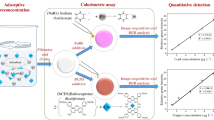
Abstract
In this work, a novel colorimetric sensor was prepared via a cellulose-polyethylenimine-based Schiff base (cellulose-Schiff base) for capturing and detecting copper ions (Cu2+) in aquatic environments and life systems. The cellulose-Schiff base displayed continuous color changes from inherent pale yellow to deep green due to the formation of a cellulose-Schiff base-Cu2+ charge transfer complex within 30 s, indicating excellent “naked-eye” detection capability for Cu2+ ions in aqueous solution. The limit of detection (LOD) was 1.054 ppm, lower than the U.S. EPA guidelines for drinking water of 1.3 ppm. Moreover, the cellulose-Schiff base colorimetric sensor was successfully used to capture and detect exogenous Cu2+ ions in biofluids (simulated urine and bovine serum). The proposed sensing mechanism of the cellulose-Schiff base for Cu2+ ions is that N atoms (C=N and N–H) in the sensor capture and concentrate Cu2+ ions to form sensor-Cu2+ complexes, then intramolecular charge transfer occurs as a consequence. In addition, test strips based on cellulose-Schiff base were developed, and provide a convenient, cheap, sensitive, and reliable monitoring platform for on-site semi-quantitative analysis of Cu2+ ions in real samples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal ion pollution problems have had a terrible influence on human health and the environment. Therefore, the monitoring of heavy metal ions is of great practical significance. Copper ion (Cu2+) is an essential trace metal element for human health and plays a cofactor role in various enzyme functions, redox processes and pigment synthesis in all living organisms (Quintanar et al. 2016; Bian et al. 2017; Giampietro et al. 2018). However, deregulation of copper homeostasis can cause liver and kidney damage, many types of cancers, several neurodegenerative diseases such as Parkinson’s, Alzheimer’s and Wilson’s disease (Pall et al. 1987; Kaler 2011; Singh et al. 2013; Bandmann et al. 2015a, b; Barnham and Bush 2014). Cu2+ ion is produced and accumulates in the environment and the food chain with the rapid development of industry and agriculture. The daily allowance of copper intake as suggested by the National Research Council is 1.5–3.0 mg for adults, 1.5–2.5 mg for children, and 0.4–0.6 mg for infants (Jung et al. 2009; Udousoro et al. 2017). Additionally, the U.S. Environmental Protection Agency (EPA) ruled that the limit of Cu2+ ion is 1.3 ppm in drinking water (Richardson and Ternes 2011). Therefore, there is an urgent need to develop a convenient, real-time, and sensitive sensor for Cu2+ detection in the environment and life systems.
So far, a variety of detection technologies for Cu2+ ions have been exploited, such as atomic absorption/emission spectroscopy (AAS/AES), inductively coupled plasma mass spectrometry (ICP-MS), surface plasmon resonance (SPR), and high-performance liquid chromatography (HPLC) (Lin and Huang 2001; Zhao et al. 2012; Zhang et al. 2014a, b; Loegel et al. 2017; Pereira et al. 2018). These methods have greatly enhanced the trace detection for Cu2+ ions in different samples. However, these traditional methods usually require expensive equipment, complex sample preparation, and professional operators. To address the above issues, various fluorometric and colorimetric chemosensors were studied due to their potential for naked-eye detection in organic media (Jin and Han 2014; Ndokoye et al. 2014; Liu et al. 2017; Fang et al. 2017; Joo et al. 2017; Miao et al. 2017; Zhao et al. 2017; Awual et al. 2014). For example, Joo et al. synthesized a chemosensor 1 by a combination of N-aminophthalimide and 8-hydroxyjulolidine-9-carboxaldehyde for colorimetric detection of Cu2+ ions in a buffer/DMF solution (3/2, v/v, 10 mM bis–tris, pH 7.0), with a detection limit of 0.14 μM (Joo et al. 2017). Jin and Han designed hexadecyl trimethylammonium bromide (CTAB) modified CdSe/ZnS quantum dots (QDs), presenting ultrasensitive and selective fluorimetric detection of Cu2+ ions in the presence of thiosulfate (Jin and Han 2014). These methods developed highly sensitive sensors for copper detection. However, the ability to detect trace amounts of Cu2+ ions in aquatic environments and life systems remains a challenge.
Schiff bases derived from the condensation of aldehyde/ketone and amine can coordinate metal ions via azomethine nitrogen (–C=N), which are important materials in the fluorescent analysis of metal ions (Cheng et al. 2013; Horak et al. 2018; Awual et al. 2015). In our previous work, cellulose-Schiff bases were synthesized based on branched polyethylenimine (PEI) and displayed excellent selective adsorption and separation abilities for dyes (Zhu et al. 2016). Cellulose is an abundant, biocompatible, biodegradable, and renewable biomacromolecule that can be chemically modified to be various sensors (Kumari and Chauhan 2014; Schyrr et al. 2014; Nawaz et al. 2018). Compared with activated small molecule probes and nanoparticle probes, cellulose-based sensors are more stable in surrounding changes in solution. This inspired an assumption for simultaneous capture and detection of Cu2+ ions in aqueous solution and biofluid using cellulose-Schiff base as visual sensor.
