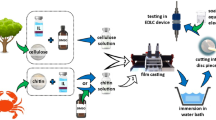
Abstract
Cellulose/poly(vinyl alcohol) (PVA) composite gels are prepared as separators for quasi-solid-state electrical double-layer capacitors (EDLCs) by a simple freeze-thawing method. Fourier-transform infrared spectroscopy, scanning electron microscopy and mechanical testing machine are used to characterize the structure and morphology. Compared with the PVA gel, the as-prepared composite gels show improved network structure and enhanced mechanical properties. The cyclic voltammetric curves, galvanostatic charge/discharge curves, electrochemical impedance spectroscopy and cycling performance of EDLCs with these composite gels are also evaluated. It is found that the EDLC with the optimum composite gel can work in a voltage window of 0–1.8 V and display a specific capacitance of 125.1 F g−1 (based on active carbon on one electrode) at a current density of 1 A g−1. Furthermore, it also has an excellent cycling stability with capacitance retention of ~ 88% after 1500 cycles. These results suggest that the composite gels can serve as a class of promising separators for EDLCs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditional supercapacitors use liquid electrolytes such as acid or alkali solution, similar to batteries (Anothumakkool et al. 2014; Li et al. 2014). However, this configuration impedes further applications for two reasons: the use of solutions makes the supercapacitor heavier, which causes difficulties in their integration, and the possibility of harmful electrolyte leakage requires that they be safely encapsulated, giving rise to increased costs (Anothumakkool et al. 2014; Niu et al. 2013). Thus, flexible gel polymer electrolytes have been developed as substitutes of liquid electrolytes (Moon et al. 2015).
Flexible devices are a mainstream direction in modern electronics and related multidisciplinary fields (Liu et al. 2014; Yang et al. 2013). Electrical double-layer supercapacitors (EDLCs) with flexible solid gel polymer electrolytes have emerged as a new class of energy storage devices and attracted considerable attention in recent years due to their small size, low weight, ease of handling, and excellent reliability.
Gel polymer electrolytes still have several defects, such as poor mechanical strength and low ionic conductivity. Poly(vinyl alcohol) (PVA) hydrogel is a widely used gel polymer electrolyte for supercapacitors (Ma et al. 2014; Wang et al. 2012). However, the PVA-based gel polymer electrolytes will became brittle and show poor mechanical strength due to the damage of inorganic ions to the hydrogen bond between PVA polymer chains and water molecules when PVA hydrogels are combined with inorganic salts, acid or alkali dissolved in water.
Cellulose is one of the most abundant biopolymers on earth, occurring in wood, cotton, hemp and other plant-based materials and serves as the dominant reinforcing phase in plant structures. It can also be adopted in gel polymer electrolytes to provide high mechanical integrity. Abitbol et al. (2011) and Lu et al. (2008) incorporate cellulose nanocrystals into poly(vinyl alcohol) (PVA) to improve the mechanical properties, structural stabilities and distinct microstructures. Chang et al. (2008) dissolved cellulose in a mixed aqueous solution of sodium hydroxide (NaOH) and urea (H2NCONH2) to uniformly disperse the cellulose in the solution. In order to solve the problem of low ionic conductivity of polymer gel supercapacitors, Zhang et al. (2015) report that Li2SO4 could be easily added into PVA aqueous solution over a large amount and the ionic conductivity could be improved further with a wide operation electrochemical window. Similarly, other lithium salts, including LiCF3SO3, LiN(SO2CF3)2, LiPF6, LiBF4, and LiClO4, have also been explored as conducting salts in PVA hydrogel (Huang et al. 2012; Moon et al. 2015; Trapa et al. 2005; Wang et al. 2012). Furthermore, the electrochemical windows of EDLCs with such gel polymer electrolytes even exceed 2.0 V (Gao et al. 2012; Huang et al. 2015; Luo et al. 2008; Wessells et al. 2012).
