Abstract
Reverse micellar dyeing of cotton fabrics with PEG-based non-ionic surfactant in heptane non-aqueous medium with the use of reactive dyes with different reactive groups was investigated. Single jersey knitted fabrics were used in this study. The experimental results reveal that samples dyed by both one-bath and two-bath heptane reverse micellar methods can achieve higher colour yield than the conventional water-based dyeing method. Among different reactive dyes, samples dyed with hetero-bifunctional vinylsulfone based reactive dyes could achieved higher colour yield (K/S) than the homo-bifunctional and monofunctional reactive dyes in non-aqueous dyeing medium. Moreover, the ranges of L*, a* and b* value for hetero-bifunctional vinylsulfone based reactive dyes were narrower than homo-bifunctional and monofunctional reactive dyes. In term of levelness, monofunctional reactive dyes generally resulted a better levelness than homo-bifunctional and hetero-bifunctional reactive dyes. Although one-bath heptane method yields high K/Ssum values, the problem of unlevelness should be considered while the use of two-bath heptane reverse micellar method can strike a balance between levelness and colour yield.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textile dyes, such as direct dyes, reactive dyes, vat dyes, sulphur dyes and azoic dyes, have long been used for dyeing of cellulosic fibres. Among these dyes, reactive dyes are the dyes most commonly used for cotton fibres. Reactive dyes, the first commercial dyes to react with cellulosic fibres with the formation of dye-fibre covalent bond, were first introduced in 1956 by ICI (Ahmed 1995; Aspland 1992).
Reactive dyes have five different interlocking features and they can be assigned different letters as S–C–B–R–X. S represents the solubilising groups; C represents the basic part of the colour molecule; B is the bridging groups linking the reactive part of the molecule to the soluble colour portion; R is reactive groups; and X is the leaving groups. The chemical entities primarily responsible for fibre reaction are mainly B, R and X, affecting reactivity of the dye. Every group has an effect on physical properties of dye molecules including colour, molecular size, ability to diffuse into fibres, solubility, substantivity, salt sensitivity and fastness (Aspland 1997; Broadbent 2001).
The first commercial reactive dyes, Procion M dyes, contained a highly reactive functional group of dichlorotriazine which could react with the hydroxyl group of cellulosic fibres at room temperature under alkaline conditions. After that, dyes containing lower reactivity (hot-type) monochlorotriazinyl (MCT) reactive group and Remazol dyes (warm-type) bearing vinyl sulphonyl (VS) reactive group were introduced by Ciba and Hoechst respectively in subsequent years. However, the initially developed reactive dyes had several drawbacks: (1) low dye fixation; (2) low colour strength; and (3) high dye residue. With the aim to maximize dye fixation, especially in long-liquor-ratio exhaust dyeing, bifunctional reactive dyes, Procion H-E ranges containing two monochloro-triazinyl groups (bis MCT), were introduced by ICI in the early 1970s (Lewis 2014; Taylor et al. 2001).
Bifunctional reactive dyes, which carry two reactive groups in dye molecules, were introduced as a means to improve dye fixation and efficiency in exhaust dyeing of cellulosic fibres (Burkinshaw and Kabambe 2011; Shore 2002) from 50–60 to 70–80% (Smith 2003). They can be further classified into two groups: (a) homo-bifunctional, consisting of two identical reactive groups, for example, C.I. Reactive Red 120, and (b) hetero-bifunctional, comprising two dissimilar reactive groups, for instance, C.I. Reactive Red 194 (Burkinshaw and Paraskevas 2011; Khatri et al. 2015; Taylor 2000).
In recent years, monofunctional and bifunctional reactive dyes have been widely used to improve dyeing quality of cotton fibres. Lewis and Vo (2007) attempted to dye cotton with the use of sulphatoethylsulphone (SES) and monofluorotriazine (MFT)-based reactive dyes under neutral conditions, without the need of alkali. Further work has been done to dye cotton under neutral conditions in the presence of a buffer (Lewis et al. 2008).
