Abstract
The gas barrier and mechanical properties are crucial parameters for packaging materials, and they are highly correlated to the molecular interactions in the polymer matrix. To improve these properties of TEMPO-oxidized cellulose nanofibers (TOCNs) composite films, we studied the effect using hydroxypropyl guar (HPG) or carboxymethyl guar (CMG) in the preparation of TOCN composite films, which were made by following the solution-casting method. The subsequent film characterizations were carried out by UV–Vis spectra, scanning electron microscopy, oxygen and water vapor permeability measurements, tensile and thermogravimetric analyses. SEM results showed that CMG-based films had denser structures than their HPG counterparts. Moreover, the improved hydrogen bonding of the CMG-based films was partially responsible for the improved gas barrier performance, tensile strength and thermal stability. These results support the conclusion that CMG had advantages over HPG when used in the preparation of TOCNs packaging composite films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, plastics dominates food packaging materials (Ghanbarzadeh et al. 2015). Bio-based materials, due to their environmental advantages, have received much attention. Nanocellulose is a promising nanomaterial having potential applications in many industries such as food, paper and medicine (Hosseinidoust et al. 2015; Klemm et al. 2011). TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl)-mediated oxidation is a well-studied method to manufacture individualized nanocellulose, known as TEMPO-oxidized cellulose nanofibers (TOCNs) with the width of 3–5 nm (Saito et al. 2006, 2009). Literature results (Fukuzumi et al. 2009; Rodionova et al. 2012; Wu et al. 2014) showed that the transparent TOCNs films possessed good mechanical strength and oxygen-barrier property, which rendered them interesting as packaging materials. Nevertheless, due to the hydrophilic characteristic of cellulose nanofiber, the pristine films have poor water vapor barrier property. Much work, including hydrophobic treatment, has been done to improve their water vapor resistance (Osterberg et al. 2013; Pan et al. 2013).
Guar gum, a natural biodegradable polysaccharide consisting of polymannan backbone with galactose side groups, has wide applications in different industrial sectors (Grzadka 2013, 2014). Previous study indicated that guar could effectively trap and retain water molecules within the matrix (Rosiaux et al. 2013). Moreover, guar coating was effective to improve the shelf life of the fresh kinnow fruit through controlling the dehydration process (Shah et al. 2015). In an early study (Dai et al. 2015), it was shown that hydroxypropyl guar (HPG) can enhance the water vapor barrier property of TOCN composite films. Specifically, the water vapor permeability (WVP) of 100 % TOCNs film was 1.17 × 10−5 g m/(m2 day Pa) while the WVP of 50 % HPG/50 % TOCN composite film was 0.70 × 10−5 g m/(m2 day Pa).
It is well known that molecular interactions (e.g. hydrogen bonding capacity) of substituents in the matrix play a very important role in materials properties, especially for composite materials (Kumar and Singh 2008; Mao et al. 2008; Way et al. 2012). Furthermore, intra- or inter-molecular hydrogen bonding always affects the miscibility, strength and other properties of the resultant films (Fujisawa et al. 2011