Abstract
The transesterification reaction between cellulose and vinyl esters is regarded as a clean and facile strategy for the tunable synthesis of cellulose esters. In this study, a series of cellulose esters with degrees of substitution from 0.58 to 3.0 have been prepared successfully under mild conditions without adding any external catalysts when cellulose was dissolved in the 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)/DMSO/CO2 solvent system and then followed by adding equimolar amounts of long chain fatty, aromatic, branched and steric vinyl esters. The optimization study of different reaction parameters like reaction time, temperature and amounts of substrates demonstrates that the reaction can proceed smoothly even at room temperature. This is evidenced by a cellulose benzoate with a DS of 2.6 obtained when the reaction is performed at 25 °C in 4 h. The as-prepared cellulose esters structure has been confirmed by 1H NMR, 13C NMR and FTIR, and material thermal properties were evaluated by DSC and TGA for an in-depth understanding the relationship between the cellulose esters structure and properties. It is believed that the DBU not only acts as a reagent for the CO2-derivative dissolution of cellulose in DMSO, but also acts as an in situ organocatalyst for the subsequent transesterification reaction.
Graphical abstract
Cellulose esters have been prepared successfully at low temperature using long chain fatty acids, aromatic, branched and steric vinyl esters as acyl donors in the DMSO/DBU/CO2 solvents system, and the DBU not only acts as reagents for the CO2-derivative dissolution of cellulose in DMSO, acts as an organocatalyst for the subsequent derivatisation.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose carboxylic esters are a class of commercially important thermoplastic or functional materials, which can be processed into fiber, film and filter materials (Klemm et al. 2005). No matter whether the derivatization were performed under homogeneous or heterogeneous condition, the cellulose carboxylic esters usually were prepared by using the strategies as shown in Scheme 1; (a): carboxylic acidic anhydrides were used as acyl donors, in which high conversion rates were usually observed when large amounts of acyl donors were used. However, this process suffered drawbacks of yielding undesirable carboxylic acid as side products and low efficiency in the cases of long-chain nonpolar fatty acid anhydrides due to their low solubility in polar cellulose solvent systems (Cumpstey 2013; Gericke et al. 2012); (b): carboxylic acidic chlorides were used as acyl donor, in which high conversion rates also were observed. However, this process suffered drawbacks, such as degradation of cellulose and using organic base scavengers to remove the formed HCl byproduct (Barthel and Heinze 2006); (c) carboxylic esters were used as acyl donor via transesterification reaction, in which a large excess of the reagents and high temperature were needed, and usually low substitution efficiency was achieved (Schenzel et al. 2014); (d) carboxylic acids were used directly via esterification reaction using strong acids as catalysts (Freire et al. 2006); (e) vinyl esters were used acyl donors via transesterification reaction in the presence of basic catalysts, which was used to prepare cellulose esters with carboxylic acids difficult in preparation of corresponding anhydrides or acidic chloride (Cao et al. 2013). Among of these, the preparation of cellulose esters by using vinyl esters as acyl donors was regarded as a relatively milder and cleaner strategy than others, and the strategy has been widely applied for the synthesis of cellulose esters, starch esters and lignocellulosic esters (Table 1) (Vicente et al. 2004).
Traditionally, the transesterification reactions were performed under heterogeneous conditions, which usually need long activation, reaction time and extra amount of vinyl esters at high temperature when inorganic basic catalysts were used. Furthermore, it was limited to short acyl chains of C2 to C4 (Cao et al. 2013, 2014). It was reported that the transesterification reaction between cellulose and vinyl esters could be fulfilled under low temperature under homogeneous conditions in DMSO/TBAF solvent system, but it still need 40 h to achieve a satisfactory substitution degree (DS). In addition, DBU have to be used as a catalyst in the DMAc/LiCl solvent system (Heinze et al. 2000).
Recently, it was found that basic ionic liquid 1-ethyl-3-metyl-imidazolium acetate ([Emim]OAc) not only had a good solubility to cellulose, but also could act as an in situ catalyst for the transesterification reaction between cellulose and vinyl esters. Although a series of cellulose esters had been prepared under relatively mild conditions, an undesirable side reaction between the acetates from [Emim]OAc and the cellulose backbone was observed (Hinner et al. 2016). Therefore, the search into new preparation method for cellulose esters by using vinyl esters is still primary important so far.
