Abstract
We developed an isocyanate group containing quaternary ammonium salt (IQAS) as a potential antimicrobial finishing agent that can be stored in a high purity form for more than a year in dry environments to permit convenient transportation and storage. Additionally, we report a facile and eco-friendly finishing technique to fabricate durable antimicrobial cotton fabrics using IQAS as an antimicrobial finishing agent by a dipping–padding–drying process. IQAS was bound onto the surface of cotton fabrics by a covalent bond to obtain a cotton fabric with excellent bactericidal activity, tearing strength, breaking elongation, bending rigidity, water vapor permeability, surface roughness and smoothness. The antimicrobial rates of these fabrics reached 92.2% and 98.5% against gram-negative bacterium Escherichia coli and gram-positive bacterium Staphylococcus aureus, respectively, even after 50 laundering cycles. These values are much higher than the reference antimicrobial rates of AAA class antimicrobial fabrics, as well as those of conventionally finished cotton fabrics, indicating that the IQAS finished cotton fabrics maintained excellent antimicrobial activity even after long-term repeated launderings. Moreover, the results indicate that the IQAS-treated cotton fabrics could improve the breaking strength (increased by 13.5% in the warp direction and 20.3% in the weft direction), the bursting strength (increased by 11.9%), the air permeability (increased by 12.6%) and the hydrophilicity compared to untreated cotton fabrics. Additionally, our non-leaching antimicrobial cotton fabrics treated with IQAS were non-toxic and showed no skin stimulation. Therefore, IQAS and the antimicrobial finishing technique reported here have great potential applications in antimicrobial fabrics used in hospitals, hotels and other susceptible situations.
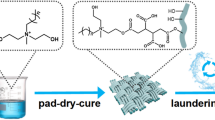
Graphical abstract
An isocyanate group containing quaternary ammonium salt that permits convenient transportation and storage was developed as a potential antimicrobial finishing agent to fabricate excellent antimicrobial cotton fabrics with well-preserved physical properties and security using a dipping–padding–drying process.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Porous and hydrophilic cotton fabrics are the most popular clothing fabrics due to their breathable and comfortable properties. However, pathogenic bacteria tend to proliferate rapidly on cotton fabrics to form bacterial plaques/biofilms resulting in reducing the fabric wearability (Chen et al. 2016; Zhang et al. 2018). Fabrics used in hospitals, hotels and other public situations are especially susceptible to pathogenic organisms, leading to transmitting infectious diseases among guests, patients and medical personnel.
To improve this inadequacy, substantial efforts have been made to develop antimicrobial cotton fabrics using antimicrobial compounds, such as chitosan and cationic compounds (Chen et al. 2018; Gao et al. 2014, 2015; Sadeghi-Kiakhani et al. 2018; Yin et al. 2018; Yu et al. 2016), zwitterionic betaines (Chen et al. 2011, 2016; He et al. 2017), N-halamine (Dong et al. 2017; Liu et al. 2015; Tian et al. 2017), poly(hexamethylene biguanide) (Amrit et al. 2013; Patil et al. 2018; Zhi et al. 2017), metals and metal salts (Bacciarelli-Ulacha et al. 2014; Ibrahim and Hassan 2016; Kang et al. 2016; Liu et al. 2018; Xu et al. 2018) and metallic oxides (Giesz et al. 2016; Wang et al. 2016). Among them, much attention has been paid to the development of cationic antimicrobial agents due to their broad-spectrum antimicrobial function based on interruption of protein activity and other essential functions of cell membranes (Li et al. 2011; Simoncic and Tomsic 2010). Due to the slow leaching of the antimicrobial agents into its surroundings, leaching antimicrobials would induce resistance in microbes; and they do not withstand repeated laundering. Additionally, leaching antimicrobial finishing agents on textiles will kill the resident flora of nonpathogenic bacteria on the skin of the wearer, which are important to the health of the skin as they can create an unfavorable environment for the growth of pathogenic bacteria (Simoncic and Tomsic 2010). Compared to leaching antimicrobial agents, bound antimicrobials rarely induce resistance in microbes, and they are much more resistant to repeated laundering. Therefore, non-leaching bactericidal surfaces with permanent bactericidal activity should be developed to rapidly kill contacted and invasive pathogenic microbes and to reduce the development of resistance to antimicrobials in microbes.
