Abstract
Carboxymethyl ethers of xylan, cellulose, and pullulan could be efficiently modified applying the Ugi-reaction in the “green” solvent water. Using different components, namely an aldehyde, an amine, and an isonitrile, the reaction led to novel polysaccharide derivatives with peptide-like substituents. The reactions were carried out applying 2-methoxyethylamine and propargyl amine, paraformaldehyde and benzaldehyde as well as tert-butylisonitrile at room temperature. The characterization of the products was performed by 13C-NMR spectroscopy. In any case, a high conversion of the carboxyl groups could be realized. The products isolated possessed a lower degree of substitution compared to the starting material, which may result from a hydrolytic cleavage of ether bonds. Nevertheless, the results indicated that carboxymethylated polysaccharides are useful starting materials for new polysaccharide derivatives in a toolbox reaction.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polysaccharides like cellulose, starch, and xylan are formed in huge amounts biosynthetically by many organisms including plant, animals, fungi, algae, and microorganism. They are renewable resources with a vast structural diversity (Heinze et al. 2006b). Chemical modifications of polysaccharides using conventional reaction schemes like esterification with organic acids (Heinze et al. 2006b; Hornig et al. 2009; Schulze et al. 2016), sulfation (Daus et al. 2011; Gericke et al. 2009; Herrero et al. 2015; Wu et al. 2017), and sulfoalkylation (Demleitner et al. 1992; Ebringerová and Pastýr 1988; Vieira et al. 2005; Zhang et al. 2011), e.g., lead to a broad variety of products with promising properties useful for many applications. Two-step reaction paths are also a common path to obtain polysaccharide-based products with promising properties. In this context, cellulose tosylates are well-investigated (Heinze et al. 2006a; Heinze and Rahn 1997; Rahn et al. 1996) useful for nucleophilic displacement reaction with amines, halides, pseudo-halides, or others to synthesize novel 6-deoxy-6-functionalized polysaccharide derivatives (Koschella and Heinze 2001; Zieger et al. 2015). Another class of reactive polysaccharide derivative are phenyl carbonates (Elschner et al. 2013a, b) that can be easily transformed to stabile polysaccharide carbamates by reaction with a broad variety of amines (Elschner and Heinze 2015).
Carboxymethylated products of cellulose, starch, and many other polysaccharides can be easily obtained under heterogeneous reaction conditions efficiently yielding one of the most important ionic polysaccharide ether, which possess a myriad of applications (Heinze 1998; Heinze and Pfeiffer 1999; Heinze et al. 1999; Konduri and Fatehi 2016; Petzold et al. 2006a, b; Tiitu et al. 2006). Although the carboxyl groups introduced are reactive, there is a limited number of chemical modifications studied up to now. Simple methyl esters of carboxymethyl cellulose (CMC) were synthesized by conversion with dimethyl sulfate in dimethylsulfoxide (Tezuka et al. 1996). Furthermore, ethyl ester of carboxymethyl (CM) polysaccharides were prepared with ethanol using an autocatalytic process (Sibikina et al. 2004b). Using additionally amines in this autocatalytic process, the corresponding amides are generated, however, with low conversion of 7–50% (Sibikina et al. 2004a, b). It may be assumed that the chemical modification of CM-polysaccharides is limited because these biopolymer derivatives are soluble in water only and do not swell significantly in organic solvents.
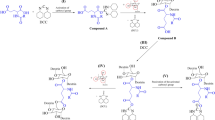
In organic chemistry of low-molecular compounds, isonitrile-based multi-step reactions are an easy and efficient path to modify carboxylic acid groups (Dömling 2006; Dömling and Ugi 2000). In this regard, the Ugi-reaction is of particular interest because diverse products can be obtained in a single reaction step (Brauch et al. 2010; Rivera et al. 2012; Ziyaei Halimehjani and Sharifi 2017). During the conversion of carboxyl group by Ugi-reaction, a carbonyl group containing compound, a primary amine and an isonitrile are included in the reaction.
In a first step, the amine and the carbonyl group form the corresponding imine, which is protonated by the carboxylic acid. The isonitrile and the carboxylic acid attack the protonated imine and form an intermediate, which undergo a Mumm-rearrangement to from a diamide derivative (Brauch et al. 2010). The mechanism of the Ugi-reaction is given in supporting information (SI Fig. 1).