It is well believed that the efficient dissolution of cellulose can significantly promote the tunable derivatisation of cellulose, particularly, ‘multifunctional solvent’ that not only owning good solubility to cellulose, but also acting as catalysts or components for its in situ subsequent derivatisation has been obtained much attention in academic fields as no external catalyst is needed for cellulose derivatisation.
For example, ionic liquid [Emim]OAc due to the basicity of acetate anion not only had good solubility for cellulose, but also had good catalytic activation for the reaction of cellulose with carboxylic anhydrides, carboxylic esters and vinyl esters (Gericke et al. 2012; Hinner et al. 2016). Taking the advantage of strong basicity of NaOH, highly efficient etherification of cellulose was achieved in the Urea–NaOH aqueous solvent system (Song et al. 2008). The in situ organocatalytic performance of the superbases/DMSO/CO2 solvent system for cellulose have been demonstrated recently and has been applied successfully to the efficient synthesis of cellulose esters by using anhydrides and cellulose-graft-poly(l-lactide), cellulose-graft-poly-(e-caprolactone) copolymers via ring polymerization (Song et al. 2015; Xu et al. 2017, 2018; Yang et al. 2014, 2015). Only cellulose esters with short acyl chains of C2 to C4 were successfully prepared using anhydrides as acylation reagents in the DBU/DMSO/CO2 solvent system under mild conditions(Yang et al. 2015), and it was hard to prepare cellulose esters deriving from long chain, aromatic, branched and steric carboxylic acids due to the difficult preparation of corresponding anhydrides.
Herein, we describe the characterization and optimization of the direct transesterification of cellulose with long chain fatty, aromatic, branched and steric vinyl esters in the DBU/DMSO/CO2 solvent system without any additional catalysts (Scheme 2) under mild conditions. The reaction conditions for the preparation of cellulose esters, such as temperature, time and the molar ratio of vinyl esters/anhydroglucose units (AGU) to cellulose were studied. The cellulose esters were characterized by Fourier transform infrared spectoscopy (FT-IR), nuclear magnetic resonance spectrascopy (1H NMR, 13C NMR), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) as well as gel permeation chromatography (GPC).
Experimental section
Materials
Microcrystalline cellulose (MCC) with a degree of polymerization (DP) of 240 was obtained from Aladdin Commerce Reagent Co., Ltd. and dried at 60 °C for 24 h in vacuum oven before use. CO2 with a purity of > 99.99% was supplied from Guiyang Shenjian Gas Company. DMSO was purchased from Tianjin Kaixin Chemcial Industry Co., Ltd and was dried by 4A molecular sieves. DBU was purchased from Aladdin Commerce Reagent Co. Ltd., was dried by KOH. The vinyl benzoate, vinyl pivalate, vinyl laurate, DMSO-d6 and CDCl3 were purchased by Sigma-Aldrich Co. Ltd. The vinyl 2-ethylhexyloate, vinyl octanoate, vinyl palmitate were obtained from J&K Scientific, and were used without purification. All chemical reagents were analytical reagents.