Bound antimicrobial compounds containing a reactive siloxane group have been developed to finish cotton fabric to endow them with good antimicrobial activities against bacteria and fungi (Chen et al. 2011; Gao and Cranston 2008). However, the siloxane-containing antimicrobials tend to self-condensate, leading to a poor storage stability. Herein, we report a rapid, eco-friendly, and cost-effective finishing technique to fabricate durable antimicrobial cotton fabric surfaces with excellent bactericidal activities using an isocyanate group (NCO)-containing reactive quaternary ammonium salt (IQAS) as an antimicrobial finishing agent using a dipping–padding–drying process. Antimicrobial quaternary ammonium salt (QAS) can be chemically bound onto the surface of cotton fabrics because the NCO group of IQAS can react easily with the OH group of cotton fabrics under mild conditions. The bound QAS on cotton fabrics can rapidly kill the invasive and contacted microbes, endowing perdurable antimicrobial activity. Additionally, perdurable antimicrobial cotton fabrics finished with IQAS showed no skin irritation, no acute oral toxicity and well-preserved the physical properties.
Materials and methods
Materials
Isophorone diisocyanate (IPDI, > 99%) and stannous octoate (C.P.) were purchased from Aladdin Industrial Corporation, China. 3-bromo-1-propanol (> 99%) was purchased from Energy Chemical Co. Ltd., China. N,N-dimethyloctadecylamine (99%) was provided by Adamas Reagent Co. Ltd., China. Tetrahydrofuran (THF, AR), N,N-dimethylformamide (DMF, AR) and other solvents were all purchased from Guangzhou Chemical Reagent Factory, China. Two commercial siloxane quaternary ammonium salts from Dow Corning Corporation (USA, Code: AEM 5700) and Beijing Jlsun High-tech Co., Ltd. (China, codes: SCJ877) were used to finish the cotton fabrics following the instructions in the user’s manual. Gram-negative bacterium Escherichia coli (E. coli, ATCC25922), and gram-positive bacterium Staphylococcus aureus (S. aureus, ATCC6538) were provided by Guangdong Institute of Microbiology and were incubated on a nutrient agar plate at 37 °C for 24 h before use. Kunming (KM) mice (half of each sex) and New Zealand rabbits (1 male and 2 females) were obtained from the Medical Laboratory Animal Center of Guangdong Province and handled with protocols approved by the Laboratory Animals Center of Shenzhen University. All the animal experiments and maintenance were approved by the Laboratory Animal Ethics Committee of Shenzhen University. KM mice (~ 4 weeks old) were housed 5 per cage. All animals were fed with water and standard laboratory chow.
Synthesis of IQAS
The reactive IQAS was synthesized using a two-step typically chemical reaction, and the synthetic principle is shown in Fig. 1a. First, a nucleophilic substitution reaction of N,N-dimethyloctadecylamine with 3-bromo-1-propanol was performed under continuous stirring for 12 h at 70 °C in a nitrogen atmosphere to obtain the intermediate product N-octadecyl-N-(3-hydroxypropyl)-N,N-dimethylammonium bromide (HO-QAS) containing the hydroxyl (OH) group. Second, the addition reaction between the OH group of OH-QAS with the NCO group of IPDI was performed under continuous stirring for 12 h at 40 °C in a nitrogen atmosphere (Fig. 1a). After the crude product was washed five times using petroleum ether, it was dried under vacuum for 24 h at 80 °C. Finally, the yellowish oily IQAS was obtained.
Cotton fabric finishing
The typical antimicrobial finishing process of cotton fabrics is briefly presented as follows. Commercial cotton fabrics were sequentially washed with DMF and water for 10 min, and then the clean cotton fabrics (defined as raw cotton fabric, 0) were obtained after they were dried in air. Thereafter, 0 were dipped in IQAS finishing agents (2.0 wt%, dissolved in water, liquor ratio 20:1) for 2 min. Then, the fabrics were sequentially cured for 10 min at 80 °C and 2 min at 130 °C. Finally, the cotton fabrics finished with IQAS (1) were obtained after they were washed three times with purified water to remove the unreacted IQAS, and no obvious color difference between 0 and 1 was observed. Two commercial siloxane-containing quaternary ammonium salts AEM 5700 and SCJ877 were used to finish the cotton fabrics following the instructions in the user’s manual with the same concentration, and the cotton fabrics finished with AEM5700 (2) and SCJ877 (3) were obtained as comparative samples.