In the field of polysaccharides, the Ugi-reaction was studied to form ionic hydrogels from CMC, hyaluronic acid, alginic acid, oxidized scleroglucan and pectin using glutaraldehyde, 1,5-diaminopentane or 1,4-bis(3-isocyanopropyl)piperazine, i.e. cross-linking of the polysaccharides derivatives were carried out (Bu et al. 2005; de Nooy et al. 1999; de Nooy et al. 2000; Mironov et al. 2013; Shulepov et al. 2016; Werner et al. 2006). Moreover, Ugi-reaction was also applied to generate a covalent bond between polysaccharides and proteins. By deprotonation of cellulose, dextran and cross-linked agarose with tert-butoxide in dimethylsulfoxide (DMSO) an isonitrile containing substituent was introduced, which afterwards was used to generate linkages between polysaccharides and trypsin or Gly–LeuNH2 (Freeman et al. 1979). The amino group of trypsin linked to CMC or alginate by an Ugi-reaction in water (García et al. 2009). Binding horseradish peroxidase via Ugi-reaction to modified sodium alginate yielded in a biosensor which showed response towards H2O2 (Camacho et al. 2007).
Up to now, there are no investigations of chemical modification of CM-polysaccharides using the Ugi-reaction to synthesize soluble biopolymer derivatives, which could offer a wide field of applications. The aim of this studies was to use common and commercially available carboxymethyl polysaccharides as “platform” to generate new soluble biopolymer derivatives by Ugi-multicomponent reaction and to analyse the structure of the products in detail.
Experimental
Materials
The solvents and reagents were obtained from Sigma-Aldrich Chemie GmbH and were used without further purification. Xylan (Mw 9200 g/mol) from beech wood was provided by Lenzing AG (Lenzing, Austria). Pullulan (Mw 261,000 g/mol) was obtained from TCI Deutschland GmbH (Eschborn, Germany). Carboxymethyl cellulose (Mw 90,000 g/mol) was purchased from Sigma-Aldrich Chemie GmbH. Before use, xylan and pullulan were dried in vacuum at 80 °C for 2 h.
Measurements
NMR spectra of polysaccharide derivatives were recorded at 25 °C in deuterium oxide or DMSO-d6 (60 mg/ml) with a Brucker Avance 250 MHz spectrometer (1H NMR: 16 scans, 13C NMR: > 10,000 scans). A VARIO EL III CHNS analyzer (Elementaranalysensysteme GmbH) was used for elemental analyses. Size exclusion chromatography (SEC) in DMSO with 0.5 wt% LiBr as solvent (65 °C, flow rate: 0.5 ml/min) was performed on a JASCO system (isocratic pump PU-980, RI-930 refractive index detector) with a Novema 3000 and a Novema 300 column in series. FT-IR spectra were recorded on a Nicolet iS5 spectrometer using translucent KBr tablets containing the solid polysaccharide samples. SEC in water with 0.1 M NaNO3/NaN3 as solvent (30 °C, flow rate: 1.0 ml/min) was performed on a JASCO system (isocratic pump PU-980, RI-2031 Plus refractive index detector) with a PSS SUPREMA guard/1000/30 column. For both SEC systems pullulan was used as calibration standard.
Carboxymethylation of xylan and pullulan (carboxymethyl xylan CMX, typical example)
To a suspension of 30 g (227.3 mmol) xylan in 900 ml isopropanol, 121.2 g (454.5 mmol) of an aqueous solution of NaOH (15%) were added slowly at 25 °C. The reaction mixture was stirred for 1 h at 25 °C and afterwards 26.5 g (227.3 mmol) of sodium monochloroacetate (SMCA) were added. After stirring of the reaction mixture for 5 h at 55 °C, the reaction mixture was filtrated and the solid residue was suspended in 1 L aqueous methanol (80%). After neutralization with dilute acetic acid, the suspension was filtrated and the residue was washed three-times with 1 L of aqueous methanol (80%) and once with 1 L ethanol. The product was dried in vacuum at 40 °C. The degree of substitution was determined by 1H-NMR measurement after hydrolysis of 100 mg polymer in 1.5 ml 25% D2SO4 (in D2O) for 1 h.
Yield: 36.6 g (178.1 mmol modified AXU) CMX (78% of the theoretical yield); DS = 0.93.
Elemental analysis found (calculated): C%: 40.05 (40.1), H%: 5.1 (3.86).
