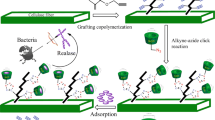
Abstract
This paper presents an environmentally friendly strategy to obtain alkynylated cellulose fibers (ACFs), a versatile platform for tailoring cellulose by robust click reaction. This strategy is based on the integration of two efficient reactions: selective oxidation of cellulose fibers by sodium periodate (NaIO4) generating dialdehyde cellulose fibers and subsequent Schiff base reaction with 3-ethynylaniline yielding alkynylated cellulose fibers (ACFs). The alkynyl moieties introduced into ACFs were simply transferred with azido compounds under Cu(I) catalysis and mild conditions. The content of alkynyl groups of ACFs was found to be as high as 3.0 mmol/g. Fourier transform infrared spectroscopy (FTIR) showed that the selective oxidation of cellulose fibers generated aldehyde groups and the Schiff base reaction resulted in the incorporation of ethynyl groups and benzene rings into cellulose fibers. FTIR and X-ray photoelectron spectroscopy results confirmed the successful click reaction between ACFs and 4-azidobenzoic acid. This clickable platform would serve as a versatile starting precursor for finely tuning cellulose fibers for advanced applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been broadly accepted that the development based on fossil resources as raw materials is unsustainable in the long run. In this context, the utilization of renewable resources has gained increasing interest due to their inherent renewability and environmental friendliness. Among these renewable materials, cellulose is considered as a green feedstock and has gained special attention due to its abundant availability. However, some intractable challenges still remain. For example, poor solubility and lack of efficient processing methods have been two of the obstacles (Schmidt et al. 2014). Nevertheless, scientists have managed to make substantial progress in harnessing this green polysaccharide. For example, scientists have developed green solvents to dissolve cellulose, like ionic liquids (Swatloski et al. 2002; Wang et al. 2012) and low temperature NaOH-based systems (including alkali/urea or thiourea/H2O) (Qi et al. 2011). On the other hand, scientists also push forward modification and functionalization of cellulose by new processing methods, one of which is click chemistry.
Click chemistry refers to reactions that are rapid, versatile, highly selective, and high yielding when connecting two molecular components (Kolb et al. 2001). Since the first click reaction copper-catalyzed azide–alkyne cycloaddition (CuAAC) was recognized, a number of reactions, including strain promoted azide–alkyne cycloadditions (SPAACs), Diels–Alder reaction and photo-initiated thiol-ene radical reaction, have been classified into the click chemistry family (Anseth and Klok 2016). With these impressive features, click chemistry is compatible with the green chemistry principles (Anastas and Eghbali 2010) in a comprehensive manner.
The first investigation on the design of cellulose derivatives through click reaction (CuAAC) was reported by Liebert et al. (2006), closely followed by Hafren et al. (2006). Ever since then, investigations on modification of cellulose materials based on click chemistry have seen remarkable increase. In micro- and nano-level sized cellulose modification and functionalization, cellulose nanoplatelet gels (Filpponen and Argyropoulos 2010), fluorescent cellulose nanocrystals (Filpponen et al. 2011), photobactericidal porphyrin-cellulose nanocrystals (Feese et al. 2011) and fluorescent nanofibrillated cellulose (Pahimanolis et al. 2011) are some of the attractive examples successfully obtained via click chemsity (CuAAC). Click chemistry was also employed as connecting approach to prepare nanocrystalline cellulose-clicked-β-casein nanoparticle (Karaaslan et al. 2013) and microcrystalline cellulose-clicked clay (Yadav et al. 2015), and assembling approach in an end-to-end assembly of nanocrystalline cellulose into nanocellulose fibers (Yang and van de Ven 2016).
Regarding cellulose fibers, click chemistry based modification is still limited. Elchinger et al. (2014) reported a new material formed by CuAAC between propargyled kraft pulp and azidated starch. Mangiante et al. (2013) proposed a green nondegrading approach to alkyne-functionalized cellulose fibers and realized postmodification of the resulting fibers with azide-functionalized molecular probes and azide end-capped PEG through CuAAC. Abd Rahman et al. (2016) studied crosslinking of cellulose fibers via azide–alkyne click chemistry.
