Abstract
Cellulose hybrid fibers (CeHFs), hybridized via graphene oxide (GO) and metal ions (Ca2+), are synthesized by dry-jet wet spinning. The synthesized GO–Ca2+-CeHFs exhibit the tensile strength and the breaking elongation of 551 ± 37.5 MPa and 5.9 ± 0.4%, respectively, while the GO/cellulose composite fibers (GO–CeFs) show the tensile strength of 403 ± 76.0 MPa and the elongation of 4.5 ± 0.5%; thus, the GO–Ca2+–CeHFs demonstrate improved mechanical properties over GO–CeFs by 37 and 31% in terms of tensile strength and elongation, respectively. These results are attributed to the metal ions that form a good interfacial interaction between the functional groups of cellulose and GO. In addition, the tensile strength of GO–Ba2+–CeHFs is as high as 580 ± 25 MPa, which is induced by the difference in the ionic radius. Therefore, the high mechanical properties of the synthesized cellulose-based fibers have the potential to be used as sustainable alternative to the synthetic fibers used in the industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing economic growth and the lack of sustainable materials and environmental concerns have led to the utilization of new materials from natural resources. Cellulose, one of the most abundant biomass on the earth, is biodegradable and environmentally friendly. Therefore, cellulose-based fibers have been gaining considerable attention and are widely used in clothing, tire cords, interior materials for automobile, and other industrial applications (Wu and Farnood 2015; Siró and Plackett 2010; Zhao et al. 2007). However, the mechanical properties of the cellulose fibers are noticeably lower than that of the synthetic fibers; furthermore, their production costs are prohibitively high which limits their industrials application (Unterweger et al. 2014; Mohanty et al. 2000). To overcome these issues, carbon materials (e.g. graphene, carbon nanotube, and carbon fiber) are used as fillers for the cellulose fibers matrix (Wang et al. 2011; Domun et al. 2015). Among the carbon materials, graphene is considered as promising filler since it exhibits exceptional mechanical, electrical and thermal properties within the confines of 2-dimensional (2D) layer (Geim and Novoselov 2007; Zhu et al. 2010; Stankovich et al. 2006). Graphene oxide (GO), which is oxygenated graphene, is thermodynamically stable than graphene and possesses oxygen functional group; thus, it can be easily dispersed and combined with the cellulose matrix (Dreyer et al. 2010). The GO-reinforced cellulose composites have been extensively reviewed to study the effects of GO for several applications including electronic devices, sensors, energy storages, ultra-lightweight materials, etc. (Wang et al. 2012; Yadav et al. 2013; Feng et al. 2012; Shi et al. 2014).
The interfacial bonding between the filler and the matrix is the key factor for the properties of composite materials. Poor interfacial bonding results in low mechanical properties due to the presence of gaps between the filler and the matrix (Terzopoulou et al. 2015). Recently, the development of a nano-hybrid material that exhibits drastically different properties has attracted great interest. Therefore research into the development of coupling agents (e.g. metal ions) as the 3rd component has been carried out in order to overcome the low interfacial bonding between two different materials (Li et al. 2015; Xu et al. 2013; Lee and Wang 2006). The remarkable observation by cross-linking on the bacterial cellulose has recently been reported (Wang et al. 2017; Yang et al. 2017). The enhanced interfacial bonding between two different materials can be attributed to the 3rd component of the nano-hybrid material. Since the 3rd component improves the interfacial bonding at a nanoscale level, nano-hybrid materials with various properties are realized through the method of interface control. Therefore, the nano-hybrid materials can be the potential alternative to the synthetic fibers for many industrial applications such as automobiles, aircrafts, and constructions, and bio-nanocomposites (Liu et al. 2012; Li et al. 2013; Charleux et al. 2015; Huang et al. 2015).
In this study, we show an environmentally friendly method to fabricate GO/cellulose fibers (GO–CeFs) by incorporating GO into the cellulose matrix using N-methylmorpholine-N-oxide (NMMO) based solvent. Likewise, metal ions are introduced as the 3rd component for the GO–CeFs in order to enhance the interfacial adhesion between the cellulose and GO (GO/cellulose hybrid fibers; GO-CeHFs). The positive effect of the metal ions to the interaction between GO and the cellulose is investigated in terms of structure related mechanical properties of the GO–CeFs.