Despite neutral pH dyeing, fluorotriazine-based (Cid et al. 2007), instead of dichlorotriazine-based (Cid et al. 2004), reactive dyes were found to be more preferable for dyeing cotton in supercritical carbon dioxide. Attempts have also been made to dye cotton (Ahmed 2005; Farha et al. 2010) and cotton/wool blend (El-Shishtawy et al. 2007) with the use of bifunctional vinyl sulfone-based reactive dyes in the presence of organic salts such as sodium edate. Kan and Fong (2017) studied the reuse of monofunctional and bifunctional vinyl sulfone based reactive dye for cotton dyeing.
Not withstanding the aforementioned, the possibility of using reverse micelle dyeing was also considered as one of the alternative methods for improving dyeing and effluent quality. The use of reverse micelle was first investigated with the application of an anionic surfactant named Aerosol-OT (AOT) (Sawada and Ueda 2003a, b, c). However, the ionic head groups of the surfactant in reverse micelle may cause a negative impact on polarity of the water pool, leading to unstable micro-environment in the dyeing process (Sawada and Ueda 2004a, b). To stabilize the micro-environment, the use of Triton X-100 (non-ionic surfactant) to facilitate the dyeing of cotton fibre in reverse micelle system was investigated (Yi et al. 2012, 2014, 2015).
Our previous work has investigated the feasibility of optimizing the use of poly(ethylene glycol) (12) tridecyl ether (PEG-12), non-ionic surfactant in nature, to facilitate reactive dyeing of cotton in heptane and octane reverse micellar non-aqueous medium (Wang et al. 2016). Experiments on computerised colour matching (CCM) of reverse micellar dyed cotton for production of colour mixture for cotton fabrics in different solvent mediums have also been conducted (Tang et al. 2017a, b, 2018a, c). The influence of HLB value on PEG-based reverse micelle dyeing system has also been studied (Tang et al. 2018b).
Inspired by Siddiqua et al. (2017), this study investigated the role of functional group of bifunctional and monofunctional reactive dyes in terms of colour strength and fastness properties of dyed cotton fabrics and the unknown effect of using different groups of reactive dyes and different dyeing methods on reverse micellar dyeing of cotton. The objectives of this study include: (a) to investigate conventional water-based dyeing and PEG-based non-ionic heptane-assisted one-bath and two-bath reverse micellar dyeing of cotton substrates with reactive dyes of different reactive functional groups; (b) to assess the colour yield and levelness of water-dyed and solvent-dyed samples; (c) to compare samples dyed with reactive dyes of different functional groups in water-based dyeing method and reverse micellar non-aqueous dyeing methods; and (d) to evaluate the relationships between different groups of reactive dyes and the reflectance value, absorption (K/Ssum) value and unlevelness of the coloured cotton samples in conventional water-based dyeing system and reverse micelle heptane-based dyeing system.
Experimental
Materials and reagents
Pure cotton single jersey fabric (40 wales per inch and 55 courses per inch, fabric weight: 140 g/m2), which was ready for dyeing, was used. Soap solution, comprising 2 g/L soda ash and 2 g/L soap, was used to clean the fabric at 90 °C for half an hour. After that, the fabric was thoroughly rinsed with cold water, dried at room temperature and finally conditioned at conditioning room with relative humidity of 65 ± 2% and temperature of 20 ± 2 °C for 24 h before subsequent experiment. Non-ionic surfactant, named poly(ethylene glycol) (12) tridecyl ether (PEG-12) with chemical formula C13H27(OCH2CH2)12OH, and co-surfactant, 1-octanol, were of reagent grade and were used to facilitate the formation of reverse micelle. Heptane solvent (reagent grade) was used as the non-aqueous dyeing medium. Sodium carbonate (Na2CO3) (reagent grade) was used as the colour fixation agent. Sodium chloride (NaCl) was used as exhausting agent for conventional water-based dyeing. Several monofunctional and bifunctional reactive dyes with different reactive groups, provided by Dystar from Shanghai, China, were directly used with no purification (Table 1).