Measurements
The IR spectra of the samples were recorded with a Fourier transform IR spectrometer (FTIR, Nicolet iS50, the United States). The test specimens were prepared by the KBr-disk method. 1H NMR spectrum were recorded on a INOVA 400 MHz NMR Spectrometer (Varian, Palo Alto, CA, USA) in DMSO-d6 or CDCl3. The DS of cellulose octanoate, -laurate and -palmitate was calculated from the 1H NMR spectrum from the integral of the terminal methyl groups (Imethyl) and the area of the AGU signals (IAGU) according to (Hinner et al. 2016):
In the case of cellulose 2-ethylhexyloate, the integral of the two terminal methyl groups (I2*methyl) was used; and in the case of cellulose pivalate, the integral of the three terminal methyl groups (I3*methyl) was used; and in the case of cellulose benzoate, the integral of the phenyl protons (Iphenyl) were used according to the following equations (Hinner et al. 2016):
The 13C NMR spectra of cellulose esters in DMSO-d6 or CDCl3 were recorded at 25 °C on a INOVA 400 MHz NMR Spectrometer (Varian, Palo Alto, CA, USA). DSC analysis of the microcrystalline cellulose and cellulose esters were conducted on a TA Q2000. In order to provide the same thermal history before the measurement, each sample was heated from − 70 to 220 °C at a scanning rate of 5 °C/min and kept at 220 °C for 5 min and quenched to − 70 °C. All the reported Tg values were observed in the second scan. TGA was carried on a TG-DTA/DSC-APPARTUS with a heating rate of 10 °C/min from 25 to 600 °C under nitrogen atmosphere. GPC measurement was carried out with waters 515 HPLC pump and waters 2414 refractive index detector, DMF (0.05 M LiBr) or CHCl3 was used as the eluent with a flow rate of 1 mL/min at 50 °C. Empower 2 software was used to calculate molecular weight on the basis of an universal calibration curve generated by a polymethylmethacrylate of narrow molecular weight distribution.
Dissolution of cellulose in the DBU/DMSO/CO2 solvent system
A mixture of microcrystalline cellulose (0.65 g, 4.01 mmol), DMSO (10.00 g, 127.99 mmol), and DBU (1.83 g, 12.04 mmol) were added into a high-pressure reactor, and then 2 bar of CO2 was introduced into the system. Subsequently, the reactor was heated to 50 °C in an oil bath for 3 h with magnetic stirring to dissolve cellulose, and a clear 5 wt% cellulose solution was obtained and ready for subsequent derivatization.
Typical procedure for the preparation of cellulose esters
The designed amount of vinyl ester was dropped into 5 g of 5 wt% of cellulose solution in a flask equipped with a mechanical stirrer at room temperature, and then the reaction was performed under designed conditions with vigorous mechanical stirring. After completion of the reaction, the cellulose esters were precipitated out with 100 mL of methanol and washed with methanol (2 × 50 mL) and water (50 mL) to remove the solvent residuals, and a serials of cellulose esters are obtained after freezing dry.
Results and discussion
After the successful dissolution of cellulose in DBU/DMSO/CO2 solvent system, the transesterification reaction between cellulose and vinyl benzoate was selected as a model reaction to optimize the reaction parameters as the vinyl benzoate is a typical acylation reagent with big steric hindrance due to the presence of phenyl group. To our delight, as shown in Table 2, a series of cellulose benzoates with different DS can be obtained when vinyl benzoate is added into the cellulose solution under various conditions. It is worth noting that a cellulose benzoate with a DS = 2.60 is obtained at 25 °C in 4 h when equimolar amounts of vinyl benzoate is added. The DS of all the products were analyzed by 1H NMR in DMSO-d6 according to previous publications (Hinner et al. 2016; Xiao et al. 2014; Zhang et al. 2009). It is found that the reaction proceeds considerably faster and a DS of 1.63 is achieved after only 0.5 h at 40 °C when the molar ratio of vinyl benzoate to the AGU unit is 3:1(Table 2, CE-8). However, it takes 4 h to achieve a comparative DS at 80 °C when the same molar ratio of vinyl benzonate to the AGU is applied in [Emim]OAc ionic liquid (Hinner et al. 2016).
The results in Table 2 show the DS controllability in this system for the synthesis of various cellulose esters by tuning reaction conditions such as reaction time, reaction temperature and the amount of vinyl esters. The transesterification reaction occurs very fast in the first 1 h and then becomes slow (CE-4, CE-6, CE-7). These is evidenced by the fact that a cellulose ester with a DS of 2.50 is achieved in 1 h (CE-7), and the DS is slightly increased from 2.50 to 2.67 and 2.73 when prolonging the reaction time from 1 h to 2 and 4 h (CE-4, CE-6), respectively. Increasing temperature cannot favor the reaction obviously, as the DS of cellulose benzoate only increases from 2.6 to 2.73 (CE-4) and 2.94 (CE-1) when the reaction is performed at 40 °C and 80 °C in 4 h, representing a conversion of 98% of cellulose hydroxyl group at 80 °C in 4 h. Further enhancing the molar ratio of vinyl benzoate to AGU to 4:1 leads to a cellulose benzoate with a DS of 3.0, which implies that lower acyl donor is needed to achieve cellulose esters with high DS values under much more mild conditions in the DBU/DMSO/CO2 solvent system comparing to other process, including the ionic liquid-based process (Hinner et al. 2016).