In this work, we developed a novel flexible cellulose/PVA composite gel by solution freeze-thawing method. Thereafter, it was doped with Li2SO4, which endows the resultant gel polymer electrolyte with high ionic conductivity and superior mechanical properties. The optimization of the raw material ratio between cellulose and PVA, mechanical properties of these composite gels and electrochemical performances of EDLCs with the resultant flexible gel polymer electrolytes were explored and analyzed in detail.
Experimental section
Synthesis of cellulose/PVA composite gel
The cellulose/PVA composite gel was prepared in solution by freeze-thawing method. Firstly, 6 g PVA was dissolved in 100 mL distilled water with agitation at 90 °C for 2 h to form a homogeneous and low-viscous solution. 4.0 g of cellulose microcrystalline, 7.0 g NaOH and 12.0 g urea were dissolved in 150 mL distilled water to form a homogeneous opalescent solution after long stirring and three freeze-thawing cycles. Secondly, 0.6 g above-mentioned homogeneous opalescent solution and 5 g PVA aqueous solution were put into a 20 mL glass bottle at room temperature under constant stirring for 1 h. Then the solution was kept at 25 °C for 2 h without stirring to remove the bubble. Next, the resultant mixture was poured into rounded glass petri dishes (diameter = 12 cm) and was freeze for over 12 h until a constant mass was arrived, followed by taking out the gel and thawing it at room temperature. The cycle was repeated for three times at least. Finally, the obtained cellulose/PVA composite gel was immersed into the deionized water for 5 days to remove the residual NaOH and urea. The schematic illustration of cellulose/PVA composite gel preparation is given in Scheme 1.
For comparison, a series of experiments were carried out by adjusting the weight ratio of cellulose to PVA. When the ratio of cellulose to PVA is 0, 0.025, 0.05, 0.075, 0.3, 0.5 and 1, the samples were named as CP-0, CP-2.5, CP-5.0, CP-7.5, CP-30, CP-50 and CP-100, respectively.
Preparation of carbon electrodes
The uniform slurry was prepared by mixing of activated carbon (8 mg, 80%), conductive carbon black (1.5 mg, 15%), and PTFE (0.5 mg, 5%) in 0.4 mL ethanol. Then, the obtained slurry with a rectangle area of 1.0 × 1.0 cm2 was coated and roll-pressed on stainless steel meshes (0.1 mm thickness and 99% purity), further vacuum dried at 80 °C overnight to obtain an activated carbon electrode.
Quasi-solid-state double layer supercapacitors assembling
The electrochemical performance of EDLC cells was analyzed using a symmetrical two-electrode capacitor configuration. Prior to assembly into a supercapacitor, the gel was soaked in a 1.5 mol L−1 Li2SO4 solution for 1 week while changing the solution once a day, a hydrogel of the cellulose/PVA composite electrolyte was obtained, and excess electrolyte was removed from the surface using absorbent tissue paper. The symmetrical supercapacitor consisted of two facing carbon electrodes, sandwiching a piece of gel polymer as electrolyte and separator. The quasi-solid-state PVA gel-based EDLC was encapsulated using EVA hot-melted glue under an ambient atmosphere. At last, quasi-solid-state EDLC can be obtained from two electrodes and a resultant cellulose/PVA composite gel.
Characterization
Sample analysis
The surface morphologies of the samples were sputtered a layer of platinum with a thickness of several nanometers and then characterized by a scanning electron microscope (SEM, JSM-IT300). The Fourier Transform infrared (FTIR) spectra were recorded using a Nicolet 6700 FTIR spectrometer. The mechanical stretch measurements of cellulose/PVA gel polymer (rectangular, 0.8 cm × 3.5 cm) were performed at 20 °C with two-column testing machine Instron 5967 (Instron Co. Ltd).
Characterization of the electrolyte uptake and leakage ratios
The electrolyte uptake and leakage ratios of the composite gels were measured by soaking composite gels in the electrolyte at room temperature for a week. The weight (Mt) of a wet composite gel was measured after sandwiching it between two pieces of filter papers and placing a 200 g glass plate on the top filter paper for 5 min. Mdry was measured after the gel freeze drying for 48 h. The electrolyte uptake (W%) and electrolyte leakage ratios (WL%) were calculated using the following equations:
For each composite gel, three samples were tested and the average value was taken from the three measurements.