Conventional water-based dyeing of cotton with salt addition
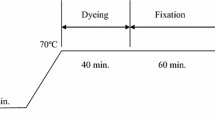
The liquor-to-goods ratio was 50:1 in conventional water-based cotton dyeing. The amount of electrolyte added in the dye liquor was based on concentration of reactive dye used (percentage on weight of fibre, % owf) as listed in Table 2. The conventional water-based cotton dyeing profile is shown in Fig. 1. To begin with, the cotton fabric was first immersed in the dye liquor containing the corresponding amount of dye and salt. The dye liquor was then put into the shaking water bath at 30 °C and agitated for 10 min. After 10 min, the temperature was gradually raised to 70 °C at a rate of 2 °C/min. Dyeing process was begun at 70 °C for 40 min. Soda ash (5 g/L), as listed in Table 2, was then added into the dye liquor for dye fixation at 70 °C for 60 min. After fixation, the cotton fabric was then immersed in soap solution (2 g/L) and rinsed for 20 min at 50 °C twice, followed by rinsed with cold water, air-dried and conditioned at relative humidity of 65 ± 2% and 20 ± 2 °C for at least 24 h prior to further experiment.
Dye encapsulated PEG-based reverse micelles preparation
The PEG-12 nonionic reverse micelles were prepared by simple injection at room temperature (Tang et al. 2017a, b; Wang et al. 2016). PEG-12 non-ionic surfactants were first premixed with co-surfactants (1-octanol) with agitation. The surfactant to co-surfactant ratio, 1:8 mol ratio (1.49 ml co-surfactant per ml of surfactant), was used. The surfactant/co-surfactant mixture was then dissolved in n-heptane non-polar solvent (regent grade with min. 99% purity), to facilitate self-assembling of reverse micelles. The solvent volume used was corresponding to cotton weight ratio, which was 8:1 (8 ml solvent per gram of cotton). Dye concentrations in aqueous solutions prepared were 0.2% w/v, 1% w/v, 3% w/v, 5% w/v and 7% w/v. Reactive dye aqueous solution of 0.5 ml was then added into the reverse micelle solution dropwise. After injecting the dye solution, the mixtures were stirred until it was well dispersed with dye encapsulated in the reverse micelle.
Heptane-based reverse micellar dyeing of cotton fabrics
Five colour depths of dyeing, 0.1%, 0.5%, 1.5%, 2.5% and 3.5% on weight of fibre, were used. Cotton fabrics was dyed with 1:25 surfactant to water mole ratio, which was 0.51 ml of water per ml of surfactant. The PEG-based reverse micelle dye liquors were freshly prepared according to “Dye encapsulated PEG-based reverse micelles preparation” section and the whole dyeing process was launched with no electrolytes addition. The dyeing profiles of one-bath and two-bath reverse micellar dyeing are shown in Figs. 2 and 3 respectively. Soda ash amount added in the heptane-assisted reverse micellar dyeing system is listed in Table 3.
With regard to one-bath reverse micellar dyeing (Fig. 2), cotton fabric was first immersed in reverse micellar dye liquor and was then poured into the shaking water bath at 30 °C agitating for 10 min. The temperature was then raised to 70 °C at a rate of 2 °C/min. Cotton fabrics were dyed at 70 °C for 40 min. Soda ash aqueous solution (0.3 ml) was then added into the dye liquor (Table 3) for dye fixation at 70 °C for 60 min. The dyed fabrics were then immersed in soap solution (2 g/L) at 50 °C for 20 min twice, thoroughly rinsed with water, air-dried and conditioned for 24 h before further experiment.
Regarding two-bath reverse micellar dyeing (Fig. 3), cotton fabric was first dipped into the prepared dye-encapsulated reverse micelle solution, put into the shaking water bath at 30 °C and agitated for 10 min. The temperature was then raised to 70 °C at a rate of 2 °C/min for dyeing. After 40 min, soda ash aqueous solution (0.3 ml) was then added into the dye liquor (Table 4). The alkali-added dye liquor was then put into another shaking water bath set at 30 °C and agitated for 10 min. Fixation process takes place when dyeing temperature is increased to 70 °C for 40 min, followed by rinsing in soap solution (2 g/L) at 50 °C for 20 min twice, rinsed with cold water, air-dried and conditioned for 24 h before subsequent measurement. In this study, two bath method did not mean the transfer of fabric samples from one dye liquor to another. It meant the transfer of dye liquor from one water-bath to another.