In modification and functionalization of other forms of cellulose materials, click chemistry is also very effective. Ringot et al. (2009) obtained new photobactericidal porphyrin-grafted cellulose fabric by click chemistry (CuAAC). Xu et al. (2013) synthesized antibacterial cellulose film using “clickable” quaternary ammonium compound. CuAAC was also proved to be a robust toolkit for preparation of cellulose derivatives with unique structures (Pohl et al. 2008). In addition, click chemistry also became appealing in biomaterials (Anseth and Klok 2016). Click chemistry in modification of polysaccharides (including cellulose) has been reviewed by Elchinger et al. (2011) and recently by Meng and Edgar (2016). Interested readers may turn to these reviews for more comprehensive information.
An important issue that needs to be emphasized is, even if the click reactions are by definition reactions with quantitative yields, high tolerance of functional groups, etc., they require particular starting reagents (Elchinger et al. 2011). For instance, the terminal alkyne or azide groups need to be “inserted” into cellulose to begin the CuAAC procedure. Thus, the availability of alkyne- or azide- bearing cellulose becomes a precondition for implementing CuAAC click reactions. Here, we name the alkyne-bearing cellulose as “clickable cellulose platform”. Synthesis of this versatile platform undoubtedly becomes a crucial premise, yet a challenging task. Synthesis methods employed traditionally for clickable platform preparation are not favorable in terms of economy and environment because they need toxic reagents, special solvents or harsh conditions. Green synthesis strategy of clickable cellulose platform is therefore highly desirable. Synthesis of clickable cellulose fibers is particularly difficult owing to the poor accessibility.
In the current study, we designed a combinational synthesis approach to obtain clickable cellulose platform, alkynylated cellulose fibers (ACFs), through selective oxidation of cellulose fibers (Scheme 1, Step 1) followed by Schiff base reaction (Scheme 1, Step 2). Click reactivity of ACFs was verified by click reaction with 4-azdiobenzoic acid (Scheme 1, Step 3). Evidences from FTIR showed that dialdehyde cellulose fibers (DAC fibers) formed after cellulose fibers were treated with NaIO4, and ethynyl and benzene groups were successfully incorporated into cellulose fibers after Schiff base reaction, characteristic peaks changed correspondingly after click reaction. XPS data also implied that the Schiff base reaction and click reaction were successful.
Our inventive methodology is green, easy to operate, efficient, simple both in the work-up stage and purification, in accordance with principles of sustainable development as well as green chemistry. The preparation procedure was performed in cellulose fibers suspension without any other solvent except water as the aqueous medium. This strategy may also provide a promising opportunity to prepare various clickable cellulose-based materials, such as lignocellulosic fibers, fabrics and textiles.
Experimental
Materials
Bleached softwood kraft pulp (produced in Canada) was kindly provided by Mudanjiang Hengfeng Paper Co. Ltd. (Heilongjiang, China) and was used as the cellulose source. 3-Ethynylaniline (3-EAn) with a purity of 97%+ was purchased from Bide Pharmatech Ltd. (Shanghai, China). 4-Azidobenzoic acid was bought from Tokyo Chemical Industrial Co. Ltd. Other chemicals and reagents were all of analytic grade unless stated elsewhere.
Synthesis of dialdehyde cellulose (DAC) fibers
To a three-neck bottle containing evenly-dispersed cellulose fibers suspension of 1% consistency (2.25 g cellulose fibers, 13.7 mmol anhydroglucose unit AGU), NaIO4 solution (2.93 g, 13.7 mmol, 50 ml) was added dropwise. The bottle was kept in a water bath of 40 °C. After 4 h, the DAC fibers were recovered by filtration and washed with tap water (2 l for 3 times) to remove the remnant chemicals. Aldehyde content of DAC fibers was determined according to the principle of previously reported reference (Sirvio et al. 2011), that is, aldehyde groups were completely converted to oxime with NH2OH∙HCl. The aldehyde content of DAC fibers can be calculated from the nitrogen content of resultant oxime, and the nitrogen content can easily be measured by Kjeldahl apparatus.
Synthesis of alkynylated cellulose fibers (ACFs)
To a 250 ml round-bottomed flask charged with 200 ml distilled water, 1.0 g DAC fibers (based on oven-dry mass) were added and dispersed into a suspension. 3-EAn (from 3 to 33.5 mmol, depending on the mole ratio of EAn to aldehyde) was added into the suspension to start the Schiff base reaction. The reaction was stopped by separating the yielded ACFs from the dispersion by filtration. The ACFs were washed with running tap water (2 l for 3 times) and then distilled water (200 ml for twice) to remove the unconverted residues for click reaction and instrumental analysis.