The molar ratio of vinyl benzoate to AGU has significant effect on the DS of cellulose benzoates, which is evidenced by the fact that decreasing the molar ratio from 3:1 to 2:1 results in a significant decreasing in the DS from 2.73 (CE-4) to 0.73 (CE-9). Only a DMSO insoluble product is obtained when the molar ratio is set to 1:1 (CE-10) in 4 h and a cellulose benzoate with a DS of 0.58 (CE-11) is obtained when the reaction time is prolonged from 4 h to 12 h. Given these results, it is reasonable to assume that the DS value of cellulose esters can be controlled by varying the stoichiometric ratio of vinyl esters/AGU, temperature and reaction time in this solvent system.
Furthermore, apart from the advantages by using vinyl esters as acyl donors over other acyl donors as aforementioned, the advantages of the DBU/DMSO/CO2 solvent system in this study over the widely concerned [Emim]OAc ionic liquid process is very obvious, such as lower cost of the solvents, higher reaction speed and without undesirable reaction like in the [Emim]OAc ionic liquid system. It was reported that a mixed cellulose benzoate acetate with a total DS of 2.4 (DSbenzoate = 2.3; DSacetate = 0.1) was obtained in [Emim]OAc ionic liquid solvent system under comparative conditions (Hinner et al. 2016). The aforementioned advantages and DS controllability of cellulose benzoate in this study will provide many opportunities in the design of cellulose esters with various DS by simply varying the reaction conditions to meet the requirement of targeted applications.
It is well recognized that the facile preparation of cellulose esters derived from long chain fatty acids, aromatic, branched and steric substrates, such as pivalate, is a challenge (Hinner et al. 2016). For example, the vinyl ester-based cellulose modification was carried out in a heterogeneous reaction system in DMSO by using NaOH or KOH as catalysts. This process requires high substrate concentrations and was limited to short acyl chains of C2 to C4 (Shogren and Biswas 2010; Xie and Hsieh 2001). A series of mixed cellulose esters with moderate DS were achieved in the [Emim]OAc ionic liquid-based process in the cases of vinyl octanoate (DSoctanoate = 1.5; DSacetate = 0.1), vinyl laurate (DSlaurate = 1.6; DSacetate = 0.2), vinyl palmitate (DSpalmitate = 1.5; DSacetate = 0.1) and vinyl pivalate (DSoctanoate = 2.1; DSacetate = 0.1). Furthermore, low DS was achieved in the case of vinyl 2-ethylhexyloate (DSvinyl 2-ethylhexyloate = 0.9; DSacetate = 0.2) at 80 °C in 4 h (Hinner et al. 2016). The outstanding performance of the present process for the transesterification reaction between cellulose and vinyl benzoate stimulates us to extend the process to other challenging acyl donors. It is found that other commercial available vinyl esters are applicable in this process. Cellulose pivalate with a DS of 2.45 (CE-12), cellulose 2-ethylhexyloate with a DS of 1.45 (CE-13), cellulose octanoate with a DS of 3.0 (CE-14), cellulose palmitate with a DS of 3.0 (CE-15) and cellulose laurate with a DS of 3.0 (CE-16) were obtained when the reactions were performed at 40 °C in 4 h with a molar ratio of vinyl esters to AGU at 3:1.