Colour yield
Colour yield of water-dyed and solvent-dyed samples using various reactive dyes was assessed by SF650 Spectrophotometer (DataColor International, USA). K/S values of dyed substrates were obtained by measurement in visible wavelengths between 400 and 700 nm under the following parameters: (1) large aperture (30 mm) with specular included; (2) folding the dyed substrates two times to assure opacity; (3) measuring face side of dyed fabrics; (4) illuminant D65 light source; and (5) 10° standard observer. Four measurements were conducted and averaged using the same parameters.
The colour yield, expressed in the form of K/S value from wavelengths between 400 nm and 700 nm at 10 nm intervals, was calculated by Eq. (1) (Xin 2006) while the K/Ssum values were calculated by summing the K/S values between wavelengths of 400 nm and 700 nm. The larger the K/S values and K/Ssum values, the higher would be the uptake of dye, and the better would be the colour yield.
where K is absorption coefficient, depending on colourant concentration, S is scattering coefficient of the dyed samples, and R is reflectance of the dyed samples.
Levelness measurement
The levelness of the dyed fabrics was measured by using Relative Unlevelness Indices (RUI). It was obtained by measuring reflectance values of the four random selected spots on dyed substrates within (λ = 400–700 nm) at 10 nm intervals. The number of repeats for measurement is four. SF650 Spectrophotometer (DataColor International, USA), set with parameters of illuminant D65, specular reflection, a standard observer of 10° and a large aperture (30 mm), was used for conducting the measurement.
Four equations were mainly used for RUI measurement: (1) standard deviation (sλ) of reflectance values at a specific wavelength (Eq. 2); (2) uncorrected relative unlevelness index (RUIu) (Eq. 3); (3) corrected relative unlevelness index (RUIc) obtained by summation of coefficients of variation of reflectance values (Eq. 4); and (4) relative unlevelness index (RUI) obtained by multiplying coefficients of variation of reflectance by the photopic relative luminous efficiency function (Vλ) (Eq. 5). The RUI values are suggested to be interpreted as listed in Table 4 (Chong et al. 1992).
CIE L*a*b* measurement
The CIE L*a*b* of conventional water-dyed and reverse micellar dyed substrates were assessed by SF650 Spectrophotometer using illuminant D65 light source, specular reflection, 10° standard observer and a large aperture (30 mm). The number of repeats for measurement is four.
Colourfastness to laundering
The colourfastness to laundering of the dyed substrates were evaluated by AATCC Test Method 61-2013 (Colourfastness to Laundering: Accelerated) with Test No. 2A.
Solvent recovery study
After reverse micellar dyeing, 40 ml of dyeing waste (containing heptane) was placed into 100 ml round-bottom flask with magnetic stirring for distillation. The round-bottom flask was immersed in oil bath and connected with a thermometer and a condenser tube via a three-way adaptor. The distillation process was controlled at a rate of 1 drop per second under appropriate heating power. The temperatures measured at the intersection region of three-way adaptor were 98 °C for heptane. The distillate was completely collected when the temperature starts to drop and no further distillates were collected after condensation. The collected distillates was then transferred into a separatory funnel, two-phase layers can be observed. A small amount of water was at the bottom layer and the upper layer was the recovered solvent. From the collection of distillate, the percentage of solvent recovery based on the ratio of amount of recovered solvent to the theoretical prediction of solvent volume used before dyeing could be determined.
Results and discussion
Colour yield
Table 5 illustrates the colour yield, in terms of K/Ssum values, of the reactive-dyed cotton fabrics using different dyeing approaches. The experimental results reveal that one-bath heptane dyeing approach can generally achieve the highest colour yield, followed by two-bath heptane approach and conventional water-based dyeing approach. Most of the K/Ssum values obtained by heptane-assisted reverse micellar dyeing approaches, whether one-bath or two-bath, are nearly 2 to 3 times higher than the conventional water-based dyeing approaches, indicating the superiority of the reverse micellar approach for dyeing of cotton fabrics.
The K/Ssum values of the dyed fabrics, to a large extent, reflected the colour yield of the dyestuff. Table 5 shows that the highest colour yield obtained was Navy-01 (TFP-VS), followed by Black-01 (DCT-VS) and Blue-02 (FCP-VS). They are hetero-bifunctional vinylsulfone-based reactive dyes which can produce fabrics with dark shades. One possible reason is that the two different reactive groups are highly compatible to each other so that both can greatly contribute to fixation of dye in fibre (Taylor 2000), resulting in high colour yield and dark shade.