The content of alkynyl groups in the ACFs was measured based on the following fact: there is equivalent mole amount of alkynyl groups and nitrogen within ACFs (Scheme 1, Step 2). So the content of alkynyl groups in ACFs can be measured by referring to the nitrogen content of the ACFs. And the nitrogen content was measured by Kjeldahl apparatus.
Click reaction of ACFs
To a 250 ml round-bottomed flask charged with the mixed solution (150 ml 95% ethanol and 50 ml distilled water) dissolving 10 mmol 4-azidobenzoic acid, catalytic amount of sodium ascorbate and CuSO4 were added separately to initiate the click reaction. The system was kept at ambient temperature and in a dark environment to protect it from light for 2 days.
The clicked products were collected by filtration and washed with 95% ethanol (200 ml × 2) then distilled water (200 ml × 2). Clicked products were dried to reach constant weight and then used for instrumental measurement.
Degree of polymerization (DP) measurement, handsheet formation and tensile index
DAC fibers were made into handsheet of 60 g/m2 by handsheet former. Both tensile measurement and degree of polymerization measurement experiments were carried out according to literature methods (Shi and He 2003). The solution for DP measurement is copper ethylenediamine solution.
XRD, FTIR and XPS analysis
X-ray diffraction (XRD) profiles of DAC fibers were recorded from 2θ = 5°–45° (Rigaku D/max2200, 30 kV, 40 mA). Fourier transform infrared (FTIR) spectra of samples were collected from Fourier Transform Infrared Spectroscopy Thermo Fisher Scientific Nicolet 6700 with accumulation of 32 scans and resolution of 4 cm−1. X-ray photoelectron spectroscopy (XPS) data were obtained using a Thermo Fisher Scientific’s K-Alpha X-ray photoelectron spectrometer (ESCALAB 250 Xi) system. An Al Ka X-ray source was used. The vacuum in the analyzing chamber was 1.0 × 10−8 Pa during analysis. The binding energies were calibrated against the C1 s binding energy at 248.8 eV.
Results and discussion
Selective oxidation
Influences of NaIO4/AGU, reaction temperature, reaction time and pH (buffer system) on the yield and aldehyde groups content of DAC fibers are shown in Fig. 1. The increase of the ratio of NaIO4/AGU led to an increase in the content of aldehyde groups in DAC fibers without noticeable yield loss (Fig. 1a). Higher ratio of NaIO4/AGU means higher reactant concentration and higher reaction rate, leading to higher products concentration (here higher content of aldehyde groups). With the increase of reaction temperature from room temperature to 50 °C, the content of aldehyde groups in DAC fibers increased but the yield had an optimum at 40 °C (Fig. 1b). Reaction rate is accelerated at elevated temperature according to Arrhenius equation. So the content of aldehyde groups in DAC fibers would become higher at elevated temperature. However, the oxidative degradation of cellulose fibers is also aggravated at elevated temperature. When prolonging the reaction time, the content of aldehyde groups in DAC fibers increased but tended to be leveling off while the yield decreased when reaction time was longer than 4 h (Fig. 1c). This is owing to the accessibility and reactivity of hydroxyls of cellulose fibers. It is easy for reagents to diffuse in the amorphous region of cellulose but relatively difficult in the crystalline region. The reactants can contact and react with the hydroxyls in the amorphous region and on the surface of the crystalline region within several hours. However, even if prolonging the reaction time, it is still difficult for reactants to further diffuse and react. At the same time, degradation becomes notable which brings the yield down. The pH (buffer system) had a significant impact on the yield and the content of aldehyde groups of DAC fibers, and the optimal reaction medium was distilled water (initial pH of 6.5) (Fig. 1d). Generally speaking, weak acidic medium is favorable for selective oxidation of cellulose by NaIO4. Nevertheless, the yielded DAC fibers are sensitive to alkaline, and molecular chain tends to be broken down. In brief, under appropriate reaction conditions, i.e., temperature of 40 °C, reaction time of 4 h, and reaction medium of distilled water (initial pH of 6.5), NaIO4/AGU mole ratio can be considered as a key process variable to prepare DAC fibers with varying levels of aldehyde groups.
