Abstract
Efficient, rapid removal of oil spills on the surface of water is technologically significant for protecting the ecological marine environment. In this work, environmentally friendly, novel, magnetic hydrophobic PVA/CNF aerogels (MHPCA) with ultralow density (13.84 kg m−3), high porosity (> 98%), magnetic responsivity and good mechanical properties were successfully prepared by vacuum freeze-drying PVA/CNF gel followed by a facile silanization reaction and dipping process. The hydrophobic but oleophilic nature of the as-prepared MHPCA (water contact angle over 138°) came from the polysiloxane coated on the fiber surface by the silanization reaction. The MHPCA had excellent oil/water selectivity, with oil absorption capacity as high as 136 g/g being observed. Additionally, the MHPCA exhibited good magnetic response and could absorb oil on the surface of water by magnetic driving. More importantly, the MHPCA showed excellent elasticity after 30 compression-release cycles, which demonstrated the reusability and durability of the super absorbent. The results of this work provide a feasible idea for easily and efficiently removal of oil pollution from the water surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of the rapid development of the petroleum industry and the necessity of marine oil transportation, oil spill accidents have frequently occurred during oil transportation and oil mining. Oil spills not only have a negative impacts on the marine environment, but also threaten the surrounding ecological environment and human health (Shafir et al. 2007; Fingas 2012). Therefore, finding an effective way to resolve oil pollution is a pressing task for the entire international community. Currently, conventional methods used for dealing with oil pollution can be mainly divided into four groups: combustion methods (Liu et al. 2016), physical methods (Ge et al. 2014), chemical methods (Syed et al. 2011; Cho et al. 2014), and bioremediation (Prince 1997). Combustion methods cause waste of the crude oil. Considering physical methods, booms and skimmers are often used, but they are inefficient, especially when the proportion of oil in the oil/water mixture is low (Rajakovic et al. 2007). Dispersant and solidifying agent are often used in chemical methods to change the physical and chemical properties of oils. However, chemical methods are costly and harmful to the environment (Page et al. 2002). Bioremediation using microorganisms to degrade oils is environment-friendly and effective, but its efficiency is significantly affected by oxygen, temperature and pH (Prince 1997).
Compared with traditional methods, physical absorption has attracted considerable attention for cleaning up oil spills from the water surface due to its easy operation, high adsorption capacity and low cost (Wang et al. 2016; Zhou and Chuai 2010). To date, for the purpose of removing oily contaminants, various types of absorbent materials have been widely used in practical applications. These include inorganic sorbents (Karakasi and Moutsatsou 2010; Bastani et al. 2006), such as silicon dioxide, fly ash and organoclay, organic sorbents (Bayat et al. 2005; Choi and Cloud 1992), such as sawdust, peat, activated carbon and plant fibers and synthetic organic sorbents (Zhu et al. 2011; Lin et al. 2012; Wahi et al. 2013), such as polyvinyl polyurethane, polyvinyl chloride and polystyrene foam materials. However, shortcomings of these absorbent materials are also obvious. For example, the absorption capacity of inorganic sorbents and organic sorbents are poor. Additionally, these sorbents have the problem of absorbing not only oils but also water during the absorption process (Bastani et al. 2006; Nakazawa and Somorjai 1995). Although synthetic organic sorbents are highly hydrophobic, these sorbents may cause secondary pollution, and degradation remains a major environmental challenge (Lee et al. 2016). Therefore, it is necessary to identify better adsorption materials with high oil absorption capacity that are environmentally friendly, hydrophobic and oleophilic.