To compare the colour yield of homo-bifunctional and hetero-bifunctional reactive dyes, Blue-03 (VS–VS), Blue-02 (FCP-VS) and Blue-01 (MFT-VS) were taken as examples. Fabrics dyed by Blue-02 obtained the highest colour yield, followed by Blue-03 and Blue-01. As pointed out by Khatri et al. (2015), hetero-bifunctional dyes provide better fixation and higher flexibility for colouration method and process parameters. Although Blue-03 is a homo-bifunctional reactive dye, its colour yield is higher than that of Blue-01. The possible reason is compatibility of reactive groups in which the reactive groups of Blue-01 (MFT-VS) are not as compatible as Blue-03 (VS–VS). Blue-03 has double VS group in which the working temperature range for dyeing application is much narrower than that of Blue-01 which has two different reactive groups, leading to wider working temperature range. It is also well known that vinyl sulphone (VS) groups generally have higher reactivity than monochlorotriazine (MCT) and monofluorotriazine (MFT) groups. Therefore, Blue-03 has reactive groups with higher compatibility (in terms of working temperature range for dyeing application and reactivity), resulting in better colour yield than that of Blue-01.
Generally speaking, the VS and FCP groups are alkali-controllable while MFT group is salt-controllable. This means the substantivity of VS and FCP groups to cotton fibre is controlled by alkali addition while the substantivity of MFT group to cotton fibre is controlled by salt addition. In the reverse micellar dyeing process in this study, no salt was added in the dyeing process. Therefore, Blue-01 (MFT-VS) has a lower substantivity when compared with Blue-03 (VS–VS) and Blue-02 (FCP-VS).
In this experiment, most of the monofunctional reactive dyes are dyes with brilliant colours, for instance, Blue-04 (MFT), Red-02 (FCP) and Yellow-02 (VS). They normally have one reactive group which has lower colour yield than dyes with more than one reactive group (bifunctional). Therefore, lower colour yield and lighter shade are generally observed for samples dyed by monofunctional reactive dyes.
Reflectance curves
Figures S1 (a) to (k) (Supplementary Information) represent reflectance curves of the dyed samples with different reactive dyes and by different dyeing methods. Generally speaking, the higher the dye concentration is, the lower would be the reflectance percentage and darker would be the shade of the fabric. From Fig. S1, samples dyed by conventional water-based method exhibit significantly higher reflectance percentage than those dyed with reverse micellar methods. For reverse micellar dyeing methods, one-bath method yields slightly higher reflectance percentage than the two-bath method, but the difference is small and not significant. Therefore, it can be concluded that samples dyed by conventional water-based method result in fabric with the lightest shade while the darkest shade of the fabric could be obtained by using one-bath reverse micellar method.
The reflectance curves show unique colour patterns for different colours of dyestuff used for dyeing. Generally speaking, no peak shifting of the curves is observed. This indicates that there is no chromatic shift or colour change when the samples are dyed by reverse micellar system in non-aqueous alkane medium. Since our previous works mainly focused on three primary colours and mixed colours for conducting computerised colour matching (Tang et al. 2017a, b, 2018a, c), this work further proves that it is feasible to apply the reverse micellar dyeing system for dyeing of cotton with different types of reactive dyes to produce fabrics with different colour appearances.
CIE L*a*b* values
Table S1 (Supplementary Information) presents CIE L*a*b* values of cotton fabric dyed with different reactive dyes and by different dyeing methods. The L* value means lightness. The a* value and b* value represent red-green and yellow-blue and vary according to the colour of the dyed fabrics. For example, if the dyed fabric is red in colour, its a* value will increase when the dye concentration used increases. The a* value will be negative when the dyed fabrics possess an increasing green element. The b* value increases when more yellow element is found while it decreases to negative value when more blue element is encountered. From Table 6, the conventional water-dyed fabrics generally have higher L* values, which means lighter in shade, than the heptane-dyed fabrics by one-bath and two-bath methods.