Therefore, in this work, we designed an alternative technology that would capture Cu2+ ions efficiently and selectively, while simultaneously enabling copper detection and readout of corresponding levels directly from aqueous solution and biofluids (urine and bovine serum) in a colorimetric analysis. This strategy depends on the utilization of cellulose-polyethylenimine-based Schiff base with abundant amino receptors (–NH). These receptors exhibit a high affinity for Cu2+ capture and concentration and simultaneous detection and evaluation of copper levels by intramolecular charge transfer (ICT). Further, more convenient test strips have also been developed for semi-quantitative analysis of Cu2+ ions from aqueous solution as well as biofluids. This work presents a promising viability of cellulose-polyethylenimine-based Schiff base for convenient, real-time, highly selective and sensitive, probe-free visual detection of Cu2+ ions in the environment and life systems.
Experimental
Reagents
Branched polyethylenimine (PEI, Mw = 600, 99%), cellulose (Mw = 20,000), NaOH, urea, NaIO4, NaCl, NH4Cl, Cu(CH3COO)2, Mn(CH3COO)2, Co(CH3COO)2, Ca(CH3COO)2, Mg(CH3COO)2, FeCl3, ZnCl2, hydrogen nitrate, creatinine, sodium orthophosphate disodium, potassium orthophosphate, anhydrous sodium sulfite, ethylene glycol, ethanol, bovine serum, and filter paper were all purchased from Shanghai Aladdin Chemical Reagent Co, Ltd., and all of the chemicals were used without further purification.
Synthesis and characterization of cellulose-Schiff base
Cellulose-polyethylenimine based Schiff base (named as cellulose-Schiff base) were prepared through sodium periodate-mediated oxidation and then PEI grafting. The synthetic process of cellulose-Schiff base is shown in Scheme 1. Briefly, 2 g of cellulose powders and 2 g of NaIO4 were dissolved into 60 mL of 7 wt% NaOH/12 wt% urea solution. The mixed solution covered using foil paper to prevent photo-induced NaIO4 decomposition, was adjusted by 1 M HCl to pH 4, then reacted for 4 h under stirring at 60 °C. 2 g of PEI was then added into aforementioned reaction system. After 3 h reaction, the mixture was centrifuged with 8000 rpm for 5 min to harvest the supernatant. Subsequently, the supernatant was extracted using anhydrous ethanol to harvest pale yellow precipitate i.e. cellulose-Schiff base, which was washed three times with anhydrous ethanol and dried at 60 °C in a vacuum oven.
The chemical structures of the samples were characterized by Fourier transform infrared spectroscopy (FTIR, Nicolet 5700, Thermo Electron Corp., USA) ranging from 4000 to 400 cm−1 at a resolution of 4 cm−1. The crystal phases of samples were detected on an ARL X’RA diffractometer (XRD, Thermo Electron Corp.) using monochromatic Cu Kα radiation (k = 1.54056 Å), operating at 40 kV and 30 mA. The elemental analysis of cellulose and cellulose-Schiff base were performed using Elemental analyzer (Vario MICRO cube, Elementar Co., Germany). 13C Nuclear magnetic resonance (NMR) was recorded on a Bruker 300 MHz solid-state NMR spectrometer. The surface chemical composition of cellulose-Schiff base before and after Cu2+ capture was recorded by X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, USA) using a monochromatic Al Kα radiation.
Capture and detection of Cu2+ ions in aqueous solution
Selective
20 mg of cellulose-Schiff bases were incubated with different metal ions (Cu2+, Mn2+, Co2+, Fe3+, Zn2+, Ca2+, and Mg2+ ions) in 20 mL aqueous solution for 1 min with/without the coexistence of Cu2+ ions, respectively. Residual concentration of metal ions in aqueous solution were measured by Atomic absorption spectroscopy (AAS, Spectr AA-220, USA). The capture efficiency and capture capacity was calculated on the following Eqs. 1 and 2:
where Co and Ct are the initial concentrations (mg/L) and residual concentrations (mg/L) of metal ions in solution at t time, respectively. V is the volume of the aqueous phase (L), and m is the weight of dry sample (g).