Cellulose-based aerogels showed potential in oil absorbents due to their large specific area and high porosity (Chin et al. 2014; Feng et al. 2015; Innerlohinger et al. 2006; Jin et al. 2015). Several efforts have been made to develop cellulose-based aerogels with high hydrophobicity by coating them with hydrophobic polymer or metallic oxide. For instance, cellulose-based aerogels were treated with methyltrimethoxysilane to obtain hydrophobic surfaces with a water contact angle over 153° (Feng et al. 2015). In another research, cellulose-based aerogels were coated with titanium dioxide (TiO2) (Innerlohinger et al.) for oil removal from the water surface. C. D. Jin and S. J. Shen recently reported superhydrophobic and superoleophilic aerogels that were prepared from waste newspaper treated with trimethylchlorosilane (Jin et al. 2015). These studies demonstrated that cellulose-based aerogels with hydrophobic modification have potential applications in the removal and recovery of pollutants from oil spills.
Although cellulose-based aerogels treated with hydrophobic modification from renewable cellulose fibers are desirable materials for oil spills, inherent inferior strength and modulus have limited their practical applications. PVA, as an excellent water solubility and biocompatibility filler, could enhance the mechanical properties of cellulose-based aerogels owning to the strong hydrogen bonding (Zheng et al. 2014). Besides, driving oil absorption materials to the polluted surface of water and collecting absorption materials rapidly after absorption also needs to be solved. Some researchers have proved that magnetic composite materials can solve this problem effectively (Wang et al. 2016; Raj and Joy 2015; Ge et al. 2013; Yu et al. 2017; Reddy et al. 2016; Chen et al. 2013; Gu et al. 2014). Therefore, we have sufficient reasons to believe that magnetic aerogel can be a promising candidate for oil pollution.
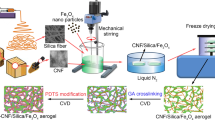
In this work, high selectivity, high oil absorption capacity and environmentally friendly magnetic CNF/PVA aerogels were developed using a facile freeze-drying process followed by hydrophobic modification and simply dipping into Fe3O4 nanoparticles solution. The aerogels had low densities (13.84 kg m−3), high porosities (> 98%) and good magnetic response. After treatment with methyltrichlorosilane (MTS) by a simple thermal chemical vapor deposition method, the aerogels showed excellent absorption abilities for several kinds of oils with hydrophobic characteristic. The aerogels could be easily collected from water surface by applying an external magnetic field after absorption. Furthermore, the aerogels exhibited excellent elasticity and high mechanical durability. The characterization of the aerogels were performed with scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, and X-ray diffraction (XRD). The oil absorption capacity, relationship of oil absorption capacity to different time intervals (0, 5, 10, 15, 20, 30 and 60 s), magnetic removal process and reusability were also investigated in this paper, which described a potential material for the removal of oils from the water surface.
Experiments
Materials
All chemicals were of reagent grade and used without further purification. Analytical grade iron (II) chloride tetrahydrate (FeCl2·4H2O), iron (III) chloride hexahydrate (FeCl3·6H2O), 25% ammonia solution, sulfuric acid (H2SO4), polyvinyl alcohol (PVA, MW:145,000 g mol−1), glutaraldehyde (GA, crosslinker, 25 wt% in H2O), anhydrous ethanol and methyltrichlorosilane (99 wt%) were purchased from Alddin. Bamboo powder was sieved by a 60-mesh filter and later vacuum-dried and set aside.
Preparation of cellulose nanofibers (CNFs)
The chemical purification of bamboo fibers were carried out based on the previous studies (Chen et al. 2011), and the CNFs slurry was mechanically lapped by a grinder (MKCA6-2, Masuko Sangyo Co., Ltd., Japan) at 1500 rpm (Abe and Yano 2010).
Preparation of Fe3O4 nanoparticles
In this study, Fe3O4 nanoparticles were synthesized by a chemical co-precipitation method according to previous report (Lin et al. 2016). Briefly, approximately 5.838 g FeCl3·6H2O and 2.147 g FeCl2·4H2O were uniformly mixed in 100 ml deionized water. Next, the mixed solution was added into a three-necked flask with vigorous stirring at 60 °C under nitrogen. Then NH3·H2O (25 wt%) was added to the solution and pH of the solution was controlled between 10 and 11. The temperature of the whole system was increased to 80 °C and sustained for 1 h. At the final stage, the resulting Fe3O4 nanoparticles were isolated from the solvent by an external magnetic field and washed many times with deionized water until they became neutral. The Fe3O4 powder was dried at room temperature.
