Abstract
Cellulose nanocrystals were successfully oxidized with sodium hypochlorite using catalytic amounts of sodium bromide and 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radical at pH 10 in water. Carboxylate groups were selectively introduced at the surface of the crystals up to a total acid content of 1200 mmol kg−1 without damaging the integrity of the crystals. The final acid content can easily be tuned by varying the amount of oxidant introduced. The effect of temperature, the quantity of oxidant and co-catalyst on the reaction kinetics were studied. Several methods were used for the characterization of the oxidized material like field emission scanning electron microscopy, diffuse reflectance infrared spectroscopy and thermogravimetric analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose nanocrystals (CNCs, formerly called NCC) are a nanomaterial constituted of linear homopolymer chains of β-(1 → 4) linked d-glucose units. CNC particles are extracted from naturally occurring cellulose sources such as wood pulp, cotton, bacteria, and tunicates (sea animals). The wide variety of sources leads to CNCs which exhibit different average length and cross sectional dimensions.
Within wood nanofibrils, native cellulose consists of alternating amorphous and crystalline regions. CNCs are obtained by well-defined strong acid hydrolysis conditions, which preferentially dissolve the amorphous cellulosic parts present along the cellulosic fibers thus releasing the unaffected crystalline parts in the aqueous medium. The use of sulfuric acid imparts negatively charged acidic sulfate half-ester groups located at the surface of the crystals (sulfated CNCs). These charges cause electrostatic repulsion between the CNC particles and confer a timely robust stability to the aqueous CNC suspensions (Revol et al. 1992; Dong et al. 1996). On the contrary, hydrochloric acid treatment does not introduce stabilizing negative charges onto the CNC surface, resulting in an unstable colloidal system. To overcome this feature, a subsequent process is required to make the CNCs stable in suspension (Araki et al. 2001; Azzam et al. 2010).
In a context of an emerging bioeconomy, demand for CNCs is expected to grow rapidly due to its biodegradability, non-toxic nature combined to its remarkable properties. Moreover, sulfated CNCs can form chiral nematic assembly in aqueous suspension leading to unique optical properties once a dried film has been formed. The main challenge remains its hydrophilicity which limits its incorporation into hydrophobic matrices such as thermoplastics. There is then a need to alter its surface chemistry to make the CNCs more compatible with hydrophobic media.
The TEMPO-mediated oxidation is an efficient route to provide negatively charged carboxyl groups which are not as labile as sulfate half-ester groups. Known for the past two decades, TEMPO-mediated oxidation selectively converts primary alcohol groups into carboxylic acid groups in the presence of secondary alcohol groups in polysaccharides such as starch (Kato et al. 2002), pullulan (de Nooy et al. 1994, 1995a, 1996), cyclodextrins (Fraschini and Vignon 2000) and cellulosic substrates like rayon, cotton linters, wood pulp, etc. using sodium hypochlorite as a primary oxidant (Chang and Robyt 1996; Isogai and Kato 1998; Tahiri and Vignon 2000; Da Silva et al. 2003; Saito and Isogai 2004; Saito et al. 2005; Montanari et al. 2005; Saito et al. 2006; Habibi and Vignon 2008). A variety of oxidants like peracid/bromide (Bragd et al. 2002), copper salts/O2 (Semmelhack et al. 1984), NaBrO2 (Inokuchi et al. 1990), NaClO2/NaClO (Zhao et al. 1999; Saito et al. 2009, 2010) have also been used. To date this oxidation is viewed as the most efficient way to carry out surface oxidation of cellulosic materials and to isolate nanofibrils from fibers.
TEMPO-mediated oxidation of CNCs, obtained from HCl hydrolysis of cellulose fibers, was first reported by Araki et al. (2001). Then the same reaction was applied to CNCs produced from HCl hydrolysis of cotton linters and microfibrils from sugar beet pulp (Montanari et al. 2005) and tunicate (Habibi et al. 2006). Significant amounts of carboxylate groups can be selectively introduced onto the CNC surface without damaging the integrity of the core of CNCs (Habibi et al. 2006), thus providing new chemical functionalities for enhancing material hydrophobicity or barrier properties for packaging applications (Kato et al. 2005; Fukuzumi et al. 2009) or for further surface modification by chemical/physical grafting.