Structural characterization
Fourier transform infrared spectroscopy (FTIR) spectra of the raw materials, intermediate and IQAS were recorded using a thermo Nicolet 6700 FTIR spectrometer (Thermo Scientific, USA) with the KBr method with 32 scans at a 4 cm−1 resolution. The FTIR spectra of 0, 1, 2 and 3 were measured using a thermo Nicolet 6700 FTIR spectrometer with a smart iTR attachment in the range of 650–4000 cm−1 with 32 scans at a 4 cm−1 resolution. The proton nuclear magnetic resonance (1H-NMR) spectra of the IQAS and its intermediate were recorded using a 600 MHz spectrophotometer (AVANCE AV600, Bruker) at room temperature using deuterochloroform (CDCl3) as the solvent (Fig. 1c).
The surface composition of 1 compared to 0 was analyzed using X-ray photoelectron spectroscopy (XPS, ULVAC-PHI 1800) with an Al KR as the radiation source. A survey scan was collected over the range of 0–1300 eV, and the high-resolution spectra were collected for N1 s and Br3d. The take-off angle of the photoelectron was set as 75°. The surface profile 0 was observed using a thermal field-emission scanning electron microscope (TFE-SEM, Hitachi Su-70) to compare that of 0, 2 and 3, and all the samples were coated with gold before observation.
Comprehensive performance evaluation
The mechanical properties of 0, 1, 2 and 3 were evaluated according to the standard test method of ISO 9073 part 3 (Strip Method), and the hydrophilicities of 0, 1, 2 and 3 were evaluated using the textile-test method for the capillary effect [ISO 9073-6 (2000)] (Chen et al. 2011). The water vapor permeability (WVP) and air permeability (AIP) were evaluated according to the method of our previous reference (Chen et al. 2016).
Antimicrobial activities
The antimicrobial properties of 1 compared with 0, 2 and 3 against gram-negative bacterium Escherichia coli (E. coli, ATCC25922) and gram-positive bacterium Staphylococcus aureus (S. aureus, ATCC6538) were comparatively evaluated using the agar diffusion method and the viable cell count method (Chen et al. 2016). The antimicrobial rates of 0, 1, 2 and 3 were investigated after 50 laundering cycles to evaluate the washing durability of the antimicrobial activity, and the leachability of antimicrobial agents from the finished cotton fabrics was determined using the diffusion method on an agar plate after washing once according to method from our previous reference (Chen et al. 2011).
Toxicity evaluation
The acute oral toxicity and animal skin irritation are two important requirements of antimicrobial textiles. Antimicrobial rates of 0 and 1 were applied directly to the back skin of three rabbits to evaluate their animal skin irritation according to the method of previous references (Chen et al. 2016; Yu et al. 2016). The fabrics with antimicrobial rates of 0 and 1 were immersed in water and extracted at 60 °C for 2 h, and then the extracted solution was administered orally to 20 male and 20 female mice to evaluate their animal acute oral toxicity. The mice were observed every 2 days for 2 weeks. The monomer was replaced by the extracted solution because the toxicity of the reactive antimicrobial may be quite different from that of its chemically combined form (Yu et al. 2016).
Results and discussion
The antimicrobial activity of leaching antimicrobials based on the controlled-release mechanism is attributed to their gradual and persistent release from fabrics into their surroundings. Therefore, the antimicrobial activity decrease can even expired completely after a period of use and repeated launderings, since the concentration of the active substance in the fabrics decreases and gradually falls under the limit of effectiveness. Additionally, the antimicrobial residues leaching from the fabrics may kill the resident skin flora of nonpathogenic bacteria, which are important to the health of the skin as they can help create an unfavorable environment for the growth of pathogenic bacteria, and lead to the outgrowth of pathogenic bacteria due to change in the ecology of the skin resident flora (Gao and Cranston 2008). In contrast, bound antimicrobials do not leach into the surroundings of the fabrics, so the antibacterial activity of bound antimicrobials can be maintained due to the persistent existence of antibacterial groups on the fabrics’ surfaces. Therefore, bound antimicrobials have better repeated laundering properties compared to leaching antimicrobials, and the probability of microbes developing resistance to them is also small (Simoncic and Tomsic 2010). Notably, AEM 5700 (a siloxane-involved quaternary ammonium salt) developed by Dow Corning has been widely used as an antimicrobial finishing agent because it can be chemically bound to the cotton backbone to endow durable antimicrobial activities. However, the storage stability of the siloxane group is poor due to its easy self-condensation (Chen et al. 2016; He et al. 2017), so it should be stored in a large amount of solvent containing organic alcohol. Furthermore, the cellulose –O–Si–O– linkage may undergo reversible hydrolysis, which can strip off some of the anchored silane from the surface (Brochier Salon et al. 2005). Herein, IQAS was developed as a potential antimicrobial to replace siloxane-involved QAS because the NCO group can more easily react with the OH groups of the cotton backbone than the siloxane group can under mild conditions, resulting in being strongly chemically bound to the cotton fabric to render durable antimicrobial activities. Additionally, IQAS can be stored in a high purity form for more than a year in dry environments to permit convenient transportation and storage.