Characterization of the gel ionic conductivity
The ionic conductivity (σ) values of the gel samples were determined by alternating current (AC) impedance spectroscopy (electrochemical workstation model: CHI 660E, Shanghai Chen Hua Co., Ltd), which was performed in the frequency range of 100 kHz–1 Hz. The impedance data were obtained by sandwiching the gel with electrolyte between two parallel Pt electrodes at room temperature. The σ was calculated using the following equation:
where Rb (ohm) is the resistance of the bulk electrolyte, L (cm) is the thickness of the gel membrane, and S (cm2) is the effective area between the gel and the Pt electrodes. For each membrane sample, the average of three measurements was taken.
Electrochemical measurements
The electrochemical performance of the as-assembled solid-state flexible EDLC was evaluated with an electrochemical workstation (CHI 660E, Shanghai Chen Hua Co., Ltd). The specific capacitance of the single electrode was calculated from the galvanostatic charge/discharge data by using the equation of C = 4IΔt/(mΔV), where I is the constant current (A), m is the total mass of AC for both electrodes (g), Δt is the discharge time (s), and ΔV is the voltage range after the IR drop during the discharge process (V).
Results and discussion
The FTIR spectra of PVA gel, cellulose/PVA composite gels and cellulose gel are shown in Fig. 1. For cellulose sample, several characteristic absorption peaks are observed at 3320, 2896, 1649 and 894 cm−1, which could be attributed to the O–H stretching vibrations, C–H stretching vibrations, deformation vibrations of the absorbed water molecules and –CH bending vibrations, respectively (Hai and Sugimoto 2018; He et al. 2018; Liang et al. 2018). The characteristic cellulose signals of C–O–C glycosidic ring vibration at 1057 and 1020 cm−1 (indicated by the dotted circle) is observed in the spectrum (Castro et al. 2014). For PVA sample, the characteristic peaks centered at 3264 cm−1 and range from 2908 to 2940 cm−1 were assigned to the stretching vibrations of –OH groups and the –CH2– stretching vibrations, respectively. It is also visible at peaks of 1087 and 831 cm−1, which could be associated with the stretching of C–O groups and rocking of –CH, respectively (Liu et al. 2013; Wu et al. 2010). For cellulose/PVA composite gels, characteristic peaks of cellulose and PVA were observed, but some of them were overlapped. Furthermore, the peaks corresponding to –OH groups were broadened and strengthened with increasing the content of cellulose, which could be ascribed to the overlapping effect and increased intermolecular hydrogen bonds between cellulose and PVA (Wang et al. 2018).
Figure 2 shows the SEM images of freeze-thawing cellulose/PVA composite gels at high and low magnification. The cellulose/PVA gel sample was frozen for over 12 h, and then put into the lyophilizer for 48 h to sublimate the water from the solid to gaseous without change the original morphology. All of them showed a mixture of micro-sized fibers with mainly a web-like structure. Figure 2a presented the typical SEM images of the CP-0, where it could be seen that the distribution of the gel surface morphology vary approximately circular. Many pores exhibited a diameter higher than 1 µm, which was faverable for electrolyte diffusion, but unfaveralbe for the enhancement of mechanical property. Figure 2b also displayed network structure, but the pore size and porosity were greatly decreased compared to the CP-0, which might be due to increased intermolecular hydrogen bonds with the incorporation of the cellulose. The network structure might not only maintain the electrolyte diffusion, but also enhance the mechanical property. Figure 2c suggested that the CP-5.0 sample exhibited homogeneous porous architecture, indicating good or certain miscibility between cellulose and PVA. At the same time, the micron-sized pores are in the range of a few hundred nanometers to up to several micrometers. The web-like morphology was also observed at low magnification. From Fig. 2d, e, it could be seen that the interconnection between the holes led to the serious destruction of the mesh structure in CP-7.5 and CP-50 samples because of excessive cellulose, which was disadvantageous for electrolyte diffusion. As shown in Fig. 2f, the pure cellulose (CP-100) gel had nearly no pore structure.