In this work, we studied oxidation parameters such as temperature, sodium bromide and sodium hypochlorite ratios were varied to yield a variety of oxidized derivatives of sulfated CNCs materials produced from wood pulp. We investigated the effects that the various oxidation parameters such as reagent quantities and reaction temperature have on reaction rate, total yield, carboxyl content introduced, and resulting morphology of the colloidal particles.
Experimental section
Chemicals
2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO), sodium bromide (NaBr) and sodium hypochlorite (NaClO) ca. 15% (w/w) were purchased from Sigma-Aldrich (Oakville, Canada). The concentration of sodium hypochlorite solution was determined by iodometry as follows: the commercial solution is diluted 10-fold then 5 mL of this solution is mixed with 25 mL of deionized water and 10 mL of a 100 g L−1 potassium iodide solution. A few drops of concentrated sulfuric acid are added to the mixture, followed by titration of released iodine with 0.1 M sodium thiosulfate solution. All chemicals were of commercial grade and used without further purification. Deionized water was used for all experiments.
Acid hydrolysis of wood pulp and preparation of CNCs suspensions
Aqueous CNC suspensions were prepared in the FPInnovations’ pilot plant from a dry commercial bleached softwood kraft pulp according to a procedure modified from the literature (Dong et al. 1998). Milled dried pulp (2 kg o.d.) was added to 64 wt% sulfuric acid (17.5 L) heated to 45 °C with mixing at 200 rpm for 25 min. The reaction was quenched with deionized water (200 L) at room temperature, centrifuged and subjected to multiple passes through a hollow fibre membrane to remove acid and soluble carbohydrates and increase the solid content of the CNC suspension which was then homogenized and filtered (1 μm) to ensure a good dispersion. The final pH of the purified suspension was 2.5 and the solid content was around 3.40% (w/w) as measured by gravimetry. The sulfate half-ester content was 214 ± 10 mmol kg−1 CNC as measured by ICP-AES and the carboxylate content was 30 ± 5 mmol kg−1 CNC as determined by conductometric titration.
TEMPO-mediated oxidation of CNCs
The generally accepted oxidation mechanism of cellulose by the TEMPO/NaBr/NaClO system (Bragd et al. 2000; Sun et al. 2005; Saito et al. 2006) is shown in Scheme 1. The substrate to be oxidized is represented by the formula RCH2OH, corresponding to CNCs in our case. The first step is the in situ formation of the hypobromite ion (BrO−) from the hypochlorite ion (ClO−). The second step is the formation of the nitrosonium salt (2) from the TEMPO radical (1) by the hypobromite ion. The hypobromite and nitrosonium ions are the active oxidizing species in the process. The third step is the oxidation of the primary alcohol group into a sodium carboxylate by the nitrosonium ion releasing hydroxylamine (3) in the reaction medium. The reaction of the hydroxylamine (3) with the nitrosonium ion (2) allows the continuous regeneration of the TEMPO radical (1). The oxidation of the primary alcohol group proceeds via a reactive aldehyde intermediate to the carboxylate final moieties: 2 mol of NaClO are required for the oxidation of one mole of primary alcohol. It is important to note that the amount of the primary oxidant NaClO introduced determines the conversion yield of the reaction. The optimum pH for the selective TEMPO/NaBr/NaClO-mediated oxidation of primary alcohol groups in the presence of sodium bromide is around 10 (de Nooy et al. 1995a). The TEMPO-mediated oxidation can also be carried out under acidic conditions leading to a decrease in the selectivity as the oxidation rates of primary and secondary alcohol groups become comparable (de Nooy et al. 1995b). When carried out under neutral conditions using the TEMPO/NaClO/NaClO2 system, the selectivity is maintained (Saito et al. 2009).