13C-NMR (101 MHz; D2O; 298 K): δ [ppm] = 181.71, 181.23, 180.55 (C=O), 104.48 (C1), 84,90, 82.99, 79.02, 76.56, 75.67 (C2–C4), 73.87 (CH2COONa), 65.61 (C5).
Ugi-reaction with CM-polysaccharides (2a, typical example)
For preparation of Ugi-derivatives, 0.5 g (2.42 mmol) of CMX were dissolved in 25 ml water. The pH value of the solution was adjusted to 1 with conc. hydrochloric acid. 0.203 g (6.75 mmol) paraformaldehyde and 0.51 g (6.75 mmol) 2-methoxyethylamine were added. The pH value of the reactions mixture is increased sa result of the addition of the amine. A pH value above 5 should be adjusted. The reaction mixture was stirred for 30 min at room temperature. After addition of 0.56 g (6.75 mmol) tert-butyl isonitrile, the mixture was allowed to react for 20 h at room temperature under stirring. The mixture was poured into 200 ml acetone. The precipitate formed was removed by filtration and washed three times with 100 ml acetone. The crude product was dissolved in 20 ml water, dialyzed against water, and finally freeze dried. The DS was determined by elemental analysis applying the following equation.
Product 2a: DS = 0.68, Yield: 0.30 g (1.05 mmol modified AXU) (43% of the theoretical yield).
Elemental analysis found (calculated): C%: 50.50 (52.37), H%: 7.52 (7.20), N%: 6.62 (6.66).
13C-NMR (101 MHz; D2O; 298 K): δ [ppm] = 175.49 (C12), 172.16, 171.25 (C7), 104.49 (C1), 84,55, 79.16, 76.51, 75.59 (C2–C4), 72.38 (C9), 65.75 (C5), 61.41, 61.04 (C10), 54.42, 54.23 (C6), 23.53, 23.06 (C13), 50.63 (C11), 49.92 (C15), 30.72 (C14).
Results and discussion
Carboxymethylation of polysaccharides
As illustrated in Fig. 1, the carboxymethylation of the polysaccharides were carried out heterogeneously with sodium monochloroacetate (SMCA) in an alkaline medium using aqueous sodium hydroxide (15%) and isopropanol as slurry medium, i.e. in the conventional synthesis path (Heinze 1998; Petzold et al. 2006b). As known, the sodium hydroxide activated the hydroxyl groups of the polysaccharide to increase their nucleophilicity. The activated hydroxyl group attacked the SMCA by a SN2 reaction to form the corresponding CM-polysaccharide derivative (Konduri and Fatehi 2016; Petzold et al. 2006b). While the CMC was commercial available, the carboxmethyl xylan (CMX) and the carboxymethyl pullulan (CMP) had to synthesized. The CMC possessed a DS of carboxymethyl groups (DSCM) of 0.96. Xylan converted with a equimolecular amount of SMCA and a twofold molar excess of aqueous NaOH gave a CMX with a DSCM of 0.93. In the case of pullulan, a twofold molar excess of SMCA and aqueous NaOH was used. The CMP received possessed a DSCM of 1.17.
13C-NMR spectra of CMX and CMP clearly showed the signal of the carbonyl carbon of the carboxymethyl group at typical chemical shift of 181.2 ppm (Fig. 2). The methylene group of the CM-substituent was observed at 73.9 ppm. The signal belonging to position 1 of the CMP appeared as a broad peak at a chemical shift 100 ppm (Fig. 2a). For CMP, the anhydroglucose unit (AGU) can be assigned to signals between 83 and 73 ppm. The peaks at 68.4 and 63.8 ppm were assigned to the different positions 6 of the pullulan backbone. In case of CMX, the 13C-NMR signal related to position 1 appeared at 104.5 ppm. The further signals assigned to the anhydroxylose unit (AXU) were found in the range of 86 to 74 ppm. Position 5 of the CMX was related to the signal at 65.7 ppm (Fig. 2b).
Ugi-reaction of carboxymethyl polysaccharides
To investigate appropriate conditions of the Ugi-reaction of CM-polysaccharides, tert-butylisonitrile, paraformaldehyde, and 2-methoxyethylamin were used. Due to the lower degree of polymerization, CMX was chosen for the experiments to get a higher resolution of the NMR spectra of the corresponding products. According to the reaction mechanism, a proton is needed for the Ugi-reaction. Therefore, CH2COONa was converted into the acid form (CH2COOH) by addition of hydrochloric acid (Fig. 3).