Influence of a NaIO4/AGU, b reaction temperature, c reaction time, d pH (buffer system) on the yield and aldehyde groups content of DAC fibers. Process conditions: a 40 °C, 4 h, distilled water; b NaIO4:AGU = 3:1, 4 h, distilled water; c NaIO4:AGU = 3:1, 40 °C, distilled water; d NaIO4:AGU = 3:1, 40 °C, 4 h
The tensile index of handsheets made from DAC fibers followed a general tendency of increasing first and then decreasing (Fig. 2a). The tensile of paper is contributed by both the strength of individual fibers and the bonding contribution among fibers (i.e., hydrogen bonds). When the mole ratio of NaIO4/AGU was below 2:1, the DAC had an increasing aldehyde content (Fig. 1a) and relatively higher DP (Fig. 2b). The increase in tensile index can be ascribed to the increased bonding contribution of condensation reaction (between aldehyde groups and vicinal hydroxyls) and increased hydrogen bonds. Condensation reactions closed the distance between the adjacent fibers due to the linkages between different anhydroglucose units (AGU) either in the same chain or between different chains. Correspondingly, the numbers of hydrogen bonds between the contiguous fibers increased (Hou et al. 2007). Therefore, the tensile index increased with the increase of the mole ratio of NaIO4/AGU. However, cellulose fibers underwent severe degradation with further increase of the mole ratio of NaIO4/AGU (Fig. 2b). In this case, the strength of individual fibers played a major role in the tensile behavior of paper. So it was observed that the tensile strength of handsheet decreased when the mole ratio of NaIO4/AGU was beyond 2:1 (Fig. 2a).
As shown in the spectra of DAC fibers (Fig. 3a), the adsorption peak centered around 1735 cm−1 corresponding to C=O stretch vibration was observed, which indicated the presence of aldehyde groups (Dash et al. 2012; Hou et al. 2007). Characteristic peaks of hydroxyls in cellulose at 1055 and 1160 cm−1 were weakened at the same time. Variations in these characteristic peaks were more pronounced at a high ratio of NaIO4/AGU. These observations are consistent with the previously reported results (Hou et al. 2007).
As shown in the XRD profiles of pristine fibers and DAC fibers (Fig. 3b), amorphous region shrunk with the increase of NaIO4/AGU mole ratio while the crystalline region also diminished. At a high NaIO4/AGU ratio, amorphous region almost disappeared. This is because the reagents can access the amorphous regions and cause the degradation. Microcrystalline and crystalline regions are relatively difficult for reagent to penetrate and diffuse. So the crystalline region showed a relatively smaller degradation.
Schiff base reaction
Schiff base reaction is a reversible reaction and the hydrolysis rate depends on pH and the surrounding structure of the base itself. Actually, when C=N—connecting aromatic groups, the stability of Schiff base could be notably improved. Schiff base reaction has been successfully employed to graft amine to cellulose nanowhisker after periodate oxidation (Dash et al. 2012) and to form cellulose nanofibers (CNF) hydrogels-scaffolds after CNF production using TEMPO mediated oxidation (Syverud et al. 2015). Here, Schiff base was also proved to be a simple and efficient grafting approach. All of the resultant ACFs had a pale yellow appearance, which is usually regarded as the characteristic color of Schiff base. And the only byproduct of Schiff base reaction is environment benign water.
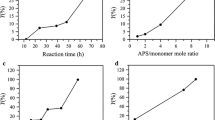
Influences of 3-EAn dosage, reaction time and reaction temperature on the Schiff base reaction results are shown in Fig. 4. It is easy to understand that the content of alkynyl groups in ACFs increased with increasing mole ratio of EAn/aldehyde (Fig. 4a, d). For DAC fibers with high content of aldehyde groups (7.52 mmol/g), the influences of reaction temperature and reaction time on Schiff base reaction were significant (Fig. 4e, f). However, for DAC fibers with medium aldehyde content (3.92 mmol/g), the influences of reaction temperature and reaction time were insignificant (Fig. 4b, c). For DAC fibers with medium aldehyde content, the aldehyde groups mainly located in the amorphous region, and were easily accessible. So the Schiff base reaction proceeded and reached equilibrium easily. However, for DAC fibers with high aldehyde content, the aldehyde groups located in both amorphous and crystalline regions; so elevated temperature and prolonged time were favorable to diffuse reactant (3-EAn) and access the reaction sites. At elevated temperature and prolonged time, the content of alkynyl groups of ACFs as high as 3.0 mmol/g was obtained.