Experiments were carried out according to literature references with minor modifications (de Nooy et al. 1994; Habibi et al. 2006). Never-dried CNC samples (1 g o.d., ~6.17 mmol total anhydroglucose units) were diluted in 100 mL of deionized water and suspensions were sonicated using a Sonics vibra-cell™ 130-watt 20-kHz sonifier with a 6-mm diameter probe at 60% amplitude (8 watts). TEMPO radical (29 mg, 0.185 mmol) and sodium bromide (319 mg, 3.10 mmol) were added to the suspension under constant mechanical stirring at room temperature unless otherwise specified. An amount of sodium hypochlorite solution (previously adjusted to pH 10 with dilute HCl, 1.49 M), corresponding to 0.1–2.0 mol of NaClO/mol primary alcohol group (Table 1), was then added by 1 mL fractions. The pH was continuously adjusted at 10 by addition of 0.2 M NaOH until no more variation was observed (ca. 30 min). Time and NaOH consumption were recorded. Methanol (2 mL) was finally added to react with excess NaClO in order to quench the reaction. Then, pH was adjusted to 7 with 0.1 M HCl. Oxidized CNC suspensions were transferred into dialysis membrane tubes (Spectrum Laboratories Inc., CA, USA) having a molecular weight cut-off of 12,000-14,000 Da and were dialyzed against deionized water for at least 3 days. The concentration of the final suspensions was determined by gravimetry and ultimately used to determine the mass recovery ratios. The slight increase in the average molecular weight of the CNCs due to the introduction of carboxylate groups has been taken into account.
Counterion-exchange treatment
Oxidized CNC suspensions were treated to convert sodium carboxylate and sulfate half-ester groups into their protonated acid form. Aqueous suspensions were placed over Dowex® Marathon C™ cation-exchange resin and gently stirred for at least 2 h at room temperature. Resin beads were then removed by filtration with Whatman GF/F glass microfibre filters (pore size 0.7 µm). CNC-COONa samples converted into their acid form will be further referred as CNC-COOH samples. The sulfur content of the CNCs and TEMPO-oxidized CNC samples are reported in Table 2. The content of sulfate half-ester groups shows a marginal decrease when the amount of primary oxidant is increased (i.e., the amount of carboxylate introduced) which seems to indicate the preservation of the crystal surface integrity, assuming that the sulfate-half ester groups are located at the surface of the material.
Conductometric titration
The carboxyl content of the oxidized CNC samples was determined using conductometric titration according to a method derived for the titration of cellulosic fibres (Katz et al. 1984). The CNC-COOH suspensions containing 0.15 g of solid content were titrated with 0.01 M NaOH using a 809 Titrando automatic titrator (Metrohm, Canada) in the presence of 1.0 mM NaCl. Typical titration curves (Fig. 1) exhibit two discontinuities assigned to the presence of a strong acid (i.e. sulfate half-ester groups introduced during the CNC production process) and a weak acid (i.e. carboxylic acid groups introduced during the TEMPO-mediated oxidation reaction). Increasing the content in carboxylic acid groups has a significant effect on the dissociation constants. When the difference in the dissociation constants of the two types of acids becomes smaller, their differentiation gets more complex. Practically speaking, increasing the acid strength leads to an increase of proton dissociation of the weak acid which in turn will cause the curvature near the first equivalence point to get more pronounced. It becomes then impossible to graphically determine the first equivalence point. One has to rely on an alternative method such as elemental analysis (Inductively Coupled Plasma Atomic Emission Spectroscopy, ICP-AES) to get an accurate value of the sulfur content, thus the strong acid content. Therefore, both the sulfate half-ester content and the carboxyl content of the sample can be given by the following equations:
Vi is the volume of NaOH (in mL), CNaOH is the exact NaOH concentration (in mol L−1) and mCNCs is the dry weight of the sample (in kg).
Diffuse reflectance infrared fourier transform spectroscopy (DRIFT)
Freeze-dried samples of CNC-COOH were preferred to CNC-COONa to avoid the overlap of carbonyl and the adsorbed water bands (~1650 cm−1). Samples were ground in a steel ball vibratory mill (Wig-L-Bug, Crescent Co.) for 30 s with dried potassium bromide (KBr, FTIR grade, Sigma-Aldrich). The final concentration in CNCs/KBr samples was 2–3% (w/w). Samples were loaded into the DRIFT cup and the surface was gently leveled off using a razor blade. The reference spectrum was obtained with pure ground KBr. DRIFT measurements were carried out with a Nicolet 6700 spectrometer (Thermo Scientific, USA) equipped with a Smart Diffuse Reflectance unit. The spectra were recorded in the 4000–400 cm−1 wavenumber range at a resolution of 8 cm−1, and 200 scans were averaged. The spectral intensities were expressed in Kubelka–Munk units and were normalized to the intensity of the 1150 cm−1 band.