IQAS was synthesized using a two-step typically chemical reaction, as shown in Fig. 1a. First, the intermediate product N-octadecyl-N-(3-hydroxypropyl)-N,N-dimethylammonium bromide (HO-QAS) was obtained using a nucleophilic substitution of N,N-dimethyloctadecylamine with 3-bromo-1-propanol. Then, the addition reaction was performed between the NCO group of IPDI and the OH group of OH-QAS, and the yellowish oily IQAS was finally obtained after proper purification. Analyzing the molecular structures of IQAS compared to the raw materials and intermediate were characterized using FTIR (Fig. 1b) and 1H-NMR (Fig. 1c). Compared to N,N-dimethyloctadecylamine and 3-bromopropanol, the intermediate, a new peak at 1465 cm−1 assigned to methyl group of quaternary ammonium salt (N+–CH3) appeared in the FTIR spectra of the IQAS and the intermediate (HO-QAS) (Beyth et al. 2008). Compared to HO-QAS, two new peaks at 1540 cm−1 (N–H group of amide linkage bonds) and 1710 cm−1 (carbonyl group, C=O) appeared in the FTIR spectrum of the IQAS, and a strong peak at 2260 cm−1 appeared, which is assigned to the NCO group, and was clearly observed in the FTIR spectra of the IQAS and IPDI (Chen et al. 2016). The 1H-NMR data of the intermediate and IQAS are shown as follows.1The H-NMR of the intermediate (600 MHz, CDCl3) δ 4.45–4.30 (m, 1H), 3.85–3.67 (m, 4H), 3.46–3.33 (m, 2H), 3.33–3.26 (m, 6H), 2.21–2.04 (m, 2H), 1.87–1.58 (m, 2H), 1.43–1.19 (m, 30H), 0.91–0.82 (m, 3H). 1H-NMR of IQAS (600 MHz, CDCl3) δ 4.35–3.95 (m, 2H), 3.90–3.55 (m, 4H), 3.50 (s, 1H), 3.47 (m, 1H), 3.39–3.27 (s, 6H), 3.10–2.77 (m, 2H), 1.86–1.55 (m, 4H), 1.45–1.22 (m, 36H), 1.20–0.79 (m, 12H). Therefore, we formed that the IQAS was prepared successfully.
The resultant NCO-containing IQAS can be tightly bound to cotton fibers by formation of covalent bonds under mild conditions (Fig. 2a), imparting the cotton fabrics with permanent antimicrobial activity. The successful finishing of cotton fabrics with IQAS was demonstrated using FTIR spectra (Fig. 2b) and XPS (Fig. 2c, d). Compared to 0, two new peaks appeared in the FTIR spectrum of 1 at 1710 cm−1 (C=O) and 1540 cm−1 (N–H group of amide linkage bonds) (Fig. 2b). A new N1s peak at 402.5 eV (N+) and the Br3d peak at approximately 67.6 eV (Fig. 2c) can only be observed in the XPS spectrum of 1 (Zhang et al. 2018). Additionally, the EDS characterization showed that a new weak signal for Br appeared in 1 (Fig. 2d). Therefore, we can confirm that IQAS was successfully bound to cotton fabrics by chemical bonds. TFE-SEM images show that the fibers of all the finished fabrics were not obviously damaged during the finishing process (Fig. 2e). A well-preserved fiber structure is beneficial to maintaining the physical properties of cotton fabrics, such as the mechanical properties and surface roughness.
Comprehensive performance evaluation
As shown Fig. 3a, b, the breaking strength and bursting strength of the cotton fabrics were improved to different extents after being finished. For example, the breaking strengths increased from 276.6 N (0) to 313.9 N (1, increased by 13.5%), 328.8 N (2, increased by 18.9%) and 311.2 N (3, increased by 12.5%) in the warp direction, and the breaking strengths increased from 263.1 N (0) to 316.4 N(1, increased by 20.3%), 276.4 N (2, increased by 5.1%) and 276.4 N (3, increased by 4.7%) in the weft direction, (Fig. 2a). Additionally, the bursting strengths were improved considerably from 573.7 N (0) to 642.0 N (1, increased by 11.9%), 585.7 N (2, increased by 2.1%) and 587.9 N (3, increased by 2.1%), (Fig. 3b). While the tearing strength (Fig. 3c) and breaking elongation (Fig. 3d) of 1, 2 and 3 all had no obvious change compared with that of 0. Additionally, the bending rigidity (Fig. 3e), surface roughness and smoothness (Fig. 3f) of the cotton fabrics were well maintained after being finished. These results indicate that the finishing process had no obviously negative effect on the tearing strength, bending rigidity, surface roughness or stiffness of the cotton fabric due to the well-preserved fiber structure (Fig. 2e).