To get a complete conversion of carboxymethyl moieties, the influence of excess of the amine, aldehyde, and isonitrile was studied and the pH value of medium were changed. The following pH values were only the values after acidifying the CM-polysaccharide solutions. Through the excess of the amine the Ugi reaction itself was performed at pH values from 5 to 7. Otherwise a hydrolyses of the isonitrile is possible. In the first set of experiments, a molar excess of 1.2 mol of the amine, aldehyde, and isonitrile to carboxylic group at a starting pH value of 3.5 were applied. The product obtained showed typical but small signals of the corresponding Ugi-product in the 13C-NMR spectrum. At a starting pH of 1 and a molar excess of 1.2 mol per mol acid group, no reaction occurred. In Fig. 4, the 13C-NMR spectra of the products obtained using a molar excess of 3 mol amine, aldehyde, and isonitrile per mol acid group are shown. At a starting pH of 3.5, the signals of the Ugi-product were clearly visible, but the reaction was incomplete (Fig. 4b). Based on this result, the starting pH value was decreased to 1. The 13C-NMR spectrum of the product obtained showed a complete conversion of the carboxy groups (Fig. 4c). Both signals of the carboxymethyl moiety at 181.2 and 73.9 ppm disappeared, on the one hand. On the other hand, two new signals occurred at 175.5 and 172.2 ppm, which can be assigned to the carbonyl atoms of the two amide moieties of the structure formed by the Ugi-reaction. In addition to the signals of the AGX between 84.6 and 75.6 ppm, peak at 72.4 ppm occurred, which can be assigned to the position 8 of the new substituent. At a chemical shift of 65.8 ppm, the signal of position 5 appeared. The methyl group at position 10 yield a peak at 61.2 ppm in the 13C-NMR spectrum. The methylene groups of the carboxymethyl groups was shifted to 53.1 ppm. The small signal at 52.1 ppm can be assigned to the quaternary C-atom of the tert-butyl group (position 13). At chemical shifts of 49.9 and 50.6 ppm, two signals appeared that could be assigned to position 9 and 11. The methyl groups of the tert-butyl group (positon 14) led to a signal at 30.7 ppm.
13C-NMR spectra of starting carboxymethyl xylan (CMX) 1a with a degree of substitution (DSCM) of 0.93 (a) and Ugi-products obtained by conversion of 1a with a molar excess of 3 mol 2-methyoxyethylamine, paraformaldehyde, and tert-butyl isonitrile per mol AXU at a starting pH value of 3.5 (b) and at a starting pH value of 1 (c, DSUgi 0.68), recorded in D2O at 25 °C
Elemental analysis of product 2a indicated a DSUgi of 0.68, which revealed a transformation of 73% of the CM moieties. Nevertheless, there were no remaining carboxyl groups in the sample, which indicated a complete formation of the novel polymeric structure based on CMX. Compared to the starting CMX, lower DS is explained by some splitting of ether moieties under the acidic conditions of the reaction.
In addition to the reaction of the CM-polysaccharides discussed above, the Ugi-reaction was studied with CMC and CMP applying same reagents, namely 2-methoxyethylamine, paraformaldehyde, and tert-butylisonitrile applying the aqueous reaction system with a pH value of 1 and an excess of the amine, aldehyde, and isonitrile of 3 mol per mol AGU (Table 1). The Ugi-product 2b obtained with CMC (DSCM 0.96) possesses a DSUgi of 0.57. Starting with CMP (DSCM 1.17), a Ugi-product 2c could be synthesized with a DSUgi of 0.92.
Figure 5 shows the 13C-NMR spectra of the CM-cellulose based product 2b and the CM-pullulan based product 2c. The spectra clearly indicated the successful conversion due to the fact that in both cases no signals of the carboxymethyl moiety were visible, on one hand. On the other hand, carbonyl groups of the Ugi-product could be assigned to signals at 174.9 ppm and 171.7 ppm. The further signals of the new substituent appeared, comparable to the xylan product 2a, at 72.4, 61.3, 54.2, 53.4, 50.8, 49.9, and 30.7 ppm.