As shown in Fig. 3a, the first three gel samples have close ionic conductivities because all of them have similar network structures. It is worth noting that the ionic conductivity of CP-5.0 is up to 1.85 × 10−2 S cm−1 when the ratio of cellulose to PVA reaches 0.05. But the ionic conductivity of gel samples displayed obvious reduction with further increase of cellulose content to 7.5% due to the destroyed porous structures. Furthermore, as shown in Fig. 2, the damage becomes more and more serious with the increase of the cellulose content. In order to study the influence of the different electrolyte concentration in the gel on the ionic conductivity, the CP-5.0 gel was soaked in Li2SO4 solution with different concentrations, and then the ionic conductivity was measured. The results show that the ionic conductivity will no longer increase when the Li2SO4 concentration exceeds 1.0 mol L−1 (Fig. 3b).
Figure 4a, b shows the cellulose/PVA composite gel (CP-5.0) tested on a universal testing systems, suggesting its good toughness. The stress–strain behaviors of CP-0, CP-2.5, CP-5.0 and CP-7.5 composite gels are shown in Fig. 4c. It could be observed that the cellulose/PVA composite gels were easily elongated without fracture. With the increase of cellulose content, the tensile stress and the elongation at break of the cellulose/PVA composite gels were improved, indicating that the cellulose/PVA composite gel became tougher. When the ratio of cellulose to PVA reaches 0.05, the cellulose/PVA composite gel could be stretched more than 2.0 times compared to the original length. The results clearly demonstrated that this flexible electrolyte was mechanically robust. The flexibility of CP-5.0 composite gel was shown in Fig. 4d by rolling around a glass rod. The CP-5.0 gel electrolyte uptake (W%) and electrolyte leakage ratios (WL%) were also calculated using the Eqs. (1) and (2), and the values of the W% and WL% were 839.04% and 7.66%, respectively, which indicated the CP-5.0 composite gel showed a potential to provide high ionic conductivity.
The charge–discharge curves of quasi-solid-state EDLCs with CP-5.0 composite gel at different current densities were presented in Fig. 5a. Within a voltage window of 0–1.8 V, the charge–discharge plots were very symmetrical, confirming a reversible ion adsorption/desorption process at the surface of porous-activated carbon (Yang et al. 2014). The specific capacitance with respect to the mass of the active material on one electrode was calculated. The specific capacitance of the quasi-solid-state supercapacitor at 1.5 A g−1 was 114.5 F g−1, which was over 91% of the value at 0.25 A g−1, suggesting the high stability and the high rate capability. For comparison, the specific capacitances of the previously reported EDLCs using gel separators are summarized in Table 1. The specific capacitance of the quasi-solid-state EDLC with CP-5.0 gel is comparable to or higher than those values in literatures at a relatively large discharge current, which is perhaps due to the hierarchical and porous structure of the cellulose/PVA gel.
a Galvanostatic charge–discharge (GCD) curves of the EDLC with CP-5.0 composite gel at various current densities. b CV curves for the EDLC with CP-5.0 composite gel at different scan rates from 1 to 100 mV s−1. c EIS of EDLCs with different components of cellulose/PVA composite gels. The embedded figure is the close-up view of the left plot in high-frequency region. d Specific capacitance evolution during cycling test with cellulose/PVA gels at current density is 1.0 A g−1, in which the voltage range is 0–1.8 V. e A LED powered by two EDLCs with CP-5.0 composite gel in series
Cyclic voltammetries (CVs) for the quasi-solid-state EDLC fabricated with CP-5.0 composite gel and activated carbon at the scan rate from 1 to 100 mV s−1 were shown in Fig. 5b. The voltage window of the EDLC was 0–1.8 V because Li2SO4 could enhance the electrochemical window of aqueous system from 0.8 to 1.8 V (Gao et al. 2012; Huang et al. 2015; Luo et al. 2008; Wessells et al. 2012). The resultant supercapacitor with CP-5.0 composite gel exhibited nearly rectangular CV responses. It indicated that the charge–discharge process was highly reversible and kinetically facile. The capacitance was stored by an accumulation of ions between the interfaces of electrolyte and electrode which was known as electric double-layer capacitance.