Comprehensive properties evaluation of (0) raw cotton fabric and the cotton fabrics finished with (1) IQAS, (2) AEM5700 and (3) SCJ877. a Breaking strength; b bursting strength; c tearing strength; d breaking elongation; e bending rigidity; f SMD and smoothness; g air permeability; h water vapor permeability; i hydrophilicity
The AIP and WVP of fabrics are critical issues for the thermophysiological comfort of the human body. The AIP was improved from 253 mm/s (0) to 285 mm/s (1, increased by 12.6%), 262 mm/s (2, increased by 3.6%) and 264 mm/s (3, increased by 4.3%) (Fig. 3 g) due to the enhanced hydrophilicity of 1 (Fig. 3i). Additionally, the WVPs of 1, 2 and 3 were well retained compared with that of 0 (Fig. 3 h). This fact may be because the long alkyl chains-containing antimicrobial compounds were bound onto the surfaces of the cotton fabrics in a method similar to ribbons fixed at only one end and no dense coating film that would reduce their air and water permeability could be formed, so 1 had good air and water permeability.
Capillary penetration was utilized to comparatively study the hydrophilicities of 0, 1, 2 and 3. The growth rates and final water wicking height of 1, 2 and 3 were all much greater than that of 0 (Fig. 3i). Especially, the water wicking height and growth rates of 1 was significantly greater than that of the other fabrics. These results indicate that the hydrophilicity of cotton fabrics may be remarkably improved by this antimicrobial finishing process due to the different driving forces of capillary penetration(Zhang et al. 2018).
The quantitative antimicrobial activities of 1, 2 and 3 against E. coli and S. aureus were estimated using the viable cell count method and were compared with that of 0. As shown in Fig. 4a, both E. coli and S. aureus could proliferate well on the surfaces of the unfinished cotton fabrics, while no viable bacteria could be observed on the surfaces of 1, 2 and 3, and the antimicrobial rates of 1, 2 and 3 were greater than 99.99% against both E. coli and S. aureus, indicating that 1, 2, 3 had excellent antimicrobial activities against both tested bacteria. The laundering durability of the antimicrobial activities of 1, 2 and 3 were further evaluated after 50 laundering cycles compared with 0 (Chen et al., 2016). The antimicrobial rats of 0 were only 19.0 and 23.0% against E. coli and S. aureus, respectively. While the antimicrobial rates were 92.2% (1), 77.6% (2) and 71.1% (3) for the gram-negative bacterium E. coli, and 98.5% (1), 94.5% (2) and 92.0% (3) for the gram-positive bacterium S. aureus after 50 laundering cycles (Table 1). The durable antimicrobial rates of 1 are much higher than those of 2, 3 and the reference antimicrobial rates of the AAA class antimicrobial fabrics, indicating that 1 maintained excellent antimicrobial activity even after long-term repeated launderings. Additionally, the antimicrobial activities of 1, 2 and 3 against S. aureus were all higher than those against E. coli, probably because QAS can induce generation of reactive oxygen species (ROS) (Jin et al. 2017), and the oxidative stress tolerance of S. aureus is lower than that of E. coli (Ghosh et al. 2012). Excess destructive ROS may trigger lipid peroxidation, leading to cell death (Chen et al. 2014), and the bacterial with a lower oxidative stress tolerance is easily damaged by the destructive ROS. A similar result was reported in a previous work (Ghosh et al. 2012).
