observed for any of other three maturity measurements (i.e., MIA, MIR and Micronaire). Likely, cellulose content index is more sensitive to reflect the cellulose content in developing cotton fibers (Table 1) than in developed cotton fibers (Tables 4 and 5). Also, RSD of three maturity measurements in Table 1 versus Tables 4 and 5 are similar regardless of developing or developed fibers, suggesting that the three maturity values are sensitive and reliable. In addition, both MIA and MIR show strong correlations with cellulose content among developing cotton fibers (Table 3) rather than within developed cotton fibers (Table 6). Overall, cellulose content in fibers does not agree with MIA or MIR or MIC values all the time. Undoubtedly, further study is necessary to better understand not only cellulose content determination, but also cellulose molecular weight distribution and degree of polymerization. For instance, the cellulose in the primary cell walls (PCW) has lower degree of polymerization (DP) and broader molecular weight distribution (MWD) than that in the SCW (Hsieh 2007). Apparent differences in both MWD and DP were noted between two fibers having similar HVI MIC (Timpa and Ramey 1994).
Conclusion
The result validates the potential effectiveness of simple algorithmic analysis of ATR FT-IR spectra in the determination of cotton fiber MIR index. The MIR values on diverse fibers were related to the referenced MIA index from the cross-sectional IA method. The linear regressions plotting MIA against MIR revealed a similarity in the slope, the intercept, and the R2 among the fiber sets examined. In other words, fiber maturity determined from ATR FT-IR measurement are consistent and in good agreement with that from cross-section IA method. The results not only could provide cotton scientists an alternative tool for measuring fiber maturity, but also could serve as an independent tool for verifying fiber maturity measurements from other methods due to rapid and direct fiber measurement of ATR FT-IR method. Essentially, accurate and consistent fiber maturity measurement by one method is more important than fiber sampling mode in a non-destructive way by other methods.
At this stage of our systematic investigations, it seems that the ATR FT-IR method can be used in such scenarios when a small number of fibers (single boll sample for example) are available or the developing fibers (composed of high levels of physiological sugars for example) are difficult to be analyzed by conventional instrumental methods.
References
Abidi N, Hequet E, Cabrales L (2010) Changes in sugar composition and cellulose content during the secondary cell wall biogenesis in cotton fibers. Cellulose 17:153–160
Abidi N, Hequet E, Cabrales L (2011) Applications of Fourier transform infrared spectroscopy to study cotton fibers. In: Nikolic G (ed) Fourier transforms—new analytical approaches and FTIR strategies. InTech, Rijeka, pp 89–114
Abidi N, Cabrales L, Haigler CH (2014) Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr Polym 100:9–16
Adedoyin AA, Li C, Toews MD (2010) Characterization of single cotton fibers using a laser diffraction system. Text Res J 81:355–367
Anthony WS, Meredith WR, Williford JR (1988) Neps in ginned lint: the effects of varieties, harvesting, and ginning practices. Text Res J 58:633–640
ASTM (2012a) Standard test method for measurement of physical properties of cotton fibers by high volume instruments, D5867. Annual book of ASTM Standards. ASTM International, West Conshohocken, PA
ASTM (2012b) Standard test method for micronaire reading of cotton fibers, D1448. Annual Book of ASTM Standards. ASTM International, West Conshohocken, PA
Ayele AG, Kelly BR, Hequet EF (2018) Evaluating within-plant variability of cotton fiber length and maturity. Agron J 110:47–55
Boylston EK, Thibodeaux DP, Evans JP (1993) Applying microscopy to the development of a reference method for cotton fiber maturity. Text Res J 63:80–87
Bradow JM, Hinojosa O, Wartelle LH, Davidonis G, Saaaenrath-Cole GF, Bauer PJ (1996) Applications of AFIS fineness and maturity module and X-ray fluorescence spectroscopy in fiber maturity evaluation. Text Res J 66:545–554
Campbell BT, Weaver DB, Sharpe R, Wu J, Jones DC (2013) Breeding potential of elite Pee Dee germplasm in upland cotton breeding programs. Crop Sci 53:894–905
Fang H, Zhou H, Sanogo S, Flynn R, Percy RG, Hughs SE, Ulloa M, Jones DC, Zhang J (2013) Quantitative trait locus mapping for Verticillium wilt resistance in a backcross inbred line population of cotton (Gossypium hirsutum × Gossypium barbadense) based on RGA-AFLP analysis. Euphytica 194:79–91
Gordon S (2007) Cotton fiber quality. In: Gordon S, Hsieh Y-L (eds) Cotton: science and technology, part 1.3. Woodhead Publishing Limited, Cambridge, pp 68–100
Guo X, Ouyang W, Xu B (2014) Assessing cotton maturity using distributional parameters of fiber cross-section measurements. Text Res J 84:1666–1676
Hequet E, Wyatt B, Abidi N, Thibodeaux DP (2006) Creation of a set of reference material for cotton fiber maturity measurements. Text Res J 76:576–586
Hsieh Y-L (2007) Chemical structure and properties of cotton. In: Gordon S, Hsieh Y-L (eds) Cotton: science and technology, part 1.1. Woodhead Publishing limited, Cambridge, pp 3–34
Kim HJ (2015) Fiber biology. In: Fang DD, Percy RG (eds) Cotton, 2nd edn. Agronomy monograph. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, WI, pp 97–127