Sensitivity
The relationship between sensitivity and condition parameters, including sensor dosage, solution pH, reaction time, Cu2+ ion concentration was investigated. Desired amount of cellulose-Schiff base (5, 10, 15, 20 and 25 mg) as sensor were added into 20 mL Cu2+ ions aqueous solution, the color changes of sensor-Cu2+ complex were observed by naked-eye. Here, effects of solution pH (2.0, 3.0, 4.0, 5.0, 6.0 and 7.0), reaction time (10–90 min), and Cu2+ ion concentration (10, 15, 20, 25, 50, 100, 150, 200, 250 and 300 ppm) on sensitivity of sensor for Cu2+ ions were investigated. In addition, the capture efficiency and capture capacity of sensor for Cu2+ ions were calculated by Eqs. 1 and 2, respectively.
UV–Vis titration
2 mg of cellulose-Schiff bases were added into 2 mL stock solution of Cu2+ ions (0–100 ppm). UV–Vis spectra of mixed solution were recorded at room temperature (UV, U-3900 spectrophotometer, Japan).
Job plot measurement
Cellulose-Schiff base sensors were added into Cu2+ solution to obtain a series of mixed solutions with a total concentration of 1.0 × 10−4 M, in which the molar ration of [Cu2+]/[Sensor] + [Cu2+] was 0.1–1.0. The absorbance of each solution is measured at room temperature.
Capture and detection of Cu2+ ions in biofluids
Simulated urine and bovine serum were added with different concentrations of Cu2+ ions to evaluate the practicability of sensor for real samples. Simulated urine was made according to the U.S. patent #7109035 (Lock et al. 2013) listed in Table S1 to avoid component interference in individual differences. Briefly, 20 mg of cellulose-Schiff bases were added into 20 mL simulated urine, while 10 mg of cellulose-Schiff bases for 10 ml bovine serum. The color changes were recorded and the capture capacity was calculated by Eq. 2.
Preparation of test strips and semi-quantitative detection
As described in Synthesis and characterization of cellulose-Schiff base section, the harvested supernatant was dialyzed using dialysis bag (MWCO 8000) for 48 h to obtain cellulose-Schiff base solution. Commercial filter paper was immersed into cellulose-Schiff base solution for 2 min, then dried at 25 °C. The dipping process was repeated 50, 100, and 150 times to prepare test strip. Finally, the detecting ability of test strip for Cu2+ visualization was performed in aqueous solution, simulated urine and serum with exogenous copper (0, 2, 4, 6, 8, 10 ppm).
Results and discussion
Synthesis and characterization of cellulose-Schiff base
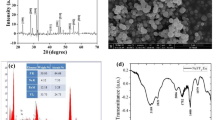
Briefly, cellulose was oxidized at the C2–OH and C3–OH positions with NaIO4 to 2,3-dialdehyde cellulose, followed by treatment with polyethylenimine to produce the corresponding Schiff base (Scheme 1). FTIR spectrum confirmed the successful synthesis of the cellulose-Schiff base according to the appearance of the characteristic peaks at 1460 cm−1 (C–N stretching vibration) and 1670 cm−1 (C=N stretching vibration), as well as the peak of the N–H stretching vibration at 3490 cm−1 (Fig. 1a) (Park et al. 2014; Lindh et al. 2016; Ruan et al. 2016; Hu et al. 2018). In addition, the crystal phase structure of the resultant cellulose-Schiff base was determined by XRD (Fig. 1b). For raw cellulose, the diffraction peaks at 15.5°, 22.7° and 34.5° corresponding to (110), (200) and (004) planes are indexed to the cellulose I crystal (Luis et al. 2016; Yang et al. 2016). After PEI modification, the diffraction peaks at 2θ = 15.5° and 34.5° in cellulose-Schiff base obviously weakened, while the diffraction peaks at 2θ = 22.7° became broader, which was ascribed to the introduction of PEI resulting in the destruction of (110) and (004) crystal planes for cellulose. Further, the solid-state 13C CP/MAS NMR spectrum monitored the signals of C2/3/5 in cellulose-Schiff bases, which have an obvious shift compared to pure cellulose. In addition, broad signals of PEI at 38.53–58.49 ppm appeared, revealing successful grafting of PEI to the C2/C3–OH positions in cellulose (Fig. S1) (Harpe et al. 2000; Kim et al. 2005). Finally, the elemental analysis presented N element content with 9.18% in cellulose-Schiff base (Table 1), providing further evidence of successful grafting of amino groups.