Preparation of PVA/CNF aerogels (PCA)
To prepare PVA/CNF aerogels (Mw: 14,500 g mol−1), 5.0 g PVA was dissolved in 100 ml deionized water and stirred for 8 h at 85 °C until the PVA completely dissolved. Then, 4 ml PVA solution (0.05 g ml−1) and 30 g CNFs (0.67 wt%) were mixed together and stirred for 1 h. The weight ratio of PVA and CNF was 1:1. Then, the crosslinking agent glutaraldehyde (200 µl, 25 wt%) and sulfuric acid (50 µl, 2.0 vol%) were added to the PVA/CNFs solution. The mixture was stirred for 1 h. Finally, the resulting mixture was placed in an ultrasonic bath for 10 min to remove any bubbles and subsequently crosslinked/cured in a vacuum oven at 75 °C for 3 h. The PVA/CNF aerogels were obtained using a freeze-drying process for 48 h at − 60 °C.
Preparation of hydrophobic PVA/CNF aerogels (HPCA)
In this paper, chemical vapor deposition (CVD) technique was used for the surface modification of PVA/CNF aerogels. The obtained PCA was placed in a big glass bottle with a small open glass vial containing MTS (300 µl). The glass bottle was capped and heated in an oven at 60 °C for 8 h for the silanization reaction, then the modified aerogels were obtained.
Preparation of magnetic hydrophobic PVA/CNF aerogels (MHPCA)
Approximately 20 mg Fe3O4 nanoparticles were ultrasonically and dispersed in 20 ml ethanol solution. Next, the HPCA was dipped into the mixed solution for 30 min. The MHPCA was obtained using a freeze-drying process for 12 h at – 60 °C.
Sample characterization
The density of PCA, HPCA and MHPCA were determined by measuring the volume and weight of each individual sample. The porosity of PCA, HPCA and MHPCA were calculated based on the following formula (1),
where ρ was the density of the PCA, HPCA and MHPCA, respectively and ρs was the density of the solid material. Gemini analyzer (Micromeritics, USA) was used to test the Brunauer–Emmett–Teller (BET) specific surface area of PCA, HPCA and MHPCA. The BET of all samples were determined by analyzing the amount of N2 adsorbed on the samples with the relative vapor pressure (P/P0) ranging from 0.0 to 1.0 at – 196 °C. The crystallinity of Fe3O4 was characterized by an X-ray diffraction instrument (D8-Adance, Bruker AXS diffractometer, German) with Cu–K α radiation (λ = 1.54 Å, 40 kV, 30 mA, 2θ = 10°–70°), and the magnetic measurement was performed using a Quantum Design MPMS 7T SQUID VSM. The microscopic morphology of the samples were observed by a scanning electron microscope (FE-SEM, Hitachi S-4800). Functional groups of samples were observed by a Fourier transform infrared spectrometer (Nicolet iS10, Thermo Scientific Inc, USA). The surface wettability of PCA, HPCA and MHPCA were measured by water contact angle (WCA) (OCA40, Micro, Germany) at ambient temperature.
Oil absorption experiments
The oil absorption capacity of the MHPCA was determined based on the method reported in previous study (Zhou et al. 2016). All tests were performed at 25 °C. Briefly, the oil was poured on the surface of water in a beaker. Then, a piece of MHPCA (~ 50 mg) was gently placed on the surface of the oil–water mixture. After entirely wetted by oils, the MHPCA with the absorbed oils were collected by sharp needle tweezers. The MHPCA was weighed after removal of excess oils using a filter paper. The oil absorbent capacity k of MHPCA was calculated according to formula (2),
where m1 and m2 are the weights of MHPCA before and after absorption, respectively.