Field emission scanning electron microscopy (FE-SEM)
After sonication up to 1000 J/g CNC using a Sonics vibra-cell™ 130-watt 20-kHz sonifier with a 6-mm diameter probe at 60% amplitude, one drop of aqueous CNC-COOH suspension (0.001% w/w) was deposited onto a hydrophilic and positively charged carbon-coated grid (NanoPlus™, Dune Sciences, USA). The drop was left in contact with the grid for 15 min, time for the negatively charged CNCs particles to be adsorbed onto the grid, the remaining liquid was then absorbed with blotting paper. A drop of a 2% uranyl acetate solution was deposited onto the grid for 2 min then the excess solution was absorbed with blotting paper and the remaining thin liquid film was left to air-dry for at least 1 h. The grids were analyzed in the transmission mode with a Hitachi SU-70 Field Emission Gun-Scanning Electron Microscope (FE-SEM) operated at an accelerating voltage of 10–20 keV. At least twenty micrographs were recorded and 500 objects were counted to get a representative distribution profile for both width and length. The determination of the CNC particle size was done using Image Pro analysis software. Only the well individualized CNC particles were measured and added to the inventory.
Thermogravimetric analysis (TGA)
Freeze-dried samples were analyzed with a Q5000IR thermogravimetric analyzer (TA Instruments, New Castle, USA). About 15 mg of sample was set in a platinum pan. Experiments were carried out under an inert atmosphere of nitrogen at a constant flow rate of 25 mL min−1. TG curves were recorded between 45 and 600 °C at a heating rate of 10 °C min−1.
Results and discussion
Oxidation of cellulose nanocrystals
Effect of reaction temperature on oxidation kinetics
Figure 2 shows the kinetics of carboxylate group formation at 4 and 25 °C for the CNCs/NaBr/NaClO/TEMPO system. The molar amount of NaClO was 0.4 mol/mol of total primary alcohols of CNCs. It is known that the oxidation of the alcohol to the aldehyde intermediate (Scheme 1) is the rate-determining step since the oxidation of the aldehyde to the carboxylic acid proceeds more rapidly (de Nooy et al. 1995b). The formation of carboxylic acid groups can be monitored online by the amount of sodium hydroxide consumed throughout the oxidation reaction. Even if not perfectly accurate, this technique is a straightforward and convenient way to monitor the total carboxylate group formation as a function of oxidation time. At time zero, the initial acid content (214 mmol kg−1) corresponds to the amount of sulfate half-ester groups grafted onto the surface during the acid hydrolysis of the bleached wood pulp.
Earlier publication on the oxidation kinetics of regenerated cellulose shows that the rate constants significantly increase with reaction temperature (Sun et al. 2005). As expected, a higher reaction temperature leads to faster oxidation of CNCs.
At 4 °C (ice bath), a short induction period precedes the carboxylic acid formation. This is probably due to the buildup of the nitrosonium ion from TEMPO radical and subsequent formation of the aldehyde intermediate (de Nooy et al. 1995b). At room temperature, no induction period is observed and the total acid content reaches a plateau that levels off at around 1500 mmol kg−1 within 10 min reaction time.
The rate constant for the oxidation of primary hydroxyl groups was calculated using Eq. (3) assuming that the nitrosonium ion and the aldehyde intermediate concentration to be constant (de Nooy et al. 1995b).
where [OH0] and [COOHt] represent the concentration of primary hydroxyl groups at time zero and the concentration of carboxyl groups at time t, respectively.
After integration and further simplification, Eq. (3) leads to Eq. (4). The rate constant k can then be easily determined from the plot ln([OH0] − [COOH t ]) versus time.
The plot of ln([OH]0 − [COOH]t) versus time (Fig. 3) clearly indicates that the carboxylate formation is first order with respect to the substrate at 25 °C, as previously reported (Bragd et al. 2000, Sun et al. 2005), with a first order rate constant kobs of 3.1 × 10−4 s−1. At 4 °C, the reaction rate is rapid at the beginning with a rate constant of kobs of 1.4 × 10−4 s−1 and then slows down after 10 min. Deviations from the first-order kinetics suggest a slower regeneration of TEMPO radical at low temperature conditions.
Estimation of CNC particle size squared cross-section
For simplification, the cross-section of CNC particles can be modeled as squares with an averaged dimension of 5 nm in width, value that was measured experimentally by FE-SEM imaging. The two lattice parameters of the unit cell of cellulose \({\text{I}}\beta\) are a = 0.801 nm and b = 0.817 nm (Sugiyama et al. 1991). In this model the spacing between the planes \(\left( {1\overline{1} 0} \right)\) and (110) are 0.61 and 0.54 nm, respectively. The total number of cellulose chains (n) packed side-to-side in a single crystal is equal to (5 × 5)/(0.61 × 0.54) = 76. Consequently, the number of cellulose chains per side is \(\sqrt n\).