Experimental
Materials
The wood pulp (Buckeye, V-60) was used to fabricate the cellulose fibers. The wood pulp (α-cellulose content of 94% and degree of polymerization of 855) was dissolved in the N-methylmorpholine-N-oxide (NMMO) solvent (BASF, Germany). The n-porpyl gallate (PG) was purchased from Sigma–Aldrich, USA. For the preparation of the GO, natural graphite flake with a purity of 99% was supplied by Alfa Aesar, USA. Calcium chloride (CaCl2) and magnesium chloride (MgCl2), barium chloride (BaCl2), Copper II chloride (Cu(II)Cl2), iron II chloride (Fe(II)Cl2) were also used from Sigma–Aldrich, USA for the post-treatment of fibers.
Fabrication of CeFs and GO–CeFs
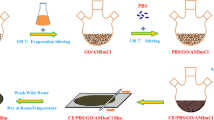
To prepare a spinning dope, 50% NMMO aqueous solvent was evaporated under a reduced pressure (to increase its concentration up to 86.7%. After the wood pulp was dried at 120 °C for 4 h, they were mixed with the prepared 87.6% NMMO solvent. The PG of 1 wt% was added into the dope as an antioxidant to prevent an oxidative decomposition during the dissolution. The mixture was stirred for 10 min before placing in an oil bath at 120 °C for 40 min under vacuum. In the preparation of the GO–CeFs, 0.2 wt% of the GO to cellulose ratio was added to the 50% NMMO solution before increasing its concentration to 86.7%. GO was prepared using a modified Hummers methods (Hummers and Offerman 1958). The solution of GO–CeFs was fabricated in the same way as that for the production of CeFs.
The CeFs and GO–CeFs were synthesized by dry-jet wet spinning with a 10 cm distance between the spinning nozzle and coagulation bath at 105 °C. The fabricated fibers were wound after passing through the coagulation and washing bath. Wound fibers were immersed in the de-ionized water for 24 h to remove any NMMO residue before drying at room temperature for 24 h.
Fabrication of GO-CeHFs
Fabricated CeFs and GO–CeFs were immersed in 0.1 M CaCl2 solutions for 30 min. Afterwards, the CeFs and GO–CeFs were washed using the di-ionized water and then dried out thoroughly at 50 °C under tension. Post-treatment using other metal chloride was performed in the same way as described above mentioned.
Characterization
Raman spectrum was acquired using a LabRAM HR (Horiba, Japan) with laser wavelength of 514 nm to confirm the presence of GO. The mechanical properties of the fibers were determined using the FAVIMAT + single-fiber tester (Textechno, Germany). The tests were performed at a gauge length of 25 mm and a cross-head speed of 2 mm/min. In addition, liner densities of GO/Cellulose with ion treatment fibers measured by vibroscope attached to the FAVIMAT + following the ASTM D1577 (2012) procedure. Transmission electron microscopy (TEM) (Titan, FEI, USA) was used to investigate the morphology of GO in the cellulose fibers. Samples for TEM analysis were prepared by the ultra-microtome (PT-PC PowerTome, RMC, USA) and mounted on the 300-mesh lacey-carbon copper-grid (Ted pella, USA). Energy dispersive spectroscopy (EDS) (Super-X EDX, Bruker, Germany) analysis was performed by a 4-silicon drift detector system to determine their elemental compositions. The introduced surface metal ions and functional groups were investigated by x-ray photoelectron spectroscopy (XPS) (K-Alpha, Thermo Fisher Scientific, USA).
Results and discussion
Comparison of CeFs and GO–CeFs
Figure 1 shows the photographs of the CeFs and GO–CeFs. The photographs indicated that the white CeFs darkens by the addition of GO (Tian et al. 2014).
Raman spectroscopy was used to investigate the existence of GO in the GO–CeFs. As shown in Fig. 2, both CeFs and GO–CeFs exhibit the Raman spectra in the range of 200 to 1250 cm−1; indicating characteristic bands from cellulose II (Zhbankov et al. 2002). The two main peaks around 1350 cm−1 and 1580 cm−1 in the GO–CeFs correspond to the D-band (associated to disorder or defect by oxidation of sp2 carbon bonds) and G-band (arising from the in-plane vibration of sp2 carbon bonds), respectively (Kudin et al. 2008).