The values of a* and b* vary according to different dye concentrations used and colour elements encountered. Concerning the a* value, samples dyed by using heptane generally obtained higher a* value than that of using water. It indicates that the use of reverse micellar heptane methods can result in fabrics with more red elements (redder) than that of the conventional water-based method and the difference in a* value is obvious for samples dyed using conventional method and heptane methods except the red reactive dyes. When compared between one bath heptane and two-bath heptane methods, one bath heptane method can generally achieve higher a* value (more red elements) than that of the two-bath method.
Regarding the b* value, heptane dyed samples generally achieved higher b* value than water-dyed samples. It indicates that more yellow elements can be found on heptane-dyed samples and large difference in b* value can be observed when the fabric samples were dyed in red and yellow colours. When compared one bath method with two-bath method, slightly higher b* value can be achieved by one bath method since one bath method does not require the transfer of one water-bath to another and the samples can thus gain benefit from continuous heating in a single bath at the same temperature.
To compare between vinylsulfone-based reactive dyed samples, it is found that the range of a* and b* values is getting broader when heptane methods were used for dyeing of samples in 5 colour depths while conventional water-dyed method result in narrower range in a* and b* values. It is also observed that hetero-bifunctional reactive dyes, such as Blue-01 (MFT-VS), generally have the narrowest range in a* and b* values, followed by homo-bifunctional reactive dyes, such as Blue-03 (VS–VS), and monofunctional reactive dyes, such as Yellow-02 (VS).
Levelness
Table 6 illustrates unlevelness indices of dyed fabrics. The measured results reveal that conventional aqueous dyeing approach and two-bath heptane dyeing approach can gain good to excellent levelness while one-bath heptane approach results in large variations in levelness (from poor to excellent levelness). Most of the poor levelness results are found on fabrics dyed with navy and black colours by one-bath heptane method. Although most of them have high colour yield, the problem of unlevelness may be encountered. The typical example is Black-01 (DCT-VS). One of the possible reasons is that fabrics with different properties are used in this study. Instead of using cotton interlock knitted fabrics used in our previous studies (Tang et al. 2017a, 2018a, c; Wang et al. 2016), this work used single jersey knitted fabrics which are much thinner and have a higher tendency for curling. Another reason is related to the planarity of the dye chemical structure (Siddiqua et al. 2017). For the two-bath heptane method, levelness of the dyed samples is good to excellent partly due to the temperature control by transferring the dye liquor from the bath at 70 °C to a bath set at 30 °C so that the temperature rises in a controlled and gradual manner, resulting in better levelness than the one-bath heptane method. The two-bath heptane methods in this case is more suitable for dyeing of thin knitted fabric with reasonable colour yield and levelness.
In general, conventional water-based dyeing method can gain better levelness since 50:1 liquor ratio is used in this study so that the chance of unlevel dyeing is highly minimized while heptane methods, due to the use of low liquor ratio (8:1), have particularly higher chance of unlevelness problem under objective instrumental measurement. In terms of dye concentration, 1.5% colour depth in one-bath method obtained poor levelness in some colours, such as Black-01, Blue-02 and Blue-04. However, dye concentration generally does not have significant effect towards levelness. In terms of dye structure, it is found that monofunctional reactive dyes generally have the best levelness, followed by homo-bifunctional reactive dyes while hetero-bifunctional reactive dyes have the poorest levelness which require more effort and control to alleviate the unlevelness problem especially in one bath heptane method.
Washing fastness results
Tables S2 (Supplementary information) and 7 represent the results of colour change and colour staining after the washing fastness test, respectively and they reveal that most of the dyed samples have excellent washing fastness (rating 5) against colour change and colour staining, except some substrates coloured in high concentration with dark shade such as Black-01 and Blue-02 which scored ratings of 4–5. This implies that dyed samples are adequately washed and rinsed during a series of dyeing operations and nearly no unfixed dyes remained in the dyed samples (Table S2 and Table 7).
Solvent recovery study
In the solvent recovery study, 38.2 ml heptane can be recovered from 40 ml dyeing waste after reverse micellar dyeing. A solvent recovery percentage of 95.5% can be achieved. Although solvent is involved in the reverse micellar dyeing process, the high solvent recovery percentage makes the dyeing process becomes sustainable.