Consequently, each side of the crystal cross-section is made of 8.7 cellulose chains packed side-to-side. The ratio of the surface can be calculated by the following equation (Montanari et al. 2005):
Assuming only half of the primary hydroxyl groups are accessible to the oxidation, the other half being buried inside the crystalline core of CNCs due to the two-fold screw axis of the cellulose chain, about 20% of the total anhydroglucose units can be oxidized leading to a theoretical carboxylic acid content around 1200 mmol kg−1 which is in good agreement with the experimental value of 1089 and 1240 mmol kg−1 obtained. The total sulfur content of the material slightly decreases as the content of carboxylic groups introduced is increased (Table 2). Since the sulfate half-ester groups are located at the surface of the crystals, it seems reasonable to believe that the integrity of the crystals is mostly preserved. This will be discussed later. Since the TEMPO-mediated oxidation of CNCs is faster at room temperature than at low temperature, we deliberately set the reaction temperature at 25 °C in the subsequent experiments.
Effect of co-catalyst (NaBr) amount on oxidation kinetics
As depicted in Scheme 1, TEMPO-mediated oxidation reaction uses NaBr as a co-catalyst which allows the regeneration of the nitrosonium ion when used in combination with NaClO. NaBr (5–30% w/w with respect to the substrate) is used to accelerate the reaction rate.
Figure 4 shows the kinetics of carboxylate group formation as a function of the NaBr co-catalyst amount. As expected, the conversion yield is not dependent on the amount of NaBr (in the range of 0.10–1 mol NaBr/mol primary alcohol) as it systematically leads to a total acid content (both strong and weak acids) around 1500 mmol kg−1. The NaBr-free oxidation kinetics curve at room temperature is similar to the NaBr-catalyzed reaction at low temperature (Fig. 2). An increase in temperature would allow the bromide-free reaction to proceed faster but it is known that high temperature negatively affects the TEMPO-catalyzed oxidation, resulting in a severe damage/degradation of the cellulose chains. In the case of the bromide-free oxidation, the nitrosonium ion is generated by direct activation of TEMPO radical by the hypochlorite ion and the reaction was proven to be efficient on starch and monosaccharide derivatives provided the temperature is kept below 20 °C and pH around 8.5 (Bragd et al. 2000).
Figure 5 shows that the oxidation rate is clearly driven by the amount of the co-catalyst introduced. A linear relationship is observed in the range 0–0.25 mol/mol primary alcohol groups which slope is less pronounced when the amount of NaBr is further increased. These results are consistent with those reported on the oxidation of rayon (Sun et al. 2005).
Effect of primary oxidant (NaClO) amount on reaction kinetics
Figure 6 shows the kinetics of carboxylate group formation for various amounts of NaClO (from 0.3 to 0.6 mol/mol primary alcohol groups, Table 1). An increase in the amount of NaClO introduced leads to the formation of a greater number of carboxylate groups. In all cases, the oxidation reaction is fast and completed in less than 25 min at the highest charge of NaClO. From Fig. 6, we can state that the reaction proceeds in two steps. Up to a carboxylate content of ~1200 mmol kg−1, only the surface primary alcohol groups are oxidized and the reaction kinetics is rapid. It then slows down, suggesting a lower accessibility of the primary alcohol groups and/or a progressive degradation of the cellulose crystals due to the penetration of reagents within the crystalline core of CNCs. The latter was confirmed by examining the reaction yield (Fig. 7). In the range 0–0.4 mol NaClO/mol primary alcohol, the mass recovery ratios were all above 92% and decreased almost linearly as a function of the amount of NaClO introduced. For excess amounts of NaClO above 0.4 mol/mol primary alcohol, the mass recovery ratio quickly decreases down to 80%. Mass loss during oxidation is more pronounced at room temperature.