In this study, we measured MHPCA oil absorption capacity for four different types of oil (soybean oil, corn germ oil, pump oil, used pump oil, gasoline, motor oil, ethanol and DMF). All tests were repeated three times, and the average capacity was reported. To investigate the oil absorption capacity of MHPCA, the aerogels were immersed in four types of oil (soybean oil, pump oil, motor oil and DMF), lifted from the oil, drained and weighed at different time intervals (0, 5, 10, 15, 20, 30 and 60 s). The compression strain rate was tested at 2 mm/min. The samples (with a diameter of 10 mm and height of 15 mm) were used for the test.
Results and discussion
Physical properties, crystallinity, magnetization and morphology
Table 1 summarizes the physical properties of PCA, HPCA and MHPCA. All of the samples had low densities (< 14 kg m−3) and high porosities (> 98%). Although the values of BET is very low, there was no great influence on the oil-absorption capacity of MHPCA. BET is usually used to test the micropores and mesopores of materials (Zhu et al. 2017), but the main pore structure of PCA, HPCA and MHPCA were macropores. Such pore structure can also provide similarly huge storage space and adsorption sites (Feng et al. 2015).
As shown in Fig. 1a, Fe3O4 nanoparticles had a spherical shape with rough surface, and the particle size of the Fe3O4 nanoparticles ranged between 20 and 30 nm. The crystalline structure of the magnetic Fe3O4 nanoparticles were characterized by an XRD pattern. The 2θ angles at 30.1° (2 2 0), 35.5° (3 1 1), 42.8° (4 0 0), 53.4° (4 2 2), 56.9° (5 1 1) and 62.3° (4 4 0) are typical diffraction peaks of Fe3O4 (Fig. 1b) (Anbarasu et al. 2015). Figure 1c summarizes the magnetic properties of Fe3O4 nanoparticles. The magnetization curve is a convincing proof (zero coercivity and magnetization increasing linearly at high fields) that the Fe3O4 nanoparticles have superparamagnetic properties. In order to make the HPCA show good magnetic response, Fe3O4 nanoparticles were added. From previous studies, superparamagnetic Fe3O4 nanoparticles would endow composite materials with good magnetic responsivity (Wang et al. 2016; Chin et al. 2014; Raj and Joy 2015; Ge et al. 2013; Yu et al. 2017; Reddy et al. 2016; Chen et al. 2013; Gu et al. 2014). Figure 1d–f shows the microstructure of PCA, HPCA and MHPCA at the cross-section in the middle of the samples. All samples were highly porous and a three-dimensional (3D) assembly of random nanofilaments by PVA and cellulose common structure that contributed the high liquid absorption capacities to the all aerogels. Actually, PVA and cellulose nanofibers were dispersed in water equably during the preparation of PVA/CNF hybrid mixture. After freeze-drying, both of them composed the sheet structure of aerogels owning to powerful hydrogen bond interaction between them (Han et al. 2016). The similar hole structure also illustrated that the MTS treatment and magnetization process had small effects on the primitive porous structure of PCA. However, there were differences in terms of what was observed on the PCA (Fig. 1g) cellular walls because numerous nanofiber-like structures were formed on the HPCA (Fig. 1h) and MHPCA (Fig. 1i), which was attributed to the formation of silicone nanofilaments (Artus et al. 2006; Zhang and Seege 2011). Because of the unique load mode, the Fe3O4 nanoparticles distributed in the surface of sheet structure randomly and some of them have obvious reunification. It can be observed that the Fe3O4 nanoparticles were loaded onto the fiber surface in Fig. 1i.
FTIR spectrum
Successful hydrophobization on the porous surface and magnetization of PVA/CNF aerogels were further evaluated by FITR analysis (Fig. 2). Two absorption bands located at 780 and 1272 cm−1 were observed, which are ascribed to the characteristic vibrations of Si–O–Si and C–Si asymmetric stretching, respectively (Zhu et al. 2013). It was also observed that the spectra of MHPCA vibration intensity increased at 586 cm−1 which may caused by the characteristic peak of Fe3O4 around 586 cm−1 (Anbarasu et al. 2015). This observation further confirmed that magnetite nanoparticles are strongly anchored on aerogels.