The de-convoluted XPS analysis was applied to determine the surface functional groups of CeFs and GO–CeFs (Fig. 3). The C1 s spectrum of CeFs presented in Fig. 3a represents three different functional groups. The major peak at about 284.6 eV is attributed to the C–C bonding. The peaks at about 285.6 and 286.7 eV correspond to the C–OH and C–O–C bonding, respectively (Belgacem et al. 1995). Figure 3b showed the C1 s spectrum of GO–CeFs. Compared to the C1 s spectrum of CeFs, two additional peaks are shown: –C=O (~ 288.2 eV) and O–C=O (~ 289.1 eV) bonding. These two peaks were generated by the GO (Chen et al. 2014).
The morphology of GO in the cellulose was examined by TEM. The bright field (BF) TEM image shows the typical nanostructure of GO in the cellulose (Fig. 4a). Figure 4b shows the scanning transmission electron microscopy (STEM) image of the high angle annular dark field (HAADF) detector with EDS point profile of GO–CeFs. From the atomic percentage data of C–K and O–K edge in Table 1, the compositions of the nanostructure (point 1) indicate high atomic proportion of carbon and oxygen compared to the cellulose (point 2). Thus, it is reasonable to believe that the nanostructure is GO due to the remarkable oxygenated functional groups of GO in comparison with the cellulose.
The typical tensile stress–strain curves of CeFs and GO–CeFs are presented in Fig. 5. It can be seen that the tensile strength of GO–CeFs 403 ± 76.0 MPa, which is 11% higher than that of the CeFs (362 ± 46.6 MPa). Although the tensile strength of GO–CeFs is higher, its breaking elongation (4.5 ± 0.5%) is 33% lower than that of the CeFs (6.0 ± 0.6%).
The effect of the metal ions post-treatment for the GO–CeFs
To further improve the mechanical properties of the GO–CeFs, metal ions (Ca2+) were used to improve the interfacial bonding between the functional groups of GO and the cellulose. Figure 6 shows the mechanical properties of CeFs, GO–CeFs and GO–Ca2+–CeHFs. The tensile strength of GO–Ca2+–CeHFs (551 ± 37.5 MPa) increased about 37% over that of the GO–CeFs. Furthermore, the elongation of GO–Ca2+–CeHFs increased up to 5.9 ± 0.4%, which is comparable to the breaking elongation of CeFs. This can be attributed to the van der waals interactions or electrostatic interactions between the oxygenated functional groups of GO, cellulose and metal ions (Öztürk et al. 2009; Kramar et al. 2014).
The morphology of GO/Cellulose composite fiber and GO–Ca2+–CeHFs was shown at Fig. 7. The diameters of both samples are around ~ 10 μm, which means the ion treatment did not affect on the surface and morphological configuration. The Linear density and diameters of ion-treatment fiber are presented in Table 2. From the measured values, there were no significant change of the liner densities and diameters after ion treatment.
TEM EDS mapping was carried out to observe the distribution of atoms in the GO–Ca2+–CeHFs to confirm that the metal ions affect the interfacial bonding between GO and cellulose (Fig. 8). Figure 8a shows the STEM HAADF image of the GO–CeFs which confirms the existence of nanometer-sized GO on the cellulose fiber. In Fig. 8b and 8c, the GO region shows slightly higher concentrations of carbon and oxygen elements than that in the cellulose region. Thus, it is confirmed that the nanoparticle shown in the TEM image (Fig. 4, Table 1) is indeed GO. Figure 8d depicts the distribution of calcium (Ca) elements showing denser distribution of Ca within the GO region. Therefore, it is observed that Ca ions (Ca+) distribution led to more densely packed interface between GO and cellulose.
In order to verify the Ca2+ distribution in the hybrid fibers, the XPS analysis was applied on the surface functional groups of calcium ion bonding between GO/Cellulose fiber. The Ca spectrum of GO–Ca2+–CeHFs presented in Fig. 9. The peaks at about 346.8 and 350.5 eV correspond to the Ca 2p3/2 and Ca 2p1/2 bonding. It revealed that Ca is well distributed after ion treatment in the GO–Ca2+–CeHFs sample.