We note that the conditions used in this study were much milder than those when anhydrides were used as acylation reagents in the same solvent system. This may be due to the in situ consumption of DBU catalyst to form a proton ionic liquids (e.g. [DBUH]OAc in the case of acetic anhydride) when anhydrides were used as acylation reagents. However, only low vapor point ethenol (in situ tautomerization to acetaldehyde) was produced when vinyl esters were used as acylation reagents. The acetaldehyde could not react with DBU and the concentration of DBU catalyst did not change during the reaction, thus presenting a high catalytic activity even at low temperature. A recent publication demonstrated that although [DBUH]OAc could act as solvent for cellulose and in situ catalyze the reaction of cellulose with anhydride, it need higher temperature and longer reaction time to achieve high DS value, indicating the better catalytic activity of DBU than [DBUH]OAc (Hanabusa et al. 2018). Furthermore, the boiling point of acetaldehyde is only 20.8 °C, and its potential evaporation from the reaction system during the reaction will destroy the balance of the transesterification reaction, thus further promoting the transesterification reaction in this study.
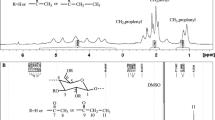
1H NMR and 13C NMR analyses were used to elucidate the cellulose esters structure and evaluate the distribution of substitution in the as-prepared cellulose esters (Zhang et al. 2009). Taking the cellulose benzoate as an example, the results (Fig. 1) show that strong peaks assigned to phenyl protons within the chemical shift range from 6.56 to 8.29 ppm (Fig. 1a) is observed; correspondingly, a peak of the chemical shift assigned to carbonyl carbons at 164.88 ppm, peaks of the chemical shift assigned to phenyl carbons at 133.25 ppm, 129.06 ppm and 128.51 ppm are observed (Fig. 1b). In Fig. 1b, only a peak at 62.58 ppm assigned to the C6´ carbon bearing a substituted phenyl group is observed, and an expected peak at 59.00 ppm assigned to the unmodified C6 is not observed, indicating a full substitution at C6 occurred in this study. Only a peak around 78.85 ppm marked as C4´ can be assigned to C4 carbons adjacent to C3 carbons bearing a substituted hydroxyl group, indicating also a full substitution at C3 occurred. Two peaks at 101.86 ppm and 98.98 ppm are observed, which are assigned to C1 and C1´ adjacent to C2 carbon bearing an unsubstituted and substituted hydroxyl group, respectively, indicating a partial substitution at C2. The peak at 75.17 ppm is assigned to the C2 carbon, and the heavily overlap resonance peaks around 71.98 ppm are assigned to C3 and C5 carbons for a high DS sample. The results are consistent with the results in a previous publication (Zhang et al. 2009). The 1H NMR and 13C NMR of other cellulose esters was included in the supporting information (Fig. S1-17).
The successful transesterification reaction of cellulose with vinyl benzoate was further confirmed by comparative FT-IR spectra of native cellulose and cellulose benzoates with different DS values, and the results are shown in Fig. 2. In comparison, a decrease in O–H band (3500 cm−1) and increase in the three major ester bands of benzoate cellulosic sample [i.e. the C=O band (1723 cm−1), the C–O band (1275 cm−1), and the phenyl group band (713 cm−1)] provide evidence for the successful transesterification reaction (Çetin et al. 2009; Gao et al. 2016; Tsuboi 1957; Zhang et al. 2009). Furthermore, the intensities of the relative bands are increasing with the increase in the DS values.
The thermal properties of raw cellulose and selected cellulose esters were characterized by TGA and DSC measurements under N2 atmosphere. As shown in Fig. 3, the thermal degradation of native cellulose starts at 327.6 °C following a single weight-loss step, whereas the three CE-4, CE-8, CE-9 start to decompose at 329 °C, 339 °C and 343 °C, respectively. This indicates that the thermal stability of cellulose is slightly increased due to the benzoation. Furthermore, the thermal stability increases slightly with the increase in the DS value. The increased thermal stability of the as-prepared cellulose esters in comparison to raw cellulose is also observed in the other cases. It appears that the thermal stability is related to the structure of acyl donors applied, and the comparative results are summarized in Table S1 and Figure S18.