Surface wettability
Water contact angles were measured to determine the surface wettability of PCA, HPCA and MHPCA. As shows in Fig. 3a, the unmodified PCA exhibited hydrophilicity as the aerogels could absorb water droplets on the aerogels surface immediately, and the water contact angle of PCA was 0°. However, the modified HPCA and MHPCA had hydrophobic properties which exhibits WCA up to 140° and 138° owing to the use of MTS (Fig. 3b, c). In previous reports, surface wettability was usually controlled by surface energy and surface roughness (Yang et al. 2015; Li et al. 2007). In this study, the silanization reaction caused decreased surface energy and high surface roughness by formation of polysiloxane particles during the silanization reaction process. The state of the water drops and dripping gasoline drops on the surface of the PCA, MPCA and MHPCA were also observed at the same time. It was observed that the PCA absorbed gasoline and water due to their amphiphilic properties (Fig. 3d). Conversely, the HPCA and MHPCA only absorbed gasoline, whereas water drops remained on the surface, revealing the selective absorption of oil/water (Fig. 3e, f). The tests also illustrated that the surface wettability of aerogel was only slightly changed by introducing Fe3O4 nanoparticles. To Further confirm the hydrophobicity of MHPCA, aerogels were evaluated by dispersing the aerogels in water and measuring the internal surface wettability. The MHPCA was wrapped around by a layer of bubbles when immersed in water completely by an external force and floated on the water surface quickly after the external force was released (Fig. 3g–i).
Absorption capacity
To investigate the oil absorption capacity of MHPCA, a series of oils including soybean oil, corn germ oil, pump oil, used pump oil, gasoline, motor oil, ethanol and DMF, were tested and the results are shown in Fig. 4a. Absorption capacity (g/g) can be defined as the weight of the absorbed liquid per weight of original MHPCA. The oil absorption capacity of MHPCA for all kinds of oils ranges from 59 to 136 times of its own weight, which is higher than other magnetic oil absorbing materials (Wang et al. 2016; Chin et al. 2014; Raj and Joy 2015; Ge et al. 2013; Reddy et al. 2016; Chen et al. 2013; Gu et al. 2014). Compared with other hydrophobic aerogels (Korhonen et al. 2011; Zhang et al. 2014; Wang et al. 2015; Feng et al. 2015; Jin et al. 2015; Yin et al. 2016; Zhou et al. 2016; Jiao et al. 2016; Zhu et al. 2017), MHPCA showed good performance for oil absorption as well as other advantage of magnetic responsivity. Different absorption capacity for oils was attributed to both surface properties of MHPCA and characteristics of oils, such as hydrophobicity, density and surface tension (Kim et al. 2012). The high organic solvent absorption capability exhibited by the MHPCA could be attributed to low density, developed pore structure, uniform hydrophobic and oleophilic silane coated on the fiber surface.
As shown in Fig. 4b, oil absorption capacity of MHPCA for soybean oil, pump oil, motor oil and DMF are plotted as a function of absorption time. The relationship between oil absorption capacity and absorption time can divided into two stages: (1) a pure absorption stage and (2) a mixed absorption/desorption stage (Cheng et al. 2010). In the first stage, the oil absorption capacity increased rapidly, which can be explained by the high porosity of MHPCA. High porosity of aerogels provide a large number of fresh channels for oil particles so that they can enter the interior of the aerogel. Once the maximum absorption quantity of oil was achieved, the surface area of MHPCA was almost occupied fully by absorbed oils which prevented the aerogels from accessing free oil. In the second stage, the oil absorption capacity closed to equilibrium due to the oil absorption and oil desorption of oil molecules that happens concurrently. The maximum oil absorption capacity of MHPCA was achieved in less than 30 s for diesel oil, and the oil absorption capacity of MHPCA remained approximately the same as the removal time increased. These results demonstrated that MHPCA is of great potential for the rapid removal (Feng et al. 2015; Meng et al. 2015 Zhou et al. 2016) of oils from water surface.