Conclusion
The dyeing of cotton using PEG-based heptane-assisted reverse micellar dyeing system with different reactive groups of reactive dyes and different dyeing profiles was investigated. Single jersey knitted fabrics were used in this study. The experimental results reveal that samples dyed by both one-bath and two-bath heptane-assisted reverse micelle methods can gain higher colour yield than that in conventional water-based dyeing method. Among different reactive dyes, hetero-bifunctional vinylsulfone based reactive dyes can generally achieve higher colour yield (K/S) than homo-bifunctional and monofunctional reactive dyes in non-aqueous dyeing medium. Moreover, the ranges of L*, a* and b* value for hetero-bifunctional vinylsulfone based reactive dyes are narrower than homo-bifunctional and monofunctional reactive dyes. However, in terms of levelness, monofunctional reactive dyes generally results in better levelness than homo-bifunctional and hetero-bifunctional reactive dyes. Although one-bath heptane method yields high K/Ssum value, the problem of unlevelness should be considered while the use of two-bath heptane method can strike a balance between levelness and colour yield. The washing fastness test was conducted, and the ratings were between 4–5 and 5 which indicate that the samples were thoroughly rinsed with excellent washing fastness properties.
References
Ahmed A (1995) Reactive dyes development: a review. Text Dye Print 28:19–24
Ahmed NS (2005) The use of sodium edate in the dyeing of cotton with reactive dyes. Dyes Pigments 65:221–225
Aspland J (1992) Reactive dyes and their application. Text Chem Color 24:31–36
Aspland JR (1997) Textile dyeing and coloration. American Association of Textile Chemists and Colorists, Research Triangle Park, pp 105–124
Broadbent AD (2001) Basic principles of textile coloration. Textile coloration. Society of Dyers and Colorists, Bradford, pp 332–357
Burkinshaw S, Kabambe O (2011) Attempts to reduce water and chemical usage in the removal of bifunctional reactive dyes from cotton: part 2 bis (vinyl sulfone), aminochlorotriazine/vinyl sulfone and bis (aminochlorotriazine/vinyl sulfone) dyes. Dyes Pigments 88:220–229
Burkinshaw S, Paraskevas M (2011) The dyeing of silk: part 4 heterobifunctional dyes. Dyes Pigments 88:396–402
Chong C, Li S, Yeung K (1992) An objective method for the assessment of levelness of dyed materials. J Soc Dye Colour 108:528–530
Cid MF, Van der Kraan M, Veugelers W, Woerlee G, Witkamp G (2004) Kinetics study of a dichlorotriazine reactive dye in supercritical carbon dioxide. J Supercrit Fluids 32:147–152
Cid MF, Van Spronsen J, Van der Kraan M, Veugelers W, Woerlee G, Witkamp G (2007) A significant approach to dye cotton in supercritical carbon dioxide with fluorotriazine reactive dyes. J Supercrit Fluids 40:477–484
El-Shishtawy RM, Youssef Y, Ahmed NS, Mousa A (2007) The use of sodium edate in dyeing: II. Union dyeing of cotton/wool blend with hetero bi-functional reactive dyes. Dyes Pigments 72:57–65
Farha SA, Gamal A, Sallam H, Mahmoud G, Ismail L (2010) Sodium edate and sodium citrate as an exhausting and fixing agents for dyeing cotton Fabric with reactive dyes and reuse of dyeing effluent. J Am Sci 6:109–127
Kan C, Fong K (2017) A study of reusing vinyl sulfone based reactive dye for dyeing cotton fiber. Fiber Polym 18:2176–2186
Khatri A, Peerzada MH, Mohsin M, White M (2015) A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J Clean Prod 87:50–57
Lewis DM (2014) Developments in the chemistry of reactive dyes and their application processes. Color Technol 130:382–412. https://doi.org/10.1111/cote.12114
Lewis DM, Vo LT (2007) Dyeing cotton with reactive dyes under neutral conditions. Color Technol 123:306–311