Capture and detection of Cu2+ ions in aqueous solutions
To verify the potential of the proposed approach for detecting Cu2+ and to investigate its recognition and capture capability, 20 mg of cellulose-Schiff bases were dispersed in 10 ppm aqueous solutions containing different metal ions (Cu2+, Fe3+, Mg2+, Ca2+, Zn2+, Co2+ and Mn2+ ions). As shown in Fig. 2a, cellulose-Schiff base showed high selectively for Cu2+ ions because the color of cellulose-Schiff base changed from inherent pale yellow to green only in the presence of Cu2+ ions within < 30 s. Meanwhile, it was found that 94.2% of Cu2+ ions could be captured, a much greater amount than other metal ions. It is well known that the coexistence of various metal ions with Cu2+ ions may interfere with Cu2+ detection. Thus, the Cu2+ selectivity of cellulose-Schiff base was investigated by competitive response in mixed solution. As shown in Fig. 2b, despite the concentration of Cu2+ ions was as low as 5 times than that of other metal ions, the color of cellulose-Schiff base could still turn green within < 30 s, simultaneously the capture efficiency of Cu2+ ions reached 93.7%. UV–Vis spectra in Fig. 3 also revealed the high selectivity of cellulose-Schiff base for Cu2+ ions. Compared to Fe3+, Mg2+, Ca2+, Zn2+, Co2+ and Mn2+ ions, the initial absorption peak of cellulose-Schiff base at 320 nm shifted to 275 nm once adding Cu2+ ions. Also, a specific absorption peak at 635 nm appeared (inset in Fig. 3), indicating the formation of complexes between cellulose-Schiff base and Cu2+ ions. All the results suggested great potential of cellulose-Schiff base as a colorimetric sensor for selective capture and detection of Cu2+ ions derived from the formation of cellulose-Schiff base-Cu2+ (called the sensor-Cu2+) charge transfer complex (Kumari and Chauhan 2014; Joo et al. 2017).
To understand the relationship between the sensitivity and the condition parameters, the effects of sensor dosage, solution pH, and reaction time, Cu2+ concentration on the capture of Cu2+ ions were investigated. First, the dependence of the sensitivity for Cu2+ detection on sensor dosage is shown in Fig. 4a. After adding 5 mg of sensor into 20 mL of 10 ppm Cu2+ ion aqueous solutions, no obvious color change was observed. Once the sensor dosage exceeded 5 mg, green sensor-Cu2+ complexes were observed within 30 s because of the increase in active recognition sites (Sayed et al. 2017; Gholam et al. 2017). In addition, the capture efficiency values as a function of sensor dosage presented an equilibrium with a sensor dosage of 15 mg, indicating excellent performance in the recognition and capture of Cu2+ ions from aqueous solutions. The effect of pH on Cu2+ detection was performed in the pH range of 2.0–7.0, and the result is shown in Fig. 4b. As seen from the optical picture, light green sensor-Cu2+ complexes were found at pH 2.0 within 1 min, while obvious green complexes were observed within 30 s when increasing the pH from 3.0 to 7.0. Additionally, the increased capture ability indicated that cellulose-Schiff base could be used as a “naked-eye” colorimetric sensor for detecting Cu2+ ions under physiological conditions.
Further, the sensor-Cu2+ capture kinetics were investigated and presented in Fig. S2 and Table S2. A time-dependence in the capture process was found. When the sensor was added to the Cu2+ solution, the capture capacity (qt) sharply increased in the first 10 min because of the fast complex reaction and then reached saturation gradually. The chemical capture mechanism was confirmed based on the analysis of a pseudo-second-order kinetics model with a high correlation coefficient R2 of 0.99758 and a rate constant k2 of 0.03924 g/g/min. Thus, the N atom of the amino group and/or the O atom of free hydroxyl group of the cellulose-Schiff base bound to Cu2+ ions from aqueous solution to form a cellulose-Schiff base-Cu2+ complex, consequently exhibiting a strong response for Cu2+ ions. The overall capacity of the sensor for Cu2+ ions was further determined by fitting capture isotherms with a broad range of copper equilibrium concentration (0–110 ppm). The fitting results are summarized in Fig. S3 and Table S3. It is clear that the capture capacity of Cu2+ ions increased with the increase of Cu2+ equilibrium concentration, and the maximum capture capacity (qmax) reached 191 mg/g. In comparison to other capture agents listed in Table 2 (Zhang et al. 2013; Yu et al. 2013; Gonzalez et al. 2014), cellulose-Schiff base displays more higher capture capacity for Cu2+ ions due to its abundant amino group contents. Comparing the correlation coefficient (R2), the Freundlich model can better predict the capture behaviors of cellulose-Schiff base with a multi-molecular layer capture, indicating a strong complexation between the cellulose-Schiff base and Cu2+ ions in a wide concentration range of Cu2+ ions.
To further assess the detection performance of cellulose-Schiff base, UV–Vis titration of the sensor with Cu2+ions was carried out. After adding 2 mg of sensor into 2 mL of Cu2+ ion solution (0–100 ppm), the specific absorption peaks at 275 and 635 nm appeared and increased gradually with the increase in Cu2+ion concentrations (Fig. 5a) revealing the sensor-Cu2+ complex formation and increase in amount. In addition, Fig. 5b shows a linear correlation between absorption intensity and Cu2+ ion concentration at 635 nm. The limit of detection (LOD) was calculated to be 1.054 ppm according to the calculation formula \( {\text{LOD = 3}}\emptyset / {\text{k}} \) (where ∅ is the standard deviation of blank measurement, k is the slope between the absorption intensity versus Cu2+ ion concentration). It is clearly seen that the detection limit of the cellulose-Schiff base for Cu2+ is lower than the limit of Cu2+ of 1.3 ppm in drinking water and approaches the blood copper levels of 0.94–1.31 ppm in healthy individuals as defined by the U.S. EPA (Richardson and Ternes 2011; Zhang et al. 2014a, b; Lee et al. 2016), indicating excellent sensitivity to Cu2+ ions in solution systems.