The Ugi-reaction of CM polysaccharides was additionally studied with further amines and aldehydes. CMX was converted with benzaldehyde and propargylamine. The corresponding xylan derivative 3a isolated possess a DSUgi of 0.83. 3a showed two typical signals in the 13C-NMR spectrum at 174.2 and 170.9 ppm that were assigned to the two carbonyl groups 12 and 7 (Fig. 6a). Furthermore, the quaternary C-atom of the phenyl moiety was assigned to the signal at 138.2 ppm. The C-atoms of phenyl groups yield signals with a chemical shift of 131.2 ppm. In addition to the signals of the AXU, the quaternary C-atom (9) of the propargyl moiety had a chemical shift of 82.1 ppm. The methylene C-atom of the propargyl moiety resulted in a signal at 36.1 ppm. Signals at 61.8 ppm and 31.2 ppm resulted from the tert-butyl group. The methylene groups of the carboxymethyl moiety appeared at a chemical shift of 53.1 ppm. Using propargylamine, acetaldehyde, and tert-butylisonitrile, the CMC (DSCM 0.96) was yielded cellulose derivative 4b with a DSUgi of 0.26. 13C-NMR spectroscopy proofed the successful conversion (Fig. 6b). Both carbonyl carbons were related to signals at 180.1 and 174.4 ppm. The signals of the triple bond vanished under the signals of the AGU in the range from 70.2 to 86.4 ppm. At a chemical shift of 64.5 ppm, a signal appeared that was assigned to chiral carbon 12 of the substituent. The quaternary C-atom of the tert-butyl moiety could be assigned to a signal at 57.7 ppm. In this case, the methylene groups of the carboxymethyl moiety appeared at a chemical shift of 53.1 ppm as well. The methylene C-atom of the propargyl moiety was assigned to a signal at 36.1 ppm, too. Furthermore, the signal of the methyl groups of the acetaldehyde moiety was assigned to a signal at 17.5 ppm. The double peaks observed in all 13C-NMR spectra of Ugi-products could be explained by the tautomeric character of the amide bond (Hammaker and Gugler 1965). The infrared (IR)-spectroscopy of the products obtained showed a clear shift of the carbonyl signal of the carboxylate from 1600 to 1670 cm−1 of the amide group (SI Fig 2-4). Furthermore, the signals of the ether bond between 1040 and 1100 cm1 were present, which proof that the substituents were linked to the polymer backbone. This finding indicated additionally the success of the chemical modification.
Considering the problems of SEC of polyelectrolytes like CM-polysaccharides, i.e., the determination of a too high molar mass, some conclusions regarding acid hydrolysis of the polysaccharide chain can be made. Nevertheless, the values of the degree of polymerization (DP) of the Ugi-products (Table 1) indicates that polymer degradation occurs under reaction conditions used.
Usually, the Ugi-products synthesized possessed a lower DS value compared to the starting carboxymethylated polysaccharide. That result could be explained by an assisted acid cleavage of the ether bond at the backbone of the polysaccharide. An assisting effect of a neighbouring amide moieties may be assumed, which facilitated a hydrolysis of an ether bond as discussed in the literature (Arcelli et al. 2001, 2004; Calvaresi et al. 2008). The assisted cleavage of ether bonds is described to occur by a formation of a five-member ring after protonation of the oxygen atom of the ether bond. In case of the Ugi-products, no five-member ring could be formed, but in a similar reaction mechanism a six membered ring may be postulated. Due to the fact that the assisted mechanism could not be exactly applied, a partial cleavage of ether bonds took place only. The results led to the conclusion that the pullulan derivative 2c was the most stable regarding the cleavage of ether bonds. Xylan derivative 2a possessed a slightly higher extent of hydrolysis. Whereas the cellulose derivative 2b lost most of the ether substituents. Especially cellulose derivative 4b underwent the highest extent of hydrolysis of ether bonds. These results indicated that the stability of the resulting ether bonds depends on the structure of the polysaccharide, which is well known, and on the substituent. It may be assumed that the different structure (conformation) of CM-polysaccharides influences the accessibility of the ether bonds that will be investigated in further studies. To investigate the ether cleavage, the conversion of CMC to product 4b was done again. The DS of the product of first experiment was 0.26 and those of the second reaction was 0.24. Consequently, the ether cleavage occurred with a comparable extent and the Ugi-reaction yield to reproducible results.
The polysaccharide derivatives synthesized by conversion of the carboxymethyl groups of CM polysaccharides with paraformaldehyde, 2-methoxyethylamine, and tert-butylisonitrile (samples 2a-c) were soluble in water and organic solvents including dimethylsulfoxide, N,N-dimethylacetamide, and N,N-dimethylformamide. Due to the hydrophobic phenyl group in the substituent, xylan derivative 3a possessed solubility in the organic solvents only. Whereas the cellulose derivative 4b exhibited solubility in water only due to the low DS value of 0.26.