Electrochemical impedance spectroscopy (EIS) was carried out to understand the complex impedance and ion diffusion process of the quasi-solid-state EDLCs with cellulose/PVA composite gels in a frequency range from 0.01 Hz to 0.1 MHz. The Nyquist plot was shown in Fig. 5c. The quasi-solid-state EDLCs with different cellulose/PVA composite gels showed a line close to 90° at low frequency, indicating a good capacitive behavior (Lee et al. 2006) and it was typical for an EDLC (Gamby et al. 2001). At high frequency, the intercept point on the real axis represented the resistance of the electrolyte and the internal resistance of the electrodes, which was also called bulk resistance of the EDLCs, and the diameter of compressed semicircle was attributed to the charge transfer resistance (Di Fabio et al. 2001). The Nyquist plots for the EDLCs with cellulose/PVA composite gels showed arcs in the high frequency region. The optimum content of cellulose in cellulose/PVA composite gel would lead to a reduced series resistance which included contact resistance, ion resistance and charge transfer resistance in the electrodes (Zheng 1999). As a consequence, the quasi-solid-state EDLC with the CP-5.0 composite gel exhibited the smallest semi-circle, which suggested the lowest charge transfer resistance of the flexible EDLC. Furthermore, both the bulk resistance and charge transfer resistance of the EDLC were the lowest, which was agreed well with the results from CVs. Long term cycling stability had been studied for assessing the reliability of a system to be further implemented in a commercial device. In this sense, Fig. 5d displayed the cycling stability of EDLCs with CP-0, CP-2.5, CP-5.0 and CP-7.5. The EDLCs with CP-2.5, CP-5.0 and CP-7.5 presented an excellent cycling stability at 1.8 V under a relatively high current density of 1.0 A g−1. Compared to the EDLC with CP-0, the EDLC with CP-5.0 indicated an increased original specific capacitance, which was enhanced by ~ 11.2%. For the EDLC with CP-5.0, after equilibration of the system within the first 400 cycles, the capacitance retention ratio after 1500 cycles was as high as 98.52%, which suggested the high stability of the supercapacitor device and the feasibility of improving PVA gel by cellulose. The capacitance loss of them might be ascribed to the mechanical degradation of activated carbon electrodes during the cycle process (Liu et al. 2018). But the EDLC with CP-0 had the lowest cycling stability due to the inferior mechanical properties and mechanical degradation of activated carbon electrodes. Two quasi-solid-state EDLC with CP-5.0 composite gel in series could light up a green LED after charging as shown in Fig. 4e, indicating the potential application of the device.
Conclusions
Composite gels based on cellulose and PVA were successfully fabricated by a freeze-thawing method, and they were used as separators for quasi-solid-state EDLCs. FTIR analysis indicated the presence of interactions between cellulose and PVA chains. The as-prepared sample consisted of 5% cellulose and 95% PVA showed relatively balanced performance due to the reinforcement effect of cellulose for composite gels. The quasi-solid-state EDLC with the optimum cellulose/PVA composite gel exhibited a voltage window of 0–1.8 V and had a specific capacitance of 125.1 F g−1 (based on one electrode) at a current density of 1 A g−1. Moreover, it also demonstrated excellent cycling stability with capacitance retention of 88% after 1500 cycles at a current density of 1 A g−1. These results meant that cellulose/PVA composite gel could serve as promising separators for quasi-solid-state EDLCs.