Effective oil absorption process
It is noted that magnetic property of MHPCA is very important and desirable for hassle-free treatment of polluted water. Figure 5a–f shows the effective absorption process (oil marked by Sudan ш) using MHPCA under a magnetic field. Because of the silane treatment for aerogels, changes in chemical composition of aerogels surface endowed MHPCA hydrophobicity and lipophilicity (Cheng et al. 2017). It is predictable that MHPCA would absorb oils instead of water when exposed to oils floating on the water. The MHPCA also possessed some advantages like high porosity and unique 3-D interpenetrating network structure inherited from PCA. The high porosity gives huge storage space to MHPCA for oils and 3-D interpenetrating network structure provides fresh channels for oil particles to enter the interior of MHPCA easily (Liu and Chen 2014). Just as displayed by digital photographs, the MHPCA can be magnetically driven to the polluted region and quickly absorb oil while the sample repels the oil. After the absorption, the sample loaded with oil remained floated on the water surface while the water surface became completely clean. High selectivity, high oil absorption capacity, fast absorption rate and easy collection progress revealed the effective oil absorption of MHPCA.
Elasticity characterization and reusability
Figure 6a shows the height of recovery of MHPCA with good mechanical properties for which there is little decrease after 30 compression-release cycles. MHPCA showed excellent elasticity due to the toughness of PVA and the coated silane (Zheng et al. 2014). The cyclic compression stress-stain curves of MHPCA at a maxium strain of 70% were also shown in Fig. 6b. No obvious structural changes were found in the test and it showed excellent elastic recovery ability. The cyclic compression stress–stain curves demonstrate that MHPCA recovered almost back to their original shapes after 30 cycles, which show the good mechanical properties of MHPCA. Reusability is of great economic significance for oil absorption materials in oil clean-up applications. In this work, a simple absorption–extrusion–absorption method was used to test the reusability and durability of MHPCA. Figure 6c shows the cycle absorption process of MHPCA. After saturated with oil, most of the absorbed oils can be removed from MHPCA by mechanical squeezing easily. More interestingly, the squeezed sample can quickly absorb oil again and regain most of its volume.
Conclusions
Hydrophobic and oleophilic magnetic PVA/CNF aerogels were successfully prepared by freeze-drying PVA/CNF gel followed by hydrophobic modification and a simple dipping method. The resulting aerogels have high oil absorption capacity for a series of oils (the maximum oil absorption capacity can reach approximately 136 times of its original weight). Furthermore, good magnetic responsivity drives MHPCA to the polluted region and is helpful for post-collection work. Thus, we have reasons to believe that this material has potential as an efficient and environmentally friendly oil absorbent for treating oil pollution in water.