To evaluate the binding model of the sensor and Cu2+ ion, a Job plot model was employed to determine the stoichiometric ratio between sensor and Cu2+ ions. The total concentration of Cu2+ ions and sensor was 1.0 × 10−4 M. The absorption intensities at 635 nm were recorded and plotted against the molar fraction of the Cu2+ ions in solution. It is clearly seen in Fig. 6 that the break point appeared at a molar ratio of 0.48 [Cu2+]/[Sensor] + [Cu2+], indicating that the cellulose-Schiff base forms a 1:1 stoichiometric complex with Cu2+ ion.
To further investigate the mechanism of cellulose-Schiff base chelating Cu2+ ions, X-ray photoelectron spectra (XPS) was performed and confirmed the successful capture of Cu2+ ions, owing to the appearance of a Cu2p3/2 characteristic peak at 933.1 eV (Fig. 7a) (Xiong et al. 2016). For C1 s, the characteristic peaks of binding energy at 284.7, 286.6, 285.9 and 288.5 eV in the cellulose-Schiff base were assigned to C–C, C–O, C–N and C–N, respectively (Sui et al. 2013; Qiu et al. 2014). After capture of Cu2+ ions, the proportion of C=N peaks was reduced, revealing the Schiff base structure involved in the capture of Cu2+ ions (Fig. 7b). This was further confirmed in N1 s XPS spectra. As shown in Fig. 7c, the N1 s spectra in the cellulose-Schiff base can be deconstructed into three major peaks with binding energies at 398.7, 399.5 and 401.7 eV, corresponding to C=N, N–C and N–H bonds, respectively (Sui et al. 2013; Qiu et al. 2014; Deng et al. 2016). After binding with Cu2+ ions, the intensity of the C=N and N–H peaks was weakened. However, there was no obvious change in binding energy peak of the C–O/C–OH at 531.0 eV, suggesting that hydroxyl group of cellulose-Schiff base did not take part in capturing Cu2+ ions. Thus, it can be proposed that both N atoms of the Schiff base (C=N) and the amino group (N–H) of the cellulose-Schiff base participated in chelation with Cu2+ ions. This consists with the results of FTIR spectra and XRD pattern in Fig. 1. After Cu2+ ion capture, the characteristic peak at 1670 cm−1 (C=N stretching vibration) in sensor-Cu2+ disappeared obviously, while a new characteristic peak at 1740 cm−1 appeared and the N–H stretching vibration peak became narrower compared to these of cellulose-Schiff base. No significant change was found in XRD pattern of sensor-Cu2+, suggesting that Cu2+ capture can not influence the crystal structure of cellulose-Schiff base. On the basis of the Job plot, XPS and FTIR analysis, we speculate that the binding mode between cellulose-Schiff base and Cu2+ ions is as shown in Scheme 2.
Capture and detection of Cu2+ ions in biofluids
The applicability of the cellulose-Schiff base for Cu2+ ion detection in real samples was further assessed after verifying the ability for copper capture with good selectivity and sensitivity from aqueous solution. Generally, patients suffering from Wilson’s disease display high urinary copper (> 100 mg/day) and serum free copper (> 25 μg/dL) levels compared to healthy individuals, who have 20–40 mg/day and 11–25 μg/dL (Kaler 2011). Hence, bovine serum and urine were chosen to evaluate the recognition and capture ability of the cellulose-Schiff base by adding 2–10 ppm of exogenous copper. After adding the sensor into urinary copper solutions, the green sensor-Cu2+ complexes appeared within 60 s, and the color of the sensor-Cu2+ complexes became darker and deeper with the increase of urinary copper concentration. Moreover, the capture capacity and efficiency of the sensor for Cu2+ ions increased when exogenous copper amounts were increased to 10 ppm. Consequently, the maximum values reached 9.58 mg/g and 95.8%, respectively (Fig. 8a). The results confirm that the cellulose-Schiff base has a strong recognition and capture ability in complex simulate urine.