Figure 8 shows the evolution of the total acid content of CNCs as a function of the amount of primary oxidant introduced. Reaction times were 30 min at 25 °C and 90 min at 4 °C. The total acid content was determined by conductometric titration after dialysis. At low temperature conditions and for concentration of NaClO below 0.4 mol/mol primary alcohol, the total acid content linearly increases with the amount of primary oxidant then reaches a ‘pseudo’-plateau which value is closely related to the total number of surface alcohol groups accessible. When the oxidation reaction is carried out at room temperature, the total acid content never reaches a plateau and continues to linearly increase. This suggests that the oxidizing species can penetrate further in the core of the crystal and/or progressively digesting it and releasing individualized soluble celluronic acid chains in the media. This result is quite different from what was observed with HCl-hydrolyzed CNCs originated from tunicates (Habibi et al. 2006), cotton linters and sugar beet pulp (Montanari et al. 2005). CNCs prepared from hydrochloric acid hydrolysis do not possess sulfate half-ester groups at the surface of the crystals which could explain the difference in behaviour towards TEMPO-mediated oxidation at room temperature. Also, it is known that room temperature conditions can favor side-reactions to occur compared to low temperature conditions (Isogai and Kato 1998). This fact was confirmed by examining the total CNC mass recovered after oxidation (Fig. 7).
Characterization of oxidized cellulose nanocrystals
Field emission scanning electron microscopy
Figure 9 shows FE-SEM micrographs in transmission mode of CNCs before and after the TEMPO-mediated oxidation with 0.4 mol NaClO/mol primary alcohol groups. No significant changes in the morphology were observed. However, the degree of agglomeration seems to be lowered after oxidation (Fig. 9b). This phenomenon can result from greater electrostatic repulsion forces as the number of negative surface charges increases. In both cases, we can observe a mixture of individual and agglomerates/aggregates particles. These micrographs also confirm the fact that the TEMPO-mediated oxidation is mild enough to preserve the integrity and shape of the crystals and introduce carboxylate groups only at their surface, as previously stated (Montanari et al. 2005; Habibi et al. 2006).
Therefore this can give some information about the number of primary hydroxyl groups located at the surface and allows to indirectly retrieve the averaged size of the crystal (Okita et al. 2010). Using Eq. 5 and assuming that (1) only one-half of the primary hydroxyl groups can be oxidized and (2) the width of the crystal is equal to \(\sqrt {0.54 \times 0.61 \times n}\), a theoretical curve of the maximum carboxyl content can be plotted as a function of the width of the crystal, as shown in Fig. 10.
When oxidations were carried out at 4 °C, we observed that a maximum total acid content of 1100 mmol kg−1 was achievable (obtained by conductometric titration). Assuming that all accessible primary hydroxyl groups [corresponding to one-half of the surface primary hydroxyl groups (Montanari et al. 2005)] have been converted into carboxylate groups, this value can be correlated with a calculated crystal lateral size (or squared cross-section) of 5.8 nm, which is similar to the average size measured from the FE-SEM images.
The average length of the TEMPO-mediated oxidized crystals is 94 ± 32 nm which is close to the average value before oxidation of 102 ± 46 nm meaning that the degradation of the crystals along its main axis is negligible during the TEMPO-oxidation process.
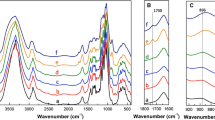
Diffuse reflectance infrared spectroscopy
Some changes in the infrared spectrum can be easily observed when CNC surface cellulose chains are selectively oxidized, as shown in Fig. 11a. The most visible change is the appearance of a carbonyl (C=O) stretching vibration at 1608 cm−1 (CNC-COONa) or 1731 cm−1 (CNC-COOH) which can be used for quantification purposes. Oxidized CNC samples require a conversion into the protonated acidic form prior to analysis to avoid the carbonyl band to overlap with that of bound water at 1660 cm−1. The intensity of reflectance of the 1731 cm−1 band expressed in Kubelka–Munk units can be linearly correlated with the total acid content as determined by conductometric titration as shown in Fig. 11b.
Thermogravimetric analysis
Figure 12 shows the thermogravimetric curves of CNCs before and after oxidation; the total acid contents are 220 and 1209 mmol kg−1, respectively. The weight loss starting below 100 °C corresponds to the evaporation of adsorbed (bulk) water. Before oxidation (blue curves), it has been proven that even a low level of protonated sulfate half-ester groups can catalyze the depolymerization of the CNCs which is followed by dehydration to volatile levoglucosan (Roman and Winter 2004). Acid forms of CNCs and oxidized CNCs (CNC-H and CNC-COOH, respectively) exhibit the same thermal behavior (blue and black dotted lines). This is an indication that the surface sulfate half-ester groups are the sole responsible for this significantly lower thermal stability as compared to the sodium form, indicating that the COOH groups do not catalyze the thermal depolymerisation like protonated sulfate half-ester groups do. However, the carboxylate groups on CNCs (CNC-COONa) induce decreased thermal stability although to a lesser extent than the acid form. This may be due to decarbonation (Fukuzumi et al. 2010), which happens at a higher temperature than the protonated sulfate half-ester-catalyzed thermal degradation.