Kim HJ, Moon HS, Delhom CD, Zeng L, Fang DD (2013) Molecular markers associated with the immature fiber (im) gene affecting the degree of fiber cell wall thickening in cotton (Gossypium hirsutum L.). Theor Appl Genet 126:23–31
Kim HJ, Rodgers J, Delhom CD, Cui X (2014) Comparisons of methods measuring fiber maturity and fineness of Upland cotton fibers containing different degrees of fiber cell wall development. Text Res J 84:1622–1633
Kim HJ, Lee CM, Dazen K, Delhom CD, Liu Y, Rodgers JE, French AD, Kim SH (2017) Comparative physical and chemical analyses of cotton fibers from two near isogenic upland lines differing in fiber wall thickness. Cellulose 24:2385–2401
Kim HJ, Liu Y, French AD, Lee CM, Kim SH (2018) Comparison and validation of Fourier transform infrared spectroscopic methods for monitoring secondary cell wall cellulose from cotton fibers. Cellulose 25:49–64
Kim HJ, Delhom CD, Rodgers JE, Jones DC (2019) Effect of fiber maturity on bundle and single fiber strength of upland cotton. Crop Sci 59:115–124
Kohel R, Richmond T, Lewis C (1970) Texas marker-1. Description of a genetic standard for Gossypium hirsutum L. Crop Sci 10:670–671
Liu Y, Kim HJ (2015) Use of attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy in direct, non-destructive, and rapid assessment of developmental cotton fibers grown in planta and in culture. Appl Spectrosc 69:1001–1010
Liu Y, Thibodeaux D, Gamble G (2011) Development of FTIR spectroscopy in direct, non-destructive, and rapid determination of cotton Fiber maturity. Text Res J 81:1559–1567
Liu Y, Thibodeaux D, Gamble G, Bauer P, VanDerveer D (2012) Comparative investigation of Fourier transform infrared (FT-IR) spectroscopy and X-ray diffraction (XRD) in the determination of cotton fiber crystallinity. Appl Spectrosc 66:983–986
Long RL, Bange MP, Gorden SG, Constable GA (2010) Measuring the maturity of developing cotton fibers using an automated polarized light microscopy technique. Text Res J 80:463–471
Lord E (1956) Air flow through plugs of textile fibers part II. The micronaire test for cotton. J Text Inst 47:T16–T47
Matic-Leigh R, Cauthen DA (1994) Determining cotton fiber maturity by image analysis, part I: direct measurement of cotton fiber characteristics. Text Res J 64:534–544
Meinert MC, Delmer DP (1977) Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol 59:1088–1097
Montalvo JG Jr, Von Hoven TM, Davidonis G (2007) Biased experimental fineness and maturity results. Text Res J 77:743–755
Paudel D, Hequet E, Noureddine A (2013) Evaluation of cotton fiber maturity measurements. Ind Crop Prod 45:435–441
Pierce FT, Lord E (1939) The fineness and maturity of cotton. J Text Inst 30:T173–T210
Robertson WC, Roberts BA (2010) Integrated crop management for cotton production in the 21st century. In: Wakelyn PJ, Chaudhry MR (eds) Cotton: technology for the 21st Century. International Cotton Advisory Committee, Washington DC, pp 63–97
Rodgers J, Montalvo JG, Gayle D, Von Hoven TM (2010) Near infrared measurement of cotton fiber micronaire, maturity and finesses—a comparative investigation. Text Res J 80:780–793
Rodgers JE, Delhom C, Fortier CA, Thibodeaux DP (2012) Rapid measurement of cotton fiber maturity and fineness by image analysis microscopy using the Cottonscope®. Text Res J 82:259–271
Rodgers JE, Delhom C, Hinchliffe DJ, Kim HJ, Cui X (2013) A rapid measurement for cotton breeders of maturity and fineness from developing and mature fibers. Text Res J 83:1439–1451
Shahriar M, Scott-Fleming I, Sari-Sarraf H, Hequet E (2013) A machine vision system to estimate cotton fiber maturity from longitudinal view using a transfer learning approach. Mach Vis Appl 24:1661–1683
Thibodeaux DP, Evans JP (1986) Cotton fiber maturity by image analysis. Text Res J 56:130–139
Thibodeaux DP, Rajasekaran K (1999) Development of new reference standards for cotton fiber maturity. J Cotton Sci 3:188–193
Timpa JD, Ramey HH Jr (1994) Relationship between cotton fiber strength and cellulose molecular weight distribution: HVI calibration standards. Text Res J 64:557–562
Turner C, Sari-Sarraf H, Hequet E, Vitha S (2015) Variation in maturity observed along individual cotton fibers using confocal microscopy and image analysis. Text Res J 85:867–883
Turner C, Sari-Sarraf H, Hequet E (2017) Training a new instrument to measure cotton fiber maturity using transfer learning. IEEE Trans Instrum Meas 66:1668–1678
Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32:420–424
Xu B, Huang Y (2004) Image analysis for cotton fibers, part II: cross-sectional measurements. Text Res J 74:409–416
Xu B, Pourdeyhimi B (1994) Evaluating maturity of cotton fibers using image analysis: definition and algorithm. Text Res J 64:330–335
Acknowledgments
The authors thank Tracy Condon of USDA-ARS-SRRC for technical assistance in collecting the experimental samples and data. This project was financially supported by the USDA-ARS CRIS project no. 6054-21000-017-00D. Authors also thank Drs. Bugao Xu of University of North Texas and Noureddine Abidi of Texas Tech University for professional insight to improve the manuscript. Mention of a product or specific equipment does not constitute a guarantee or warranty by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Kim, HJ., Delhom, C.D. et al. Investigation of fiber maturity measurement by cross-sectional image analysis and Fourier transform infrared spectroscopy on developing and developed upland cottons. Cellulose 26, 5865–5875 (2019). https://doi.org/10.1007/s10570-019-02502-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02502-0