Antimicrobial activities of the various cotton fabrics against (A) E. coli and (B) S. aureus determined using a the viable counting method and b the agar diffusion method. (0) raw cotton fabric, cotton fabrics finished with (1) IQAS, (2) AEM 5700, (3) SCJ877. Determined using a the viable cell count method and b the agar diffusion method
Meanwhile, the antimicrobial QAS leaching from 1, 2 and 3 after being washed once were evaluated by measuring the diameters of the inhibition zones (DIZs) of the E. coli and S. aureus. As shown in Tab. 1, the DIZs of 2 and 3 for S. aureus were both approximately 1 mm, while the DIZs of 1 for both bacteria were 0 mm (Fig. 4b, Table 1), indicating that no QAS was leached from 1. These results also confirm that the IQAS is a potential immobilized antimicrobial agent for cotton fabrics applications, and the antimicrobial activity of 1 can withstand repeated launderings
The covalent attachment of the QAS on cotton fabrics can provide perdurable antimicrobial activity against laundering (Table 1). However, the antimicrobial activity may decrease or even expire due to the adsorption of dirt, deadly microorganisms or complex formations of the antimicrobial cationic group and the anionic detergent during laundering, so the washing durability of antimicrobial fabrics cannot guarantee their durably antimicrobial function. To evaluate the durability of the fabrics’ antimicrobial functions, the antimicrobial activities of 1, 2 and 3 were evaluated by measuring the optical density at 600 nm (OD600) of the experimental medium with antimicrobial fabrics that were repeated put in contact with E. coli for predetermined times, as shown in Fig. 5a. Although the antimicrobial activities of all the finished fabrics were still greater than 99%, even after 5 times in contact with E. coli, the antimicrobial activities of 2 and 3 decreased obviously after contacting E. coli four times, while the antimicrobial activities of 1 did not obviously decrease even after contacting E.coli five times, indicating that the durability of the antimicrobial function of 1 is better than those of 2 and 3. The most possibly reasons for the difference between the durability of their antimicrobial functions are (1) some QAS was released from 2 and 3 because the couple interaction of siloxane with OH was to a certain degree reversible, while the force of the IQAS bound to cotton fabrics is much stronger than that of AEM5700 and SCJ877; (2) the hydrophilicity of 1 is much larger than that of 2 and 3 (Fig. 3i). The higher hydrophilicity would result in a higher repulsion force between the fabrics and bacteria because the hydrophilic surface can form a hydration layer that can reduce the adhesion of microbes (Jiang and Cao 2010; White and Jiang 2011; Zhang et al. 2013).
The antimicrobial kinetics of 1 was also evaluated and compared with 0 to investigate its bactericidal speed, as shown in Fig. 5b. Both live S. aureus and E. coli bacteria were rapidly reduced with increased contact times. More than 99.9% of the test bacteria were killed within 0.5 h of being in contact with 1 (Fig. 5b). No viable bacterial was observed when the contact time reached 2 h for S. aureus and 3 h for E. coli. 1 exhibited excellent antimicrobial activities.
Acute oral toxicity and skin irritation are two important requisite issues in the evaluation of the biosafety of the use of antimicrobial fabrics. 1 was applied directly to the back skin of rabbits to evaluate its skin irritation, and 0 was also applied directly to the back skin of the same rabbits on the other side to obtain its skin irritation as control. As shown in Fig. 6a, neither edema nor erythema was found after being in contact for 24, 48, and 72 h, and the hematoxylin and eosin (H&E) stained images showed no obvious histopathological abnormalities in all the tested regions covered with either 1 or 0 (Fig. 6b), and the primary irritation indexes of 1 and 0 are both negligible.
In vivo toxicity evaluation of the fabrics. The rabbit skin irritation results according to the ISO 10993.10-2010 standard. a The images are of the rabbits’ back that were in contact with the fabrics for 0, 1, 24, 48 and 72 h. b H & E staining images of the skin that was in contact with the samples for 72 h. Scale bar represents 50 μm. c Survival curves of mice after various treatments. Sample treated mice after oral administration showed 100% survival over 14 days. d Mice body weight gain percentages of mice after various treatments. The body weight of mice did not show a significant change within 14 days; e HE staining images of major organs collected from different groups of mice on day 14. (0) Cotton fabrics (control); (1) finished cotton with IQAS
Additionally, the acute oral toxicity test results indicate that neither death (Fig. 6c) nor asignificant body weight drop (Fig. 6d) was observed for all tested doses compared to 0. Moreover, no changes in the eating, drinking, exploratory behavior, or appearance of mice (e.g., hair glossiness and color) were noted during the test period. Compared with 0, the hematoxylin and eosin (H&E) stained images showed that no obvious histopathological abnormalities were observed in the liver, kidney, stomach, large intestine, and small intestine tissues of the mice treated with 1 at all tested doses, even after gavage administration for 14 days (Fig. 6e).