Finally, the capture and detection performances of the cellulose-Schiff base for Cu2+ ions were evaluated in serum, and the results are shown in Fig. 8b. Sensor-Cu2+ complexes formed within 5 min. The sensor presented dosage-dependent copper capture in the range of 0–10 ppm, and the color of the complexes gradually deepened from inherent pale yellow to light green to dark green. The capture efficiency of the sensor was 51.6% when the Cu2+ amount was increased to 10 ppm. It is known that serum is a more complex biofluid than urine, and it contains more iron, approximately 5 times more than copper (Lee et al. 2016). Although there is iron interference, as shown in Fig. 2, the cellulose-Schiff base still exhibits potential as a colorimetric sensor for direct copper capture from serum. The reduction of iron interference will be done by adding a highly specific affinity iron chelator in our further work to improve the sensitivity of the sensor. Even so, the cellulose-Schiff base provides a promising prospect for Cu2+-related disease diagnosis.
Test strips and semi-quantitative detection
Paper-based sensors are a convenient, cheap, and stable monitoring platform for naked-eye detection. Here, the test strips were obtained after being dipped 100 times, which displayed the strongest sensitivity for Cu2+ ion visualization. The detecting ability of test strip was shown in Fig. S4. Then, the relationships between sensitivity of the test strips and Cu2+ ion amount were evaluated, and semi-quantitative colorimetric cards were prepared. As seen from Fig. 9, with the increase of Cu2+ ions in aqueous media including a simulated urine and serum sample, the test strips displayed color changes from pale yellow to green for aqueous solution within 30 s (Fig. 9a), to light green for urine sample within 100 s (Fig. 9b), and to yellow–green for serum sample within 5 min (Fig. 9c). Even when the amount of Cu2+ ions was lowered to 2 ppm, light green strips were clearly observed, demonstrating a high sensitivity of the prepared test strips. The results demonstrated that the cellulose-Schiff base as a sensor has promising applications in on-site visual detection of Cu2+ ions for real samples without the need for complex and expensive instruments.
Conclusions
The cellulose-polyethylenimine-based Schiff base was fabricated for capture and detection of Cu2+ ions in aqueous solution, complex simulated urine as well as bovine serum. The resultant cellulose-Schiff base exhibited an obvious color change from inherent pale yellow to deep green in the presence of Cu2+ ions, which derived from the formation of cellulose-Schiff base-Cu2+ charge transfer complexes with a 1:1 stoichiometric ratio. The sensing mechanism of cellulose-Schiff base for Cu2+ ions was proposed to be such that N atoms (C=N and N–H) in the sensor capture and concentrate Cu2+ ions to form sensor-Cu2+ complexes, with intramolecular charge transfer occurring as a consequence. On the basis of sensor-Cu2+ chelation-induced color-change, semi-quantitative test strips based on the cellulose-Schiff base were developed and exhibited sensitive and visual detection capability for Cu2+ ions in aqueous solution, simulated urine and serum. Thus, the cellulose-Schiff base has potential as a convenient, cheap, sensitive, and reliable monitoring platform and can be used in environmental and biomedical applications, including on-site naked-eye detection and disease diagnosis.
References
Awual MR, Rahman IMM, Yaita T, Khaleque MA, Ferdows M (2014) pH dependent Cu(II) and Pd(II) ions detection and removal from aqueous media by an efficient mesoporous adsorbent. Chem Eng J 236:100–109
Awual MR, Eldesoky GE, Yaita T, Naushad M, Shiwaku H, AlOthman ZA, Suzuki SA (2015) Schiff based ligand containing nano-composite adsorbent for optical copper(II) ions removal from aqueous solutions. Chem Eng J 279:639–647
Bandmann O, Weiss KH, Kaler SG (2015a) Non-ceruloplasmin copper distincts subtypes in Alzheimer’s disease: a genetic study of ATP7B frequency. Lancet Neurol 14:103–113
Bandmann O, Weiss KH, Kaler SG (2015b) Wilson’s disease and other neurological copper disorders. Lancet Neurol 14:103–113
Barnham KJ, Bush AI (2014) Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev 43:6727–6749
Bian K, Chen FY, Humulock ZT, Tang Q, Li DY (2017) Copper inhibits the AlkB family DNA repair enzymes under Wilson’s disease condition. Chem Res Toxicol 30:1794–1796
Cheng JH, Wei KY, Ma XF, Zhou XG, Xiang HF (2013) Synthesis and photophysical properties of colorful salen-type Schiff bases. J Phys Chem C 117:16552–16563