Lewis DM, Broadbent PJ, Vo LT (2008) Covalent fixation of reactive dyes on cotton under neutral conditions. AATCC Rev 8(1):35–41
Sawada K, Ueda M (2003a) Adsorption and fixation of a reactive dye on cotton in non-aqueous systems. Color Technol 119:182–186
Sawada K, Ueda M (2003b) Adsorption behavior of direct dye on cotton in non-aqueous media. Dyes Pigments 58:37–40
Sawada K, Ueda M (2003c) Dyeing of protein fiber in a reverse micellar system. Dyes Pigments 58:99–103
Sawada K, Ueda M (2004a) Characteristics of aqueous microenvironments in non-ionic surfactant reverse micelles and their use for enzyme reactions in non-aqueous media. J Chem Technol Biotechnol 79:369–375
Sawada K, Ueda M (2004b) Enzyme processing of wool fabrics in a non-ionic surfactant reverse micellar system. J Chem Technol Biotechnol 79:376–380
Shore J (2002) Chemistry of reactive dyes. In: Shore J (ed) Colorants and auxiliaries, vol 1. Society of Dyers and Colourists, Bradford, pp 356–443
Siddiqua UH, Ali S, Iqbal M, Hussain T (2017) Relationship between structures and dyeing properties of reactive dyes for cotton dyeing. J Mol Liq 241:839–844
Smith B (2003) Wastes from textile processing. In: Andrady AL (ed) Plastics and the environment. Wiley, Hoboken, pp 293–295
Tang AYL, Lee CH, Wang Y, Kan CW (2017a) Octane-assisted reverse micellar dyeing of cotton with reactive dyes. Polymers 9:678
Tang AYL, Wang Y, Lee CH, Kan CW (2017b) Computer color matching and levelness of PEG-based reverse micellar decamethyl cyclopentasiloxane (D5) solvent-assisted reactive dyeing on cotton fiber. Appl Sci 7:682
Tang AYL, Lee CH, Wang Y, Kan CW (2018a) Dyeing properties of cotton with reactive dye in nonane nonaqueous reverse micelle system. ACS Omega 3:2812–2819
Tang AYL, Lee CH, Wang YM, Kan CW (2018b) Effect of hydrophilic-lipophilic balance (HLB) values of PEG-based non-ionic surfactant on reverse micellar dyeing of cotton fibre with reactive dyes in non-aqueous medium. Fiber Polym 19:894–904. https://doi.org/10.1007/s12221-018-8061-y
Tang AYL, Wang Y, Lee CH, Kan CW (2018c) Comparison of computer colour matching of water-based and solvent-based reverse micellar dyeing of cotton fibre. Color Technol 134:258–265. https://doi.org/10.1111/cote.12333
Taylor JA (2000) Recent developments in reactive dyes. Color Technol 30:93–108
Taylor JA, Pasha K, Phillips DA (2001) The dyeing of cotton with hetero bi-functional reactive dyes containing both a monochlorotriazinyl and a chloroacetylamino reactive group. Dyes Pigments 51:145–152
Wang Y, Lee CH, Tang YL, Kan CW (2016) Dyeing cotton in alkane solvent using polyethylene glycol-based reverse micelle as reactive dye carrier. Cellulose 23:965–980
Xin JH (2006) 8—Controlling colourant formulation. In: Xin JH (ed) Total colour management in textiles. Woodhead Publishing, Sawston, pp 136–159
Yi S, Dong Y, Li B, Ding Z, Huang X, Xue L (2012) Adsorption and fixation behaviour of CI Reactive Red 195 on cotton woven fabric in a nonionic surfactant Triton X-100 reverse micelle. Color Technol 128:306–314
Yi S, Deng Y, Sun S (2014) Adsorption and dyeing characteristics of reactive dyes onto cotton fiber in nonionic Triton X-100 reverse micelles. Fiber Polym 15:2131–2138
Yi S, Tong X, Sun S, Dai F (2015) Dyeing properties of CI reactive violet 2 on cotton fabric in non-ionic TX-100/Span40 mixed reverse micelles. Fiber Polym 16:1663–1670
Acknowledgments
Authors would like to thank the financial support from The Hong Kong Polytechnic University for this study (Grant Nos. RUV7, 4-ZZGK and G-UADU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, A.Y.L., Lee, C.H., Wang, Y.M. et al. A study of PEG-based reverse micellar dyeing of cotton fabric: reactive dyes with different reactive groups. Cellulose 26, 4159–4173 (2019). https://doi.org/10.1007/s10570-019-02340-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02340-0