In addition, The DSC analysis results (Fig. 4) show that the cellulose does not show glass transition temperature (Tg) at a standard heating scan under 220 °C, whereas the cellulose benzoate samples (CE-9, CE-8, and CE-4) exhibit very clear glass transitions at 210.1 °C, 192.8 °C, and 179.4 °C, respectively. The Tg of the cellulose benzoate decreases with an increase in DS, which is in accordance with the previous study and can be explained by the fact that the higher DS leads to less hydrogen bonding interaction and a lower Tg (Cao et al. 2010). The DSC curves of other cellulose ester exhibit clear glass transition as well (Fig. S19). The number average molar mass (Mn) of cellulose benzoate (CE-1, DS = 2.94) is about 113,000, and the mass average molar mass (Mw) is about 147,500 (Fig. S20), which further demonstrates the successful transesterification reaction and chemical stability of cellulose in this process.
The solubility of cellulose esters in conventional organic solvents is one of the most important properties towards their future applications. Table 2 also includes the solubility of the as-prepared cellulose esters, and the results show that the solubility is related to the functionalization patterns, and cellulose benzoates with DS larger than 0.58 and the as-prepared cellulose pivalate and cellulose 2-ethylhexyloate are soluble in DMSO, but insoluble in acetone and CHCl3, however, the as-prepared cellulose octanoate, palmitate, and laurate esters are insoluble in DMSO and acetone, but soluble in CHCl3.
Conclusions
Herein, we reported a DBU/DMSO/CO2 solvent-based process for the efficient and mild synthesis of a variety of cellulose esters via transesterification reaction between cellulose with long chain fatty acidic, aromatic, branched and steric vinyl esters. In contrast to other processes, the vinyl ester-based synthesis of cellulose esters in DBU/DMSO/CO2 solvent system presents particular characteristics, such as higher reaction speed at low temperature, good tunability of DS, and good applicable scope of vinyl esters. The outstanding performance of the DBU/DMSO/CO2 solvent-based process in this study is ascribed to the in situ organocatalytic principle of DBU both in the dissolution of cellulose and subsequent transesterification reaction.
References
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301–306
Cao Y, Zhang J, He J, Li H, Zhang Y (2010) Homogeneous acetylation of cellulose at relatively high concentrations in an ionic liquid. Chin J Chem Eng 18:515–522
Cao X, Sun S, Peng X, Zhong L, Sun R, Jiang D (2013) Rapid synthesis of cellulose esters by transesterification of cellulose with vinyl esters under the catalysis of NaOH or KOH in DMSO. J Agric Food Chem 61:2489–2495
Cao X, Peng X, Zhong L, Sun S, Yang D, Zhang X, Sun R (2014) A novel transesterification system to rapidly synthesize cellulose aliphatic esters. Cellulose 21:581–594
Çetin NS, Tingaut P, Özmen N, Henry N, Harper D, Dadmun M, Sèbe G (2009) Acetylation of cellulose nanowhiskers with vinyl acetate under moderate conditions. Macromol Biosci 9:997–1003
Chen M-J, Li R-M, Zhang X-Q, Feng J, Feng J, Liu C-F, Shi Q-S (2016) Homogeneous transesterification of sugarcane bagasse toward sustainable plastics. ACS Sustain Chem Eng 5:360–366
Cumpstey I (2013) Chemical modification of polysaccharides. ISRN Org Chem 2013:27
Dicke R (2004) A straight way to regioselectively functionalized polysaccharide esters. Cellulose 11:255–263
Freire CSR, Silvestre AJD, Neto CP, Belgacem MN, Gandini A (2006) Controlled heterogeneous modification of cellulose fibers with fatty acids: effect of reaction conditions on the extent of esterification and fiber properties. J Appl Polym Sci 100:1093–1102
Gao K, Chen Q, Hu Y, Zheng Q, Xie H (2016) Preparation and characterization of cellulose benzoate in CO2/DBU/DMSO. Sci Sin Chim 46:1337–1342
Gericke M, Fardim P, Heinze T (2012) Ionic liquids-promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17:7458–7502