Figure 13 shows the thermogravimetric curves of CNCs (sodium form) before and after oxidation with increasing amounts of primary oxidant. The total acid contents were 220, 417, 876, 1209 mmol kg−1. It is clearly seen that an increase in the carboxyl content caused a gradual decrease in the thermal stability of the oxidized CNC samples from 265 to 200 °C (at a heating rate of 10 °C min−1 under nitrogen atmosphere). The mass loss shift towards lower temperatures is typical of chemically modified cellulose (Varma and Chavan 1995).
Conclusion
The TEMPO-mediated oxidation of cellulose nanocrystals produces a variety of oxidized samples. Reaction temperature increases oxidation rate but does not have a huge impact on the final total acid content provided that the primary oxidant is not introduced in excess in respect of the surface primary hydroxyl groups (<0.4 mol NaClO/mol primary alcohol groups). Electron microscopy evidences that the oxidation conditions were sufficiently mild to retain the morphology and size of the crystals. The carboxylic acid content can be easily predicted because the reaction is stoichiometric and depends only on the amount of primary oxidant introduced. As a consequence, the amount of carboxylate groups can be tuned to a target value simply by adjusting the quantities of reagents. The total acid content of fully oxidized CNCs allowed us to estimate the dimensions of the CNC particle cross section. The data obtained were in good agreement with those obtained by imaging techniques. Oxidized CNCs have been proved to be less thermally stable than the raw CNCs which may limit their use at high temperature processing conditions, unless further modification is done.
This work has demonstrated some potential for an industrial scale up of the TEMPO-mediated oxidation, even at room temperature. TEMPO-oxidized CNCs can be viewed as a more reactive precursor than raw CNCs for further use in subsequent compatibilization processes.
References
Araki J, Wada M, Kuga S (2001) Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 17:21–27
Azzam F, Heux L, Putaux JL, Jean B (2010) Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromol 11:3652–3659
Bragd PL, Besemer AC, van Bekkum H (2000) Bromide-free TEMPO-mediated oxidation of primary alcohol groups in starch and methyl-alpha-D-glucopyranoside. Carbohydr Res 328:355–363
Bragd PL, Besemer AC, van Bekkum H (2002) Selective oxidation of carbohydrates by 4-AcNH-TEMPO/peracid systems. Carbohydr Polym 49:397–406
Chang PS, Robyt JF (1996) Oxidation of primary alcohol groups of naturally occurring polysaccharides with 2,2,6,6, tetramethyl-1-piperidine oxoammonium ion. J Carbohydr Chem 15:819–830
Da Silva Perez D, Montanari S, Vignon MR (2003) TEMPO-mediated oxidation of cellulose III. Biomacromol 4:1417–1425
de Nooy AEJ, Besemer AC, van Bekkum H (1994) Highly selective TEMPO mediated oxidation of primary alcohol groups in polysaccharides. Recl Trav Chim Pays Bas 113:165–166
de Nooy AEJ, Besemer AC, van Bekkum H (1995a) Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr Res 269:89–98
de Nooy AEJ, Besemer AC, van Bekkum H (1995b) Selective oxidation of primary alcohols mediated by nitroxyl radical in aqeous solution. Kinetics and mechanism. Tetrahedron 51:8023–8032
de Nooy AEJ, Besemer AC, van Bekkum H, van Dijk JAPP, Smit JAM (1996) TEMPO-mediated oxidation of pullulan and influence of ionic strength and linear charge density on the dimensions of the obtained polyelectrolyte chains. Macromolecules 29:6541–6547
Dong XM, Revol JF, Gray DG (1998) Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 5:19–32
Dong XM, Kimura T, Revol JF, Gray DG (1996) Effects of ionic strength on the isotropic–chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 12(8):2076–2082. doi:10.1021/la950133b
Fraschini C, Vignon M (2000) Selective oxidation of primary hydroxyl groups of β-cyclodextrins mediated by 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO). Carbohydr Res 328:585–589