The FTIR spectra and XPS spectra of DAC fibers (8.3 mmol/g aldehyde) and ACFs (2.6 mmol/g alkynyl) are shown in Fig. 5. In the FTIR spectra (Fig. 5a), paired peaks (1600 and 1500 cm−1) ascribed to benzene ring stretching, 2105 cm−1 assigned to C≡C and 3280 cm−1 assigned to C≡C–H appeared in the spectrum of ACFs. These new peaks, apparently different from those of DAC fibers, emerged as a result of the Schiff base reaction between DAC and 3-EAn. In the XPS spectra Fig. 5b, merely trace amount of nitrogen in the DAC fibers can be seen. However, nitrogen can be clearly observed in ACFs. The newly introduced nitrogen was in C=N binding state (399.2 eV). These results verified that the Schiff base reaction proceeded as expected.
Click reaction
The FTIR spectra of ACFs and clicked product and XPS spectrum of clicked product are shown in Fig. 6. In the FTIR spectra, the decrease in characteristic peak intensity of C≡C–H and C≡C was due to CuAAC reaction (cycloaddition of ethynyl groups and N3 groups, Scheme 1, Step 3), which also led to the formation of 1,2,3 triazole rings, and –COOH at 1700 cm−1 appeared correspondingly (Fig. 6a). In the XPS survey of clicked product (ACFs©4-azidobenzoic acid), one can easily differentiate N species in different chemical states before and after click reaction (Fig. 6b). For the unclicked product (i.e., ACFs), the nitrogen element was bonded in a C=N chemical state (Fig. 5b). However, the N of the clicked product located in different and complicated chemical environments. Other than C=N chemical state, N was also bonded in a triazole ring (400.9, 400.2 and 398.8 eV), which was formed as a result of CuAAC reaction. This information clearly indicated that the CuAAC between ACFs and 4-azidobenzoic acid successfully proceeded. Together with the analysis of FTIR and XPS, it is therefore reasonable to conclude that the yielded ACFs possess clickable reactivity.
Conclusions
This research established a green approach to convert cellulose fibers into clickable cellulose platform by combining two efficient reactions: selective oxidation of cellulose fibers by NaIO4 generating DAC fibers as well as Schiff base reaction between DAC fibers and 3-ethynylaniline yielding alkynylated cellulose fibers (ACFs). Clickable performance of ACFs was verified by the click reaction with 4-azdiobenzoic acid. FTIR and XPS analyses confirmed the occurrence of selective oxidation, Schiff base reaction, and click reaction.
References
Abd Rahman NS, Ahmad NA, Yhaya MF, Azahari B, Ismail WR (2016) Crosslinking of fibers via azide–alkyne click chemistry: synthesis and characterization. J Appl Polym Sci 133:43576. doi:10.1002/app.43576
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. doi:10.1039/b918763b
Anseth KS, Klok HA (2016) Click chemistry in biomaterials, nanomedicine, and drug delivery. Biomacromol 17:1–3. doi:10.1021/acs.biomac.5b01660
Dash R, Elder T, Ragauskas A (2012) Grafting of model primary amine compounds to cellulose nanowhiskers through periodate oxidation. Cellulose 19:2069–2079. doi:10.1007/s10570-012-9769-2
Elchinger PH, Faugeras PA, Boëns B, Brouillette F, Montplaisir D, Zerrouki R, Lucas R (2011) Polysaccharides: the “click” chemistry impact. Polymers 3:1607–1651. doi:10.3390/polym3041607
Elchinger PH, Awada H, Zerrouki C, Montplaisir D, Zerrouki R (2014) Kraft pulp-starch covalent linking: a promising route to a new material. Ind Eng Chem Res 53:7604–7610. doi:10.1021/ie500555g
Feese E, Sadeghifar H, Gracz HS, Argyropoulos DS, Ghiladi RA (2011) Photobactericidal porphyrin-cellulose nanocrystals: synthesis, characterization, and antimicrobial properties. Biomacromol 12:3528–3539. doi:10.1021/bm200718s
Filpponen I, Argyropoulos DS (2010) Regular linking of cellulose nocrystals via click chemistry synthesis and formation cellulose nanoplatelet gels. Biomacromol 11:1060–1066. doi:10.1021/bm1000247