Conclusion
The Ugi-multicomponent reaction is very useful to convert carboxymethyl moieties of CM-polysaccharides selectively with aldehydes, amins, and isonitriles in water. During the conversion, a cleavage of ether bonds may occur yielding a decrease in the number of CM-substituents was observed. However, the studies exhibit a new path to generate substituents with a high structural diversity starting from well-established CM-polysaccharides. Whereby the Ugi-reaction offers the possibility to use CM-polysaccharides as synthetic platform in polysaccharide chemistry. Using the Ugi-reaction, the preparation of a broad variety of polysaccharide derivatives can be expected because diverse reactive compounds can be applied. In principle, it is possible to introduce up to three reactive and orthogonal moieties in a single substituent, because every component, i.e., the isonitrile, the amine, and the aldehyde, could include a reactive moiety for further reactions. Such reactions are under investigation. Beside the CM-polysaccharides, further polysaccharides containing carboxyl groups, like alginates and pectin, seem to be suitable starting materials for the Ugi-reaction to generate soluble derivatives. This structural diversity could offer new avenues in the field of conjugation of one or more biomolecules as well as simultaneously binding of dyes to the polysaccharide backbone. In this regard, the studies about the Ugi-reaction with oligo- and polysaccharides will be expanded.
References
Arcelli A, Porzi G, Rinaldi S, Sandri S (2001) An efficient acid hydrolysis of the ether bond assisted by the neighbouring benzamide group. Part 3. J Chem Soc Perkin Trans 2:296–301. https://doi.org/10.1039/B008748N
Arcelli A, Porzi G, Rinaldi S, Sandri S (2004) Effect of neighbouring amide group bulkiness on anchimerically assisted ether bond cleavage: Part 7. J Phys Org Chem 17:289–293. https://doi.org/10.1002/poc.726
Brauch S, Gabriel L, Westermann B (2010) Seven-component reactions by sequential chemoselective Ugi–Mumm/Ugi–Smiles reactions. Chem Commun 46:3387–3389. https://doi.org/10.1039/b927388c
Bu H, Kjøniksen A-L, Nyström B (2005) Effects of pH on dynamics and rheology during association and gelation via the Ugi reaction of aqueous alginate. Eur Polym J 41:1708–1717. https://doi.org/10.1016/j.eurpolymj.2005.02.035
Calvaresi M, Rinaldi S, Arcelli A, Garavelli M (2008) Computational DFT investigation of vicinal amide group anchimeric assistance in ether cleavage. J Org Chem 73:2066–2073. https://doi.org/10.1021/jo701394z
Camacho C, Matías JC, García D, Simpson BK, Villalonga R (2007) Amperometric enzyme biosensor for hydrogen peroxide via Ugi multicomponent reaction. Electrochem Commun 9:1655–1660. https://doi.org/10.1016/j.elecom.2007.03.013
Daus S, Petzold-Welcke K, Kötteritzsch M, Baumgaertel A, Schubert US, Heinze T (2011) Homogeneous sulfation of xylan from different sources. Macromol Mater Eng 296:551–561. https://doi.org/10.1002/mame.201000390
de Nooy AE, Masci G, Crescenzi V (1999) Versatile synthesis of polysaccharide hydrogels using the Passerini and Ugi multicomponent condensations. Macromolecules 32:1318–1320. https://doi.org/10.1021/ma9815455
de Nooy AEJ, Capitani D, Masci G, Crescenzi V (2000) Ionic polysaccharide hydrogels via the Passerini and Ugi multicomponent condensations: synthesis, behavior and solid-state NMR characterization. Biomacromolecules 1:259–267. https://doi.org/10.1021/bm005517h
Demleitner S, Kraus J, Franz G (1992) Synthesis and antitumour activity of sulfoalkyl derivates of curdlan and lichenan. Carbohydr Res 226:247–252. https://doi.org/10.1016/0008-6215(92)84072-Z
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. https://doi.org/10.1021/cr0505728