References
Abitbol T, Johnstone T, Quinn TM, Gray DG (2011) Reinforcement with cellulose nanocrystals of poly(vinyl alcohol) hydrogels prepared by cyclic freezing and thawing. Soft Matter 7:2373. https://doi.org/10.1039/c0sm01172j
Anothumakkool B, Torris ATA, Bhange SN, Badiger MV, Kurungot S (2014) Electrodeposited polyethylenedioxythiophene with infiltrated gel electrolyte interface: a close contest of an all-solid-state supercapacitor with its liquid-state counterpart. Nanoscale 6:5944–5952. https://doi.org/10.1039/c4nr00659c
Castro C et al (2014) In situ production of nanocomposites of poly(vinyl alcohol) and cellulose nanofibrils from gluconacetobacter bacteria: effect of chemical crosslinking. Cellulose 21:1745–1756
Chang C, Lue A, Zhang L (2008) Effects of crosslinking methods on structure and properties of cellulose/PVA hydrogels. Macromol Chem Phys 209:1266–1273. https://doi.org/10.1002/macp.200800161
Di Fabio A, Giorgi A, Mastragostino M, Soavi F (2001) Carbon-poly(3-methylthiophene) hybrid supercapacitors. J Electrochem Soc 148:A845. https://doi.org/10.1149/1.1380254
Gamby J, Taberna PL, Simon P, Fauvarque JF, Chesneau M (2001) Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J Power Sources 101:109–116
Gao Q, Demarconnay L, Raymundo-Piñero E, Béguin F (2012) Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte. Energy Environ Sci 5:9611. https://doi.org/10.1039/c2ee22284a
Hai T, Sugimoto R (2018) Surface functionalization of cellulose with poly(3-hexylthiophene) via novel oxidative polymerization. Carbohydr Polym 179:221
He F, He X, Yang W, Zhang X, Zhou L (2018) In-situ synthesis and structural characterization of cellulose-silica aerogels by one-step impregnation. J Non-Cryst Solids 488:36–43
Huang C-W, Wu C-A, Hou S-S, Kuo P-L, Hsieh C-T, Teng H (2012) Gel electrolyte derived from poly(ethylene glycol) blending poly(acrylonitrile) applicable to roll-to-roll assembly of electric double layer capacitors. Adv Funct Mater 22:4677–4685. https://doi.org/10.1002/adfm.201201342
Huang H, Luo G, Xu L, Lei C, Tang Y, Tang S, Du Y (2015) NH3 assisted photoreduction and N-doping of graphene oxide for high performance electrode materials in supercapacitors. Nanoscale 7:2060–2068. https://doi.org/10.1039/c4nr05776g
Jiang M, Zhu J, Chen C, Lu Y, Ge Y, Zhang X (2016) Poly(vinyl alcohol) borate gel polymer electrolytes prepared by electrodeposition and their application in electrochemical supercapacitors. ACS Appl Mater Interfaces 8:3473–3481
Lee J-K, Lee Y-J, Chae W-S, Sung Y-M (2006) Enhanced ionic conductivity in PEO-LiClO4 hybrid electrolytes by structural modification. J Electroceram 17:941–944. https://doi.org/10.1007/s10832-006-7672-7
Li L, Wu Z, Yuan S, Zhang X-B (2014) Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ Sci 7:2101. https://doi.org/10.1039/c4ee00318g
Liang Y et al (2018) Surface-modified cellulose nanocrystals for biobased epoxy nanocomposites. Polymer 134:155–162
Liu D, Sun X, Tian H, Maiti S, Ma Z (2013) Effects of cellulose nanofibrils on the structure and properties on PVA nanocomposites. Cellulose 20:2981–2989
Liu X, Wu D, Wang H, Wang Q (2014) Self-recovering tough gel electrolyte with adjustable supercapacitor performance. Adv Mater 26:4370–4375. https://doi.org/10.1002/adma.201400240
Liu Y et al (2018) Understanding of carbon-based supercapacitors ageing mechanisms by electrochemical and analytical methods. J Power Sources 366:123–130
Lu J, Wang T, Drzal LT (2008) Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos A Appl Sci Manuf 39:738–746. https://doi.org/10.1016/j.compositesa.2008.02.003