References
Abe K, Yano H (2010) Comparison of the characteristics of cellulose microfibril aggregates isolated from fiber and parenchyma cells of Moso bamboo (Phyllostachys pubescens). Cellulose 17:271–277
Anbarasu M, Anandan M, Chinnasamy E et al (2015) Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim Acta Part A Mol Biomol Spectrosc 135:536–539
Artus GRJ, Jung S, Zimmermann J et al (2006) Silicone nanofilaments and their application as superhydrophobic coatings. Adv Mater 18:2758–2762
Bastani D, Safekordi AA, Alihosseini A et al (2006) Study of oil sorption by expanded perlite at 298.15 K. Sep Purif Technol 52:295–300
Bayat A, Aghamiri SF, Moheb A et al (2005) Oil spill cleanup from sea water by sorbent materials. Chem Eng Technol 28:1525–1528
Chen W, Yu H, Liu Y (2011) Preparation of millimeter-long cellulose I nanofibers with diameters of 30–80 nm from bamboo fibers. Carbohydr Polym 86:453–461
Chen MD, Jiang W, Wang HF et al (2013) Synthesis of highly hydrophobic floating magnetic polymer nanocomposites for the removal of oils from water surface. Appl Surf Sci 286:249–256
Cheng W, Tang KB, Qi YX et al (2010) One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J Mater Chem 20:1799–1805
Cheng QY, Ye DD, Chang CY et al (2017) Facile fabrication of superhydrophilic membranes consisted of fibrous tunicate cellulose nanocrystals for highly efficient oil/water separation. J Membr Sci 525:1–8
Chin SF, Romainor ANB, Pang SC (2014) Fabrication of hydrophobic and magnetic cellulose aerogel with high oil absorption capacity. Mater Lett 115:241–243
Cho YK, Park EJ, Kim YD (2014) Removal of oil by gelation using hydrophobic silica nanoparticles. J Ind Eng Chem 20:1231–1235
Choi HM, Cloud RM (1992) Natural sorbents in oil spill cleanup. Environ Sci Technol 26:772–776
Feng J, Nguyen ST, Fan Z et al (2015) Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem Eng J 270:168–175
Fingas M (2012) The basics of oil spill cleanu. CRC Press, Boca Raton
Ge B, Zhang Z, Zhu X et al (2013) A magnetically superhydrophobic bulk material for oil removal. Colloids Surf A 429:129–133
Ge J, Ye YD, Yao HB et al (2014) Pumping through porous hydrophobic/oleophilic materials: an alternative technology for oil spill remediation. Angew Chem Int Ed 53:3612–3616
Gu JJ, Jiang W, Wang FH et al (2014) Facile removal of oils from water surfaces through highly hydrophobic and magnetic polymer nanocomposites. Appl Surf Sci 301:492–499
Han SJ, Yao QF, Jin CD et al (2016) Cellulose nanofibers from bamboo and their nanocomposites with polyvinyl alcohol: preparation and characterization. Polym Compos. https://doi.org/10.1002/pc.24249
Innerlohinger J, Weber HK, Kraft G (2006) Aerocell: aerogels from cellulosic materials. Lenzing Berichte 86:137–143
Jiao Y, Wan CC, Qiang TG et al (2016) Synthesis of superhydrophobic ultralight aerogels from nanofibrillated cellulose isolated from natural reed for high-performance adsorbents. Appl Phys A 122(7):1–10
Jin C, Han S, Li J et al (2015) Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr Polym 123:150–156
Karakasi OK, Moutsatsou A (2010) Surface modification of high calcium fly ash for its application in oil spill clean up. Fuel 89:3966–3970
Kim H, Lee C, Kim MH et al (2012) Drop impact characteristics and structure effects of hydrophobic surfaces with micro-and/or nanoscaled structures. Langmuir 28:11250–11257
Korhonen JT, Kettunen M, Ras RHA et al (2011) Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl Mater Interfaces 3(6):1813–1816
Lee JH, Kim DH, Kim YD (2016) High-performance, recyclable and superhydrophobic oil absorbents consisting of cotton with a polydimethylsiloxane shell. J Ind Eng Chem 35:140–145
Li XM, Reinhoudt D, Crego-Calama M (2007) What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem Soc Rev 36:1350–1368
Lin J, Shang Y, Ding B et al (2012) Nanoporous polystyrene fibers for oil spill cleanup. Mar Pollut Bull 64:347–352
Lin CC, Lin YS, Ho JM (2016) Adsorption of Reactive Red 2 from aqueous solutions using Fe3O4 nanoparticles prepared by co-precipitation in a rotating packed bed. J Alloy Compd 666:153–158