Dömling A, Ugi I (2000) Multicomponent Reactions with Isocyanides. Angew Chem Int Ed 39:3168–3210. https://doi.org/10.1002/1521-3773(20000915)39:18<3168:aid-anie3168>3.0.co;2-u
Ebringerová A, Pastýr J (1988) Sulfoethylierung von D-Xylanen in heterogener Phase. Chem Pap 3:407–414
Elschner T, Heinze T (2015) Cellulose carbonates: A platform for promising biopolymer derivatives with multifunctional capabilities. Macromol Biosci 15:735–746
Elschner T, Ganske K, Heinze T (2013a) Synthesis and aminolysis of polysaccharide carbonates. Cellulose 20:339–353. https://doi.org/10.1007/s10570-012-9819-9
Elschner T, Wondraczek H, Heinze T (2013b) Syntheses and detailed structure characterization of dextran carbonates. Carbohydr Polym 93:216–223
Freeman A, Sokolovsky M, Goldstein L (1979) Isonitrile derivatives of polysaccharides as supports for the covalent fixation of proteins and other ligands. Biochim Biophys Acta Enzymol 571:127–136. https://doi.org/10.1016/0005-2744(79)90233-x
García A, Hernández K, Chico B, García D, Villalonga ML, Villalonga R (2009) Preparation of thermostable trypsin–polysaccharide neoglycoenzymes through Ugi multicomponent reaction. J Mol Catal B Enzym 59:126–130. https://doi.org/10.1016/j.molcatb.2009.02.001
Gericke M, Liebert T, Heinze T (2009) Interaction of ionic liquids with polysaccharides, 8—synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol Biosci 9:343–353. https://doi.org/10.1002/mabi.200800329
Hammaker RM, Gugler BA (1965) An NMR study of hindered internal rotation in N,N-dialkyl amides. J Mol Spectrosc 17:356–364. https://doi.org/10.1016/0022-2852(65)90173-6
Heinze T (1998) Ionische Funktionspolymere aus Cellulose: Neue Synthesekonzepte, Strukturaufklärung und Eigenschaften. Shaker Verlag, Aachen
Heinze T, Pfeiffer K (1999) Studies on the synthesis and characterisation of carboxymethylcellulose. Angew Macromol Chem 266:37–45
Heinze T, Rahn K (1997) Cellulose-p-toluenesulfonates: a valuable intermediate in cellulose chemistry. In: Macromolecular symposia, vol 1. Wiley Online Library, pp 103–113. https://doi.org/10.1002/masy.19971200112
Heinze U, Heinze T, Klemm D (1999) Synthesis and structure characterization of 2,3-O-carboxymethylcellulose. Macrmol Chem Phys 200:896–902
Heinze T, Koschella A, Brackhagen M, Engelhardt J, Nachtkamp K (2006a) Studies on non-natural deoxyammonium cellulose. In: Macromolecular symposia, vol 1. Wiley Online Library, pp 74–82. https://doi.org/10.1002/masy.200651206
Heinze T, Liebert T, Koschella A (2006b) Esterification of polysaccharides. Springer, Heidelberg. https://doi.org/10.1007/b98412
Herrero LJ, Foo S-S, Sheng K-C, Chen W, Forwood MR, Bucala R, Mahalingam S (2015) Pentosan polysulfate: A novel glycosaminoglycan-like molecule for effective treatment of alphavirus-induced cartilage destruction and inflammatory disease. J Virol 89:8063–8076. https://doi.org/10.1128/JVI.00224-15
Hornig S, Bunjes H, Heinze T (2009) Preparation and characterization of nanoparticles based on dextran–drug conjugates. J Colloid Interface Sci 338:56–62. https://doi.org/10.1016/j.jcis.2009.05.025
Konduri MK, Fatehi P (2016) Synthesis and characterization of carboxymethylated xylan and its application as a dispersant. Carbohydr Polym 146:26–35. https://doi.org/10.1016/j.carbpol.2016.03.036
Koschella A, Heinze T (2001) Novel regioselectively 6-functionalized cationic cellulose polyelectrolytes prepared via cellulose sulfonates. Macromol Biosci 1:178–184. https://doi.org/10.1002/1616-5195(20010701)1:5<178:AID-MABI178>3.0.CO;2-E
Mironov MA, Shulepov ID, Ponomarev VS, Bakulev VA (2013) Synthesis of polyampholyte microgels from colloidal salts of pectinic acid and their application as pH-responsive emulsifiers. Colloid Polym Sci 291:1683–1691. https://doi.org/10.1007/s00396-013-2903-3