Deng S, Zhang GS, Chen SW, Xue YN, Du ZL, Wang P (2016) Rapid and effective preparation of a HPEI modified biosorbent based on cellulose fiber with a microwave irradiation method for enhanced arsenic removal in water. J Mater Chem A 4:15851–15860
Fang XN, Liu Y, Jimenez L, Duan YK, Adkins GB, Qian L (2017) Rapid enrichment and sensitive detection of multiple metal ions enabled by macroporous graphene foam. Anal Chem 89:11758–11764
Gholam BC, Mahmood T, Ahmadreza B, Rahman H (2017) A highly sensitive and selective novel fluorescent chemosensor for detection of Cr3+ based on a Schiff base. Inorg Chim Acta 462:241–248
Giampietro R, Spinelli F, Contino M, Colabufo NA (2018) The pivotal role of copper in neurodegeneration: a new strategy for the therapy of neurodegenerative disorders. Mol Pharm 15:808–820
Gonzalez MA, Pavlovic I, Rojas-Delgado R, Barriga C (2014) Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide–humate hybrid: sorbate and sorbent comparative studies. Chem Eng J 254:605–611
Harpe AV, Petersen H, Li YX, Kissel T (2000) Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release 69:309–322
Horak E, Kassa P, Hranjec M, Steinberg IM (2018) Benzimidazole functionalised Schiff bases: novel pH sensitive fluorescence turn-on chromoionophores for ion-selective optodes. Sens Actuators B 258:415–423
Hu HZ, Wang FY, Yu LS, Sugimura KK, Zhou JP, Nishio Y (2018) Synthesis of novel fluorescent cellulose derivatives and their applications in detection of nitroaromatic compounds. ACS Sustain Chem Eng 6:1436–1445
Jin LH, Han CS (2014) Ultrasensitive and selective fluorimetric detection of copper ions using thiosulfate-involved quantum dots. Anal Chem 86:7209–7213
Joo DH, Mok JS, Bae GH, Oh SE, Kang JH, Kim C (2017) Colorimetric detection of Cu2+ and fluorescent detection of PO4 3− and S2− by a multifunctional chemosensor. Ind Eng Chem Res 56:8399–8407
Jung HS, Kwon PS, Lee JW, Kim JI, Hong CS, Kim JW (2009) Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131:2008–2012
Kaler SG (2011) ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol 7:15–29
Kim YH, Park JH, Lee M, Kim Y, Park TG, Kim SW (2005) Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release 103:209–219
Kumari S, Chauhan GS (2014) New cellulose-lysine Schiff-base-based sensor-adsorbent for mercury ions. ACS Appl Mater Interfaces 6:5908–5917
Lee M, Barin G, Ackerman CM, Muchenditsi A, Xu J, Reimer JA, Lutsenko S, Long JR, Chang CJ (2016) Copper capture in a thioether-functionalized porous polymer applied to the detection of Wilson’s disease. J Am Chem Soc 138:7603–7609
Lin TW, Huang SD (2001) Direct and simultaneous determination of copper, chromium, aluminum, and manganese in urine with a multielement graphite furnace atomic absorption spectrometer. Anal Chem 73:4319–4325
Lindh J, Ruan CQ, Stromme M, Mihranyan A (2016) Preparation of porous cellulose beads via introduction of diamine spacers. Langmuir 32:5600–5607
Liu C, Ning DH, Zhang C, Liu ZJ, Zhang RL, Zhao J, Zhao TT, Liu BH, Zhang ZP (2017) Dual-colored carbon dot rratiometric fluorescent test paper based on a specific spectral energy transfer for semiquantitative assay of copper ions. ACS Appl Mater Interfaces 9:18897–18903
Lock JY, Wyatt E, Upadhyayula S, Whall A, Nunez V, Vullev VI, Liu HN (2013) Degradation and antibacterial properties of magnesium alloys inartificial urine for potential resorbable ureteral stent applications. J Biomed Mater Res A 102:781–792
Loegel TN, Morris RN, Leska I (2017) Detection and quantification of metal deactivator additive in jet and diesel fuel by liquid chromatography. Energy Fuels 31:3629–3634
Luis A, Bruno M, Filipe EA, Daniel T, Björn L (2016) Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Carbohydr Polym 135:1–9
Miao P, Tang YG, Wang L (2017) DNA modified Fe3O4@Au magnetic nanoparticles as selective probes for simultaneous detection of heavy metal ions. ACS Appl Mater Interfaces 9:3940–3947
Nawaz H, Tian WG, Zhang JM, Jia RN, Chen ZY, Zhang J (2018) Cellulose-based sensor containing phenanthroline for the highly selective and rapid detection of Fe2+ ions with naked eye and fluorescent dual modes. ACS Appl Mater Interfaces 10:2114–2121
Ndokoye P, Ke J, Liu J, Zhao QD, Li XY (2014) L-cysteine-modified gold nanostars for SERS-based copper ions detection in aqueous media. Langmuir 30:13491–13497
Pall HS, Blake DR, Gutteridge JM, Williams AC, Lunec J, Hall M, Taylor A (1987) Raised cerebrospinal-fluid copper concentration in Parkinson’s disease. Lancet 330:238–241