Post-processing treatments using metal ions from Group 2 and Period 4 were carried out in order to determine their degree of improvement to mechanical properties (Fig. 10). Figure 10a represents the tensile strength and elongation results from the samples treated with Group 2 metal ions (magnesium, calcium, and barium) for 30 min. The graphs indicate that the tensile strength increases in the order of 515 ± 36.4, 551 ± 37.5, and 579 ± 24.9 MPa, for magnesium, calcium, and barium treated samples, respectively. The breaking elongation results, on the other hand, are similar for all three samples (~ 5.7%). The graphs in Fig. 10b illustrate the results from samples treated with Period 4 elements (calcium, copper, and iron) for 30 min. The tensile strengths for copper, iron, and calcium treated samples were 500 ± 57.3, 507 ± 35.9, and 551 ± 37.5 MPa, respectively; likewise, elongations were similar across all samples (~ 5.8%).
These results can be interpreted in terms of the size of the ionic radius. The larger the size of the ionic radius, the more space is filled at the interface between the cellulose and GO shown at Fig. 11.
Therefore, the infiltration of metal ions with larger radius to the interface can be correlated to the enhancement of mechanical properties (Matos and Arruda 2003; Okieimen et al. 1985; Martell and Hancock 1996; Park et al. 2008). The ionic radius presents in Table 3 (Chao et al. 1984; Faur-Brasquet et al. 2002; Shimada et al. 1992). This is clearly confirmed by post-treated sample with barium (the largest ion tested in this experiment); that is, the tensile strength of GO–Ba2+–CeHFs show the best improvement over the GO–CeFs by 44%.
Conclusion
The key contribution of this study is the development of the concept of graphene reinforced cellulosic fibers with minimum reduction of toughness. The fabricated GO-CeHFs were treated with various metal ions in order to form a strong interfacial bonding between GO and cellulose. The tensile strength of the fibers treated with Ca ions (GO–Ca2+–CeHFs) was around 37% higher than that of GO–CeFs. The addition of GO to the fibers recovered 31% of the reduced elongation; thus, the elongation at break was comparable to that of the conventional cellulose. In addition, the tensile strength of each fibers treated with metal ions from Group 2 and Period 4 were determined. It is observed that the improvements in the tensile strength were correlated to the larger ionic radius of the metal ions; as a result, the barium treated fibers (GO–Ba2+–CeHFs) yielded the tensile strength of 44% higher than that of GO–CeFs. Likewise, the tensile strength of GO–Ba2+–CeHFs was about 60% higher than GO–CeFs.
References
ASTM D1577-07 (2012) Standard Test Methods for Linear Density of Textile Fibers. ASTM International, West Conshohocken. https://doi.org/10.1520/D1577-07R12
Belgacem MN, Czeremuszkin G, Sapieha S (1995) Surface characterization of cellulose fibres by XPS and inverse gas chromatography. Cellulose 2:145–157
Chao SH, Suzuki Y, Zysk JR, Cheung WY (1984) Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol 26:75–82
Charleux B, Coperet C, Lacote E (2015) Chemistry of organo-hybrids: synthesis and characterization of functional nano-objects. Willey, New York
Chen L, Xu Z, Li J, Zhou B, Shan M, Li Y, Liu L, Li B, Niu J (2014) Modifying graphite oxide nanostructures in various media by high-energy irradiation. RSC Adv 4:1025–1031
Domun N, Hadavinia H, Zhang T, Sainsbury T, Liaghat GH, Vahid S (2015) Improving the fracture toughness and the strength of epoxy using nanomaterials—a review of the current status. Nanoscale 7:10294–10329
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Faur-Brasquet C, Reddad Z, Kadirvelu K, Cloirec PL (2002) Modeling the adsorption of metal ions (Cu2 + , Ni2 + , Pb2 +) onto ACCs using surface complexation models. Appl Surf Sci 196:356–365
Feng Y, Zhang X, Shen Y, Yoshino K, Feng W (2012) A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr Polym 87:644–649
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Huang FY, Wu XJ, Yu Y, Lu YH (2015) Preparation and properties of cellulose laurate (CL)/starch nanocrystals acetate (SNA) bio-nanocomposites. Polymers 7:1331–1345
Hummers WS Jr, Offerman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Kramar AD, Žekić AA, Obradović BM, Kuraica MM, Kostić MM (2014) Study of interaction between nitrogen DBD plasma-treated viscose fibers and divalent ions Ca2+ and Cu2+. Cellulose 21:3279–3289