Petzold K, Schwikal K, Günther W, Heinze T (2006a) Carboxymethyl xylan—control of properties by synthesis. Macromol Symp 232:27–36. https://doi.org/10.1002/masy.200551404
Petzold K, Schwikal K, Heinze T (2006b) Carboxymethyl xylan—synthesis and detailed structure characterization. Carbohydr Polym 64:292–298
Rahn K, Diamantoglou M, Klemm D, Berghmans H, Heinze T (1996) Homogeneous synthesis of cellulose p-toluenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Macromol Mater Eng 238:143–163. https://doi.org/10.1002/apmc.1996.052380113
Rivera DG, Pérez-Labrada K, Lambert L, Dörner S, Westermann B, Wessjohann LA (2012) Carbohydrate–steroid conjugation by Ugi reaction: one-pot synthesis of triple sugar/pseudo-peptide/spirostane hybrids. Carbohydr Res 359:102–110. https://doi.org/10.1016/j.carres.2012.05.003
Schulze P, Gericke M, Scholz F, Wondraczek H, Miethe P, Heinze T (2016) Incorporation of hydrophobic dyes within cellulose acetate and acetate phthalate based nanoparticles. Macrmol Chem Phys 217:1823–1833. https://doi.org/10.1002/macp.201600160
Shulepov ID, Kozhikhova KV, Panfilova YS, Ivantsova MN, Mironov MA (2016) One-pot synthesis of cross-linked sub-micron microgels from pure cellulose via the Ugi reaction and their application as emulsifiers. Cellulose 23:2549–2559. https://doi.org/10.1007/s10570-016-0957-3
Sibikina OV, Iozep AA, Passet BV (2004a) Acylation of aromatic amines with carboxymethyl dextran in the presence of small amounts of water. Russ J Appl Chem 77:1147–1149. https://doi.org/10.1023/B:RJAC.0000044164.62391.6b
Sibikina OV, Iozep AA, Passet BV (2004b) Reactions of carboxymethyl polysaccharides and their ethyl esters with amines. Russ J Appl Chem 77:263–266. https://doi.org/10.1023/b:rjac.0000030363.44546.42
Tezuka Y, Tsuchiya Y, Shiomi T (1996) 13C NMR determination of substituent distribution in carboxymethylcellulose by use of its peresterified derivatives. Carbohydr Res 291:99–108. https://doi.org/10.1016/S0008-6215(96)00163-2
Tiitu M, Laine J, Serimaa R, Ikkala O (2006) Ionically self-assembled carboxymethyl cellulose/surfactant complexes for antistatic paper coatings. J Coll Inter Sci 301:92–97. https://doi.org/10.1016/j.jcis.2006.04.072
Vieira MC, Klemm D, Einfeldt L, Albrecht G (2005) Dispersing agents for cement based on modified polysaccharides. Cem Concr Res 35:883–890. https://doi.org/10.1016/j.cemconres.2004.09.022
Werner B, Bu H, Kjøniksen A-L, Sande SA, Nyström B (2006) Characterization of gelation of aqueous pectin via the Ugi multicomponent condensation reaction. Polym Bull 56:579–589. https://doi.org/10.1007/s00289-006-0522-6
Wu Q-X, Wang D-D, Su T, Cheng X-D, Xu X, Chen Y (2017) Self-assembly of polyelectrolyte complexes microcapsules with natural polysaccharides for sustained drug release. Cellulose 24:4949–4962. https://doi.org/10.1007/s10570-017-1454-z
Zhang K, Brendler E, Gebauer K, Gruner M, Fischer S (2011) Synthesis and characterization of low sulfoethylated cellulose. Carbohydr Polym 83:616–622. https://doi.org/10.1016/j.carbpol.2010.08.030
Zieger M et al (2015) 6-Deoxy-6-aminoethyleneamino cellulose: synthesis and study of hemocompatibility. J Biomater Sci Polym Ed 26:931–946. https://doi.org/10.1080/09205063.2015.1068546
Ziyaei Halimehjani A, Sharifi M (2017) Synthesis of a novel category of Ugi adducts using succinic acid, succinic anhydride and maleic anhydride and their application in post-Ugi reactions for synthesis of functionalized piperazine 2,5-diones. Tetrahedron 73:5778–5783. https://doi.org/10.1016/j.tet.2017.08.028
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gabriel, L., Heinze, T. Diversity of polysaccharide structures designed by aqueous Ugi-multi-compound reaction. Cellulose 25, 2849–2859 (2018). https://doi.org/10.1007/s10570-018-1754-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1754-y