Hanabusa H, Izgorodina EI, Suzuki S, Takeoka Y, Rikukawa M, Yoshizawa-Fujita M (2018) Cellulose-dissolving protic ionic liquids as low cost catalysts for direct transesterification reactions of cellulose. Green Chem 20:1412–1422
Heinze T, Dicke R, Koschella A, Kull AH, Klohr EA, Koch W (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631
Hinner LP, Wissner JL, Beurer A, Nebel BA, Hauer B (2016) Homogeneous vinyl ester-based synthesis of different cellulose derivatives in 1-ethyl-3-methyl-imidazolium acetate. Green Chem 18:6099–6107
Itoh T, Nishimura Y, Ouchi N, Hayase S (2003) 1-Butyl-2,3-dimethylimidazolium tetrafluoroborate: the most desirable ionic liquid solvent for recycling use of enzyme in lipase-catalyzed transesterification using vinyl acetate as acyl donor. J Mol Catal B Enzym 26:41–45
Junistia L, Sugih AK, Manurung R, Picchioni F, Janssen LPBM, Heeres HJ (2008) Synthesis of higher fatty acid starch esters using vinyl laurate and stearate as reactants. Starch-Stärke 60:667–675
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Inter Ed 44:3358–3393
Mormann W, Al-Higari M (2004) Acylation of starch with vinyl acetate in water. Starch-Starke 56:118–121
Schenzel A, Hufendiek A, Barner-Kowollik C, Meier MAR (2014) Catalytic transesterification of cellulose in ionic liquids: sustainable access to cellulose esters. Green Chem 16:3266–3271
Shogren RL, Biswas A (2010) Acetylation of starch with vinyl acetate in imidazolium ionic liquids and characterization of acetate distribution. Carbohydr Polym 81:149–151
Song Y, Sun Y, Zhang X, Zhou J, Zhang L (2008) Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromol 9:2259–2264
Song L, Yang Y, Xie H, Liu E (2015) Cellulose dissolution and in situ grafting in a reversible system using an organocatalyst and carbon dioxide. ChemSusChem 8:3217–3221
Tsuboi M (1957) Infrared spectrum and crystal structure of cellulose. J Polym Sci 25:159–171
Vicente G, Martinez M, Aracil J (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour Technol 92:297–305
Xiao P, Zhang J, Feng Y, Wu J, He J, Zhang J (2014) Synthesis, characterization and properties of novel cellulose derivatives containing phosphorus: cellulose diphenyl phosphate and its mixed esters. Cellulose 21:2369–2378
Xie J, Hsieh YL (2001) Enzyme-catalyzed transesterification of vinyl esters on cellulose solids. J Polym Sci Part A Polym Chem 39:1931–1939
Xu Q, Song L, Zhang L, Hu G, Du J, Liu E, Zheng Q, Liu Y, Li N, Xie H (2017) Organocatalytic cellulose dissolution and in situ grafting of epsilon-caprolactone via ROP in a reversible DBU/DMSO/CO2 system. Chemistryselect 2:7128–7134
Xu Q, Song L, Zhang L, Hu G, Chen Q, Liu E, Liu Y, Zheng Q, Xie H, Li N (2018) Synthesis of cellulose acetate propionate and cellulose acetate butyrate in a CO2/DBU/DMSO system. Cellulose 25:205–216
Yang Y, Xie H, Liu E (2014) Acylation of cellulose in reversible ionic liquids. Green Chem 16:3018–3023
Yang Y, Song L, Peng C, Liu E, Xie H (2015) Activating cellulose via its reversible reaction with CO2 in the presence of 1,8-diazabicyclo 5.4.0 -undec-7-ene for the efficient synthesis of cellulose acetate. Green Chem 17:2758–2763
Zhang J, Wu J, Cao Y, Sang S, Zhang J, He J (2009) Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 16:299–308
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31270637 and 21574030); Science and Technology Department of Guizhou Province (Grant No. Natural Science Key Fund [2016]1402), (Grant No. Platform & Talents [2016]5652); Open research fund of Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China (08031339); Foundation of State Key Laboratory of Coal Conversion (Grant No. J17-18-907); Excellent Scientific Innovative Talent Programme from Education Department of Guizhou Province (Grant No. KY[2015]479).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Yang, F., Du, J. et al. Efficient transesterification reaction of cellulose with vinyl esters in DBU/DMSO/CO2 solvent system at low temperature. Cellulose 25, 6935–6945 (2018). https://doi.org/10.1007/s10570-018-2078-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2078-7