Luo J-Y, Liu J-L, He P, Xia Y-Y (2008) A novel LiTi2(PO4)3/MnO2 hybrid supercapacitor in lithium sulfate aqueous electrolyte. Electrochim Acta 53:8128–8133. https://doi.org/10.1016/j.electacta.2008.05.080
Ma G, Li J, Sun K, Peng H, Mu J, Lei Z (2014) High performance solid-state supercapacitor with PVA–KOH–K3[Fe(CN)6] gel polymer as electrolyte and separator. J Power Sources 256:281–287. https://doi.org/10.1016/j.jpowsour.2014.01.062
Moon WG, Kim GP, Lee M, Song HD, Yi J (2015) A biodegradable gel electrolyte for use in high-performance flexible supercapacitors. ACS Appl Mater Interfaces 7:3503–3511. https://doi.org/10.1021/am5070987
Na R et al (2016) A novel poly(ethylene glycol)-grafted poly(arylene ether ketone) blend micro-porous polymer electrolyte for solid-state electric double layer capacitors formed by incorporating a chitosan-based LiClO4gel electrolyte. J Mater Chem A 4:18116–18127. https://doi.org/10.1039/c6ta07846j
Niu Z et al (2013) Highly stretchable, integrated supercapacitors based on single-walled carbon nanotube films with continuous reticulate architecture. Adv Mater 25:1058–1064. https://doi.org/10.1002/adma.201204003
Trapa PE et al (2005) Rubbery graft copolymer electrolytes for solid-state, thin-film lithium batteries. J Electrochem Soc 152:A1. https://doi.org/10.1149/1.1824032
Wang G, Lu X, Ling Y, Zhai T, Wang H, Tong Y, Li Y (2012) LiCl/PVA gel electrolyte stabilizes vanadium oxide nanowire electrodes for pseudocapacitors. ACS Nano 6:10296–10302. https://doi.org/10.1021/nn304178b
Wang W, Liang T, Bai H, Dong W, Liu X (2018) All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohydr Polym 179:297–304
Wessells CD, Peddada SV, McDowell MT, Huggins RA, Cui Y (2012) The effect of insertion species on nanostructured open framework hexacyanoferrate battery electrodes. J Electrochem Soc 159:A98. https://doi.org/10.1149/2.060202jes
Wu S, Li F, Wang H, Fu L, Zhang B, Li G (2010) Effects of poly (vinyl alcohol) (PVA) content on preparation of novel thiol-functionalized mesoporous PVA/SiO 2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution. Polymer 51:6203–6211
Yang Z, Deng J, Chen X, Ren J, Peng H (2013) A highly stretchable, fiber-shaped supercapacitor. Angew Chem 52:13453–13457. https://doi.org/10.1002/anie.201307619
Yang L, Hu J, Lei G, Liu H (2014) Ionic liquid-gelled polyvinylidene fluoride/polyvinyl acetate polymer electrolyte for solid supercapacitor. Chem Eng J 258:320–326. https://doi.org/10.1016/j.cej.2014.05.149
Zhang X, Wang L, Peng J, Cao P, Cai X, Li J, Zhai M (2015) A flexible ionic liquid gelled PVA-Li2SO4 polymer electrolyte for semi-solid-state supercapacitors. Adv Mater Interfaces 2:1500267. https://doi.org/10.1002/admi.201500267
Zheng JP (1999) Ruthenium oxide-carbon composite electrodes for electrochemical capacitors. Electrochem Solid State Lett 2:359–361
Zhou M, Zhang H, Qiao Y, Li CM, Lu Z (2018) A flexible sandwich-structured supercapacitor with poly(vinyl alcohol)/H3PO4-soaked cotton fabric as solid electrolyte, separator and supporting layer. Cellulose 25:3459–3469. https://doi.org/10.1007/s10570-018-1786-3
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (51503161) and Hubei Provincial Natural Science Foundation of China (2018CFB267).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, Y., Liang, N., Xu, J. et al. Cellulose and poly(vinyl alcohol) composite gels as separators for quasi-solid-state electric double layer capacitors. Cellulose 26, 1055–1065 (2019). https://doi.org/10.1007/s10570-018-2123-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2123-6