Conclusion
We have developed a simple and eco-friendly finishing technique to prepare durable antimicrobial cotton fabrics using IQAS chemically immobilized onto the surface of cotton fabrics. Cotton fabrics finished with IQAS have the following functions: (1) greatly improved hydrophilicity; (2) excellent bactericidal activities; (3) enhanced breaking strength, bursting strength, and air permeability; (4) unchanged tearing strength, breaking elongation, bending rigidity, water vapor permeability, surface roughness and smoothness; and (5) no acute oral toxicity and no skin irritation in vivo. Compared with the commercially available AEM 5700 and SCJ 877, IQAS can be stored in a high purity form for more than a year in dry environments to permit convenient transportation and storage. Additionally, the durable antimicrobial activity of cotton fabrics finished with IQAS was better than those of cotton fabrics finished with AEM5700 and SCJ877. Therefore, IQAS and our finishing technique have great potential for applications in antimicrobial fabrics, including but not limited to uses in hospitals, hotels and other susceptible situations.
References
Amrit URB, Hendrix R, Dutschk V, Warmoeskerken M (2013) Time survivor study of Escherichia coli with polyhexamethylene biguanide on cotton. Text Res J 83(16):1663–1672
Bacciarelli-Ulacha A, Rybicki E, Matyjas-Zgondek E, Pawlaczyk A, Szynkowska MI (2014) A new method of finishing of cotton fabric by in situ synthesis of silver nanoparticles. Ind Eng Chem Res 53(11):4147–4155
Beyth N, Houri-Haddad Y, Baraness-Hadar L, Yudovin-Farber I, Domb AJ, Weiss EI (2008) Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials 29(31):4157–4163
Brochier Salon M-C, Abdelmouleh M, Boufi S, Belgacem MN, Gandini A (2005) Silane adsorption onto cellulose fibers: hydrolysis and condensation reactions. J Colloid Interface Sci 289(1):249–261
Chen SG, Chen SJ, Jiang S, Xiong ML, Luo JX, Tang JN, Ge ZC (2011) Environmentally friendly antibacterial cotton textiles finished with siloxane sulfopropylbetaine. ACS Appl Mater Interfaces 3(4):1154–1162
Chen SG, Guo YJ, Zhong HQ, Chen SJ, Li JN, Ge ZC, Tang JN (2014) Synergistic antibacterial mechanism and coating application of copper/titanium dioxide nanoparticles. Chem Eng J 256:238–246
Chen SG, Yuan LJ, Li QQ, Li JN, Zhu XL, Jiang YG, Sha O, Yang XH, Xin JH, Wang JX, Stadler FJ, Huang P (2016) Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Small 12(26):3516–3521
Chen S, Chen Q, Li Q, An J, Sun P, Ma J, Gao H (2018) Biodegradable synthetic antimicrobial with aggregation-induced emissive luminogens for temporal antibacterial activity and facile bacteria detection. Chem Mater 30(5):1782–1790
Dong A, Wang YJ, Gao Y, Gao T, Gao G (2017) Chemical insights into antibacterial N-halamines. Chem Rev 117(6):4806–4862
Gao Y, Cranston R (2008) Recent advances in antimicrobial treatments of textiles. Text Res J 78(1):60–72
Gao D, Chen C, Ma J, Duan X, Zhang J (2014) Preparation, characterization and antibacterial functionalization of cotton fabric using dimethyl dialyl ammonium chloride-allyl glycidyl ether-methacrylic/nano-ZnO composite. Chem Eng J 258:85–92
Gao D, Duan X, Chen C, Lyu B, Ma JZ (2015) Synthesis of polymer quaternary ammonium salt containing epoxy group/nano ZnO long-acting antimicrobial coating for cotton fabrics. Ind Eng Chem Res 54(43):10560–10567
Ghosh S, Goudar VS, Padmalekha KG, Bhat SV, Indi SS, Vasan HN (2012) ZnO/Ag nanohybrid: synthesis, characterization, synergistic antibacterial activity and its mechanism. RSC Adv 2(3):930–940
Giesz P, Celichowski G, Puchowicz D, Kamińska I, Grobelny J, Batory D, Cieślak M (2016) Microwave-assisted TiO2: anatase formation on cotton and viscose fabric surfaces. Cellulose 23(3):2143–2159
He L, Gao C, Li S, Chung CTW, Xin JH (2017) Non-leaching and durable antibacterial textiles finished with reactive zwitterionic sulfobetaine. J Ind Eng Chem 46:373–378
Ibrahim HMM, Hassan MS (2016) Characterization and antimicrobial properties of cotton fabric loaded with green synthesized silver nanoparticles. Carbohyd Polym 151:841–850