Liu PS, Chen GF (2014) Porous materials: processing and applications. Elsevier, Amsterdam
Liu H, Chen Y, Geng B et al (2016) Research progress in the cellulose based aerogel-type oil sorbents. Acta Polym Sin 2016:545–559
Meng Y, Young TM, Liu P et al (2015) Ultralight carbon aerogel from nanocellulose as a highly selective oil absorption material. Cellulose 22:435–447
Nakazawa M, Somorjai GA (1995) Coadsorption of water and selected aromatic molecules to model the adhesion of epoxy resins on hydrated surfaces of zinc oxide and iron oxide. Appl Surf Sci 84:309–323
Page CA, Bonner JS, McDonald TJ et al (2002) Behavior of a chemically dispersed oil in a wetland environment. Water Res 36:3821–3833
Prince RC (1997) Bioremediation of marine oil spills. Trends Biotechnol 15:158–160
Raj KG, Joy PA (2015) Coconut shell based activated carbon-iron oxide magnetic nanocomposite for fast and efficient removal of oil spills. J Environ Chem Eng 3:2068–2075
Rajakovic V, Aleksic G, Radetic M et al (2007) Efficiency of oil removal from real wastewater with different sorbent materials. J Hazard Mater 143:494–499
Reddy PM, Chang CJ, Chen JK et al (2016) Robust polymer grafted Fe3O4 nanospheres for benign removal of oil from water. Appl Surf Sci 368:27–35
Shafir S, Van Rijn J, Rinkevich B (2007) Short and long term toxicity of crude oil and oil dispersants to two representative coral species. Environ Sci Technol 41:5571–5574
Syed S, Alhazzaa MI, Asif M (2011) Treatment of oily water using hydrophobic nano-silica. Chem Eng J 167:99–103
Wahi R, Chuah LA, Choong TSY et al (2013) Oil removal from aqueous state by natural fibrous sorbent: an overview. Sep Purif Technol 113:51–63
Wang S, Peng X, Zhong L et al (2015) An ultralight, elastic, cost-effective, and highly recyclable superabsorbent from microfibrillated cellulose fibers for oil spillage cleanup. J Mater Chem A 3(16):8772–8781
Wang J, Geng G, Liu X et al (2016) Magnetically superhydrophobic kapok fiber for selective sorption and continuous separation of oil from water. Chem Eng Res Des 115:122–130
Yang Y, Yi H, Wang C (2015) Oil absorbents based on melamine/lignin by a dip adsorbing method. ACS Sustain Chem Eng 3:3012–3018
Yin TT, Zhang XY, Liu XY et al (2016) Cellulose-based aerogel from Eichhornia crassipes as an oil superabsorbent. RSC Adv 6(101):98563–98570
Yu L, Hao G, Xiao L et al (2017) Robust magnetic polystyrene foam for high efficiency and removal oil from water surface. Sep Purif Technol 73:121–128
Zhang J, Seege S (2011) Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv Funct Mater 21:4699–4704
Zhang Z, Sèbe G, Rentsch D et al (2014) Ultralight weight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chem Mater 26(8):2659–2668
Zheng QF, Cai ZY, Gong SQ (2014) Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J Mater Chem A 2:3110–3118
Zhou XM, Chuai CZ (2010) Synthesis and characterization of a novel high-oil-absorbing resin. J Appl Polym Sci 115:3321–3325
Zhou S, Liu P, Wang M et al (2016) Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain Chem Eng 4:6409–6416
Zhu H, Qiu S, Jiang W et al (2011) Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ Sci Technol 45:4527–4531
Zhu Q, Chu Y, Wang Z et al (2013) Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J Mater Chem A 1:5386–5393
Zhu L, Wang Y, Wang Y et al (2017) An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater 241:285–292
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31770607), the National Key Research and Development Program of China (2017YFD0600204), the Natural Science Foundation of Jiangsu Province of China (Grant No. BK20171450) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors acknowledge the Advanced Analysis and Testing Center of Nanjing Forestry University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Z., Jiang, X., Zhou, H. et al. Preparation of magnetic hydrophobic polyvinyl alcohol (PVA)–cellulose nanofiber (CNF) aerogels as effective oil absorbents. Cellulose 25, 1217–1227 (2018). https://doi.org/10.1007/s10570-017-1619-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1619-9