Kudin KN, Ozbas B, Schniepp HC, Prud’homme RK, Aksay IA, Car R (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8:36–41
Lee SH, Wang S (2006) Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos A 37:80–91
Li W, Dichiara A, Bai J (2013) Carbon nanotube-graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos Sci Technol 74:221–227
Li Y, Zhu H, Zhu S, Wan J, Liu Z, Vaaland O, Lacey S, Fang Z, Dai H, Li T, Hu L (2015) Hybridizing wood cellulose and graphene oxide toward high-performance fibers. NPG Asia Mater 7:e150
Liu LH, Métivier R, Wang S, Wang S (2012) Advanced nanohybrid materials: surface modification and applications. J Nanometer 2012:1–2
Martell E, Hancock RD (1996) Metal complexes in aqueous solutions. Plenum Press, New York
Matos GD, Arruda MAZ (2003) Vermicompost as natural adsorbent for removing metal ions from laboratory effluents. Process Biochem 39:81–88
Mohanty AK, Misra M, Hinrichsen G (2000) Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng 276–277:1–24
Okieimen FE, Ogbeifun DE, Nwala GN, Kumsah CA (1985) Binding of cadmium, copper, and lead ions by modified cellulosic materials. Bull Environ Contam Toxicol 34:866–870
Öztürk HB, Manh JV, Bechtold T (2009) Interaction of cellulose with alkali metal ions and complexed heavy metals. Lenzing Ber 87:142–150
Park S, Lee KS, Bozoklu G, Cai W, Nguyen ST, Ruoff RS (2008) Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2:572–578
Shi H, Li W, Zhong L, Xu C (2014) Methylene blue adsorption from aqueous solution by magnetic cellulose/graphene oxide composite: equilibrium, kinetics, and thermodynamics. Ind Eng Chem Res 53:1108–1118
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286
Terzopoulou Z, Kyzas GZ, Bikiaris DN (2015) Recent advances in nanocomposite materials of graphene derivatives with polysaccharides. Materials 8(2):652–683
Tian M, Qu L, Zhang X, Zhang K, Zhu S, Guo X, Han G, Tang X, Sun Y (2014) Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr Polym 111:456–462
Unterweger C, Brüggermann O, Fürst C (2014) Synthetic fibers and thermoplastic short-fiber-reinforced polymers: properties and characterization. Polym Compos 35:227–236
Wang JY, Yang SY, Huang YL, Tien HW, Chin WK, Ma CCM (2011) Preparation and properties of grpahene oxide/polyimide composite films with low dielectric constant and ultrahigh strength via in situpolymerization. J Mater Chem 21:13569–13575
Wang B, Lou W, Wang X, Hao J (2012) Relationship between dispersion state and reinforcement effect of graphene oxide in microcrystalline cellulose-graphene oxide composite films. J Mater Chem 22:12859–12866
Wang S, Jiang F, Xu X, Kuang Y, Fu K, Hitz E, Hu L (2017) Super-strong, super-stiff macrofibers with aligned, long bacterial cellulose nanofiber. Adv Mater 29:1702498
Wu T, Farnood R (2015) A preparation method of cellulose fiber networks reinforced by glutaraldehyde-treated chitosan. Cellulose 22:1955–1961
Xu Z, Sun H, Zhao X, Gao C (2013) Ultrastrong fibers assembled from giant graphene oxide sheets. Adv Mater 25:188–193
Yadav M, Rhee KY, Jung IH, Park SJ (2013) Eco-friendly synthesis, characterization and properties of a sodium carboxymethyl cellulose/graphene oxide nanocomposite film. Cellulose 20:687–698
Yang J, Chen S, Chen Y, Wang B, Pei Q, Wang H (2017) Macrofibers with high mechanical performance based on aligned bacterial cellulose nanofibers. ACS Appl Mater Interfaces 9:20330–20339
Zhao H, Kwak JH, Zhang ZC, Brown HM, Arey BW, Holladay JE (2007) Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr Polym 68:235–241
Zhbankov RG, Firsov SP, Buslov DK, Nikonenko NA, Marchewha MK, Ratajczak H (2002) Structural physico-chemistry of cellulose macromolecules. Vibrational spectra and structure of cellulose. J Mol Struct 614:117–125
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22:3906–3924
Acknowledgments
This research was supported by Nano·Material Technology Development Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2016M3A7B4900135).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ryu, J., Lim, J.S., Ahn, S. et al. Structure and properties of graphene oxide/cellulose hybrid fibers via divalent metal ions treatment. Cellulose 25, 517–525 (2018). https://doi.org/10.1007/s10570-017-1535-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1535-z