Jiang S, Cao Z (2010) Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater 22(9):920–932
Jin Y, Pei H, Hu W, Zhu Y, Xu H, Ma C, Sun J, Li H (2017) A promising application of chitosan quaternary ammonium salt to removal of Microcystis aeruginosa cells from drinking water. Sci Total Environ 583:496–504
Kang CK, Kim SS, Kim S, Lee J, Lee JH, Roh C, Lee J (2016) Antibacterial cotton fibers treated with silver nanoparticles and quaternary ammonium salts. Carbohyd Polym 151:1012–1018
Li P, Poon YF, Li W, Zhu HY, Yeap SH, Cao Y, Qi X, Zhou C, Lamrani M, Beuerman RW, Kang ET, Mu Y, Li CM, Chang MW, Leong SSJ, Chan-Park MB (2011) A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater 10(2):149–156
Liu Y, Li J, Cheng X, Ren X, Huang TS (2015) Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J Mater Chem B 3(7):1446–1454
Liu Q, Huang J, Zhang J, Hong Y, Wan Y, Wang Q, Gong M, Wu Z, Guo CF (2018) Thermal, waterproof, breathable, and antibacterial cloth with a nanoporous structure. ACS Appl Mater Interfaces 10(2):2026–2032
Patil AJ, Zhao Y, Liu X, Wang X (2018) Durable superhydrophobic and antimicrobial cotton fabrics prepared by electrostatic assembly of polyhexamethylene biguanide and subsequent hydrophobization. Text Res J 88(15):1788–1799
Sadeghi-Kiakhani M, Tehrani-Bagha AR, Safapour S (2018) Enhanced anti-microbial, anti-creasing and dye absorption properties of cotton fabric treated with chitosan-cyanuric chloride hybrid. Cellulose 25(1):883–893
Simoncic B, Tomsic B (2010) Structures of novel antimicrobial agents for textiles - A review. Text Res J 80(16):1721–1737
Tian H, Zhai Y, Xu C, Liang J (2017) Durable antibacterial cotton fabrics containing stable acyclic N-halamine groups. Ind Eng Chem Res 56(28):7902–7909
Wang C, Lv J, Ren Y, Zhou Q, Chen J, Zhi T, Lu Z, Gao D, Ma Z, Jin L (2016) Cotton fabric with plasma pretreatment and ZnO/carboxymethyl chitosan composite finishing for durable UV resistance and antibacterial property. Carbohyd Polym 138:106–113
White A, Jiang S (2011) Local and bulk hydration of zwitterionic glycine and its analogues through molecular simulations. J Phys Chem B 115(4):660–667
Xu QB, Ke XT, Cai DR, Zhang YY, Fu FY, Endo T, Liu XD (2018) Silver-based, single-sided antibacterial cotton fabrics with improved durability via an l-cysteine binding effect. Cellulose 25(3):2129–2141
Yin XZ, Weng PX, Han L, Liu JC, Tan YQ, Chen DZ, Zhou YS, Li S, Wang LX, Wang H (2018) Enhanced wettability and moisture retention of cotton fabrics coated with self-suspended chitosan derivative. Cellulose 25(4):2721–2732
Yu M, Wang Z, Lv M, Hao R, Zhao R, Qi L, Liu S, Yu C, Zhang B, Fan C, Li J (2016) Antisuperbug cotton fabric with excellent laundering durability. ACS Appl Mater Interfaces 8(31):19866–19871
Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner BD, Jiang S (2013) Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol 31(6):553–556
Zhang SB, Yang XH, Tang B, Yuan LJ, Wang K, Liu XY, Zhu XL, Li JN, Ge ZC, Chen SG (2018) New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem Eng J 336:123–132
Zhi Z, Su Y, Xi Y, Tian L, Xu M, Wang Q, Padidan S, Li P, Huang W (2017) Dual-functional polyethylene glycol-b-polyhexanide surface coating with in vitro and in vivo antimicrobial and antifouling activities. ACS Appl Mater Interfaces 9(12):10383–10397
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51773117, 51573097), the Science and Technology Project of Guangdong Province (2015A010105033), the Collaborative Innovation and Technology Project for Shenzhen-Hong Kong Innovation Circle of Shenzhen city (SGLH 20120926161415782), and the Nanshan District Key lab for Biopolymers and safety evaluation (KC2014ZDZJ0001A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gu, J., Yuan, L., Zhang, Z. et al. Non-leaching bactericidal cotton fabrics with well-preserved physical properties, no skin irritation and no toxicity. Cellulose 25, 5415–5426 (2018). https://doi.org/10.1007/s10570-018-1943-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1943-8