Fukuzumi H, Saito T, Iwaka T, Kumamoto Y, Isogai A (2009) Transparent and high gas barrier properties of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromol 10:162–165
Fukuzumi H, Saito T, Okita Y, Isogai A (2010) Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab 95:1502–1508
Habibi Y, Vignon M (2008) Optimization of cellouronic acid synthesis by TEMPO-mediated oxidation of cellulose III from sugar beet pulp. Cellulose 15:177–185
Habibi Y, Chanzy H, Vignon M (2006) TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 13:679–687
Inokuchi T, Matsumoto S, Nishiyama T, Torii S (1990) A selective and efficient method for alcohol oxidations mediated by N-oxoammonium salts in combination with sodium bromite. J Org Chem 55:462–466
Isogai A, Kato Y (1998) Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 5:153–164
Kato Y, Matsuo R, Isogai A (2002) Oxidation process of water-soluble starch in TEMPO-mediated system. Carbohydr Polym 51:69–75
Kato Y, Kaminaga JI, Matsuo R, Isogai A (2005) Oxygen permeability and biodegradability of polyuronic acids prepared from polysaccharides by TEMPO-mediated oxidation. J Polym Environ 13:261–266
Katz S, Beatson RP, Scallan AM (1984) The determination of strong and weak acidic groups in sulfite pulps. Svensk Paperstidn 6:48–53
Montanari S, Roumani M, Heux L, Vignon MR (2005) Topochemistry of carboxylated cellulose nanocrystals resulting from TEMPO-mediated oxidation. Macromolecules 38:1665–1671
Okita Y, Saito T, Isogai A (2010) Entire surface oxidation of various cellulose microfibrils by TEMPO-mediated oxidation. Biomacromol 11:1696–1700
Revol JF, Bradford H, Giasson J, Marchessault RH, Gray DG (1992) Helicoidal self-ordering of cellulose microfibrils in aqueous suspensions. Int J Biol Macromol 14:170–172
Roman M, Winter WT (2004) Effect of sulfate groups from sulfuric hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 5:1671–1677
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on the chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 5:1983–1989
Saito T, Shibata I, Isogai A, Suguri N, Sumikawa N (2005) Distribution of carboxylate groups introduced into cotton linters by the TEMPO-mediated oxidation. Carbohydr Polym 61:414–419
Saito T, Okita Y, Nge TT, Sugiyama J, Isogai A (2006) TEMPO-mediated oxidation of native cellulose: microscopic analysis of fibrous fraction in the oxidized products. Carbohydr Polym 65:435–440
Saito T, Hirota M, Tamura N, Fukuzumi H, Kimura T, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 10:1992–1996
Saito T, Hirota M, Tamura N, Isogai A (2010) Oxidation of bleached wood pulp by TEMPO/NaClO/NaClO2 system: effect of the oxidation conditions on carboxylate content and degree of polymerization. J Wood Sci 56:227–232
Semmelhack MF, Schmid CR, Cortes DA, Chou CS (1984) Oxidation of alcohols to aldehydes with oxygen and cupric ion, mediated by nitrosonium ion. J Am Chem Soc 106:3374–3376
Sugiyama J, Vuong R, Chanzy H (1991) Electron diffraction study on the two crystalline phases occurring in native cellulose from an algal cell wall. Macromolecules 24:4168–4175
Sun B, Gu C, Ma J, Liang B (2005) Kinetic study on TEMPO-mediated oxidation of regenerated cellulose. Cellulose 12:59–66
Tahiri C, Vignon M (2000) TEMPO-oxidation of cellulose: synthesis and characterization of polyglucuronans. Cellulose 7:177–188
Varma AJ, Chavan VB (1995) Thermal properties of oxidized cellulose. Cellulose 2:41–49
Zhao M, Li J, Mano E, Song Z, Tschaen DM, Grabowski EJJ, Reider PJ (1999) Oxidation of primary alcohols to carboxylic acids with sodium chlorite catalyzed by TEMPO and bleach. J Org Chem 64:2564–2566
Acknowledgments
The authors would like to acknowledge the financial support of the ArboraNano Network, the Transformative Technology Program from the Government of Canada and the Ministère des Ressources Naturelles et de la Faune du Québec.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraschini, C., Chauve, G. & Bouchard, J. TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 24, 2775–2790 (2017). https://doi.org/10.1007/s10570-017-1319-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1319-5