Filpponen I, Sadeghifar H, Argyropoulos DS (2011) Photoresponsive cellulose nanocrystals. Nanomater Nanotechnol 1:34–43
Hafren J, Zou W, Cordova A (2006) Heterogeneous ‘organoclick’ derivatization of polysaccharides. Macromol Rapid Commun 27:1362–1366. doi:10.1002/marc.200600328
Hou QX, Liu W, Liu ZH, Bai LL (2007) Characteristics of wood cellulose fibers treated with periodate and bisulfite. Ind Eng Chem Res 46:7830–7837. doi:10.1021/ie0704750
Karaaslan MA, Gao G, Kadla JF (2013) Nanocrystalline cellulose/β-casein conjugated nanoparticles prepared by click chemistry. Cellulose 20:2655–2665. doi:10.1007/s10570-013-0065-6
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004–2021
Liebert T, Hänsch C, Heinze T (2006) Click chemistry with polysaccharides. Macromol Rapid Commun 27:208–213. doi:10.1002/marc.200500686
Mangiante G, Alcouffe P, Burdin B, Gaborieau M, Zeno E, Petit-Conil M, Bernard J, Charlot A, Fleury E (2013) Green nondegrading approach to alkyne-functionalized cellulose fibers and biohybrids thereof: synthesis and mapping of the derivatization. Biomacromol 14:254–263. doi:10.1021/bm3016829
Meng X, Edgar KJ (2016) “Click” reactions in polysaccharide modification. Prog Polym Sci 53:52–85. doi:10.1016/j.progpolymsci.2015.07.006
Pahimanolis N, Hippi U, Johansson LS, Saarinen T, Houbenov N, Ruokolainen J, Seppälä J (2011) Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose 18:1201–1212. doi:10.1007/s10570-011-9573-4
Pohl M, Schaller J, Meister F, Heinze T (2008) Selectively dendronized cellulose: synthesis and characterization. Macromol Rapid Commun 29:142–148. doi:10.1002/marc.200700628
Qi HS, Yang QL, Zhang LN, Liebert T, Heinze T (2011) The dissolution of cellulose in NaOH-based aqueous system by two-step process. Cellulose 18:237–245. doi:10.1007/s10570-010-9477-8
Ringot C, Sol V, Granet R, Krausz P (2009) Porphyrin-grafted cellulose fabric: new photobactericidal material obtained by “Click-chemistry” reaction. Mater Lett 63:1889–1891. doi:10.1016/j.matlet.2009.06.009
Schmidt S, Liebert T, Heinze T (2014) Synthesis of soluble cellulose tosylates in an eco-friendly medium. Green Chem 16:1941. doi:10.1039/c3gc41994k
Shi S, He F (2003) Pulping and papermaking analysis and testing. China Light Industry Press, Beijing (in Chinese)
Sirvio J, Hyvakko U, Liimatainen H, Niinimaki J, Hormi O (2011) Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr Polym 83:1293–1297. doi:10.1016/j.carbpol.2010.09.036
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975. doi:10.1021/ja025790m
Syverud K, Pettersen SR, Draget K, Chinga-Carrasco G (2015) Controlling the elastic modulus of cellulose nanofibril hydrogels—scaffolds with potential in tissue engineering. Cellulose 22:473–481. doi:10.1007/s10570-014-0470-5
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41:1519–1537. doi:10.1039/c2cs15311d
Xu W, Gao G, Kadla J (2013) Synthesis of antibacterial cellulose materials using a “clickable” quaternary ammonium compound. Cellulose 20:1187–1191. doi:10.1007/s10570-013-9914-6
Yadav P, Chacko S, Kumar G, Ramapanicker R, Verma V (2015) Click chemistry route to covalently link cellulose and clay. Cellulose 22:1615–1624. doi:10.1007/s10570-015-0594-2
Yang H, van de Ven TGM (2016) A bottom-up route to a chemically end-to-end assembly of nanocellulose fibers. Biomacromol 17:2240–2247. doi:10.1021/acs.biomac.6b00480
Acknowledgments
The authors would like to acknowledge the financial support of the National Natural Science Foundation of China (31370579).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, C., Sun, L., Xiao, G. et al. Green and combinational method towards clickable alkynylated cellulose fibers (ACFs). Cellulose 24, 3219–3229 (2017). https://doi.org/10.1007/s10570-017-1363-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1363-1