Park JH, Choi HM, Oh KW (2014) Simultaneous crosslinking and cationization of cotton cellulose by using dialdehyde and choline chloride: comparison between the pad-dry-cure and microwave irradiation process. Cellulose 21:3107–3119
Pereira CC, de Souza AO, Oreste EQ, Vieira MA, Ribeiro AS (2018) Evaluation of the use of a reflux system for sample preparation of processed fruit juices and subsequent determination of Cr, Cu, K, Mg, Na, Pb and Zn by atomic spectrometry techniques. Food Chem 240:959–964
Qiu B, Guo J, Zhang X, Sun DJ, Gu HB, Wang Q, Wang XF, Zhang X, Weeks BL, Guo ZH, Wei SY (2014) Polyethylenimine facilitated ethyl cellulose for hexavalent chromium removal with a wide pH range. ACS Appl Mater Interfaces 6:19816–19824
Quintanar K, Domínguez-Calva JA, Serebryany E, Rivillas-Acevedo L, Haase-Pettingel C, Amero C, King JA (2016) Copper and zinc ions specifically promote nonamyloid aggregation of the highly stable human γ-D crystallin. ACS Chem Biol 11:263–272
Richardson SD, Ternes TA (2011) Water analysis: emerging contaminants and current issues. Anal Chem 83:4614–4648
Ruan CQ, Strømme M, Lindh J (2016) A green and simple method for preparation of an efficient palladium adsorbent based on cysteine functionalized 2,3-dialdehyde cellulose. Cellulose 23:2627–2638
Sayed MS, Reham A, Ibrahim AIA (2017) A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu(II). Spectrochim Acta A Mol Biomol Spectrosc 183:225–231
Schyrr B, Pasche S, Voirin G, Weder C, Simon YC, Foster EJ (2014) Biosensors based on porous cellulose nanocrystal-poly(vinyl alcohol) scaffolds. ACS Appl Mater Interfaces 6:12674–12683
Singh I, Sagare AP, Coma M, Perlmutter D, Gelein R, Bell RD, Deane RJ, Zhong E (2013) Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc Natl Acad Sci USA 110(36):14771–14776
Sui ZY, Cui Y, Zhu JH, Han BH (2013) Preparation of three-dimensional graphene oxide-polyethylenimine porous materials as dye and gas adsorbents. ACS Appl Mater Interfaces 5:9172–9179
Udousoro I, Ikem A, Akinbo OT (2017) Content and daily intake of essential and potentially toxic elements from dietary supplements marketed in Nigeria. J Food Compos Anal 62:23–34
Xiong SQ, Ye SD, Hu XH, Xie FZ (2016) Electrochemical detection of ultra-trace Cu(II) and interaction mechanism snalysis between amine-groups functionalized CoFe2O4/reduced graphene oxide composites and metal ion. Electrochim Acta 217:24–33
Yang RT, Yu HY, Song ML, Zhou YM, Yao JM (2016) Flower-like zinc oxide nanorod clusters grown on spherical cellulose nanocrystals via simple chemical precipitation method. Cellulose 23:1871–1884
Yu B, Xu J, Liu JH, Yang ST, Luo J, Zhou Q, Wan J, Liao R, Wang H, Liu Y (2013) Adsorption behavior of copper ions on graphene oxide-chitosan aerogel. J Environ Chem Eng 1:1044–1050
Zhang H, Xu M, Wang HJ, Lei D, Qu D, Zhai YJ (2013) Adsorption of copper by aminopropyl functionalized mesoporous delta manganese dioxide from aqueous solution. Colloids Surf A 435:78–84
Zhang ZY, Chen ZP, Qu CL, Chen LX (2014a) Highly sensitive visual detection of copper ions based on the shape-dependent LSPR spectroscopy of gold nanorods. Langmuir 30:3625–3630
Zhang N, Si YM, Sun ZZ, Chen LJ, Li R, Qiao YC, Wang H (2014b) Rapid, selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold-silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal Chem 86:11714–11721
Zhao LL, Zhong SX, Fang KM, Qian ZS, Chen JR (2012) Determination of cadmium(II), cobalt(II), nickel(II), lead(II), zinc(II), and copper(II) in water samples using dual-cloud point extraction and inductively coupled plasma emission spectrometry. J Hazard Mater 239–240:206–212
Zhao Z, Chen HD, Zhang H, Ma LN, Wang ZX (2017) Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens Bioelectron 91:306–312
Zhu WJ, Liu L, Liao Q, Chen X, Qian ZQ, Shen JY, Liang JL, Yao JM (2016) Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. Cellulose 23:3785–3797
Acknowledgments
The work was financially supported by the Public Technology Research Plan of Zhejiang Province (LGF18E030003, LY15E030003), National Natural Science Foundation of China (51672251), and 521 Talent Project of Zhejiang Sci-Tech University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, R., Liu, R. et al. Rapid capture and visual detection of copper ions in aqueous solutions and biofluids using a novel cellulose-Schiff base. Cellulose 25, 6947–6961 (2018). https://doi.org/10.1007/s10570-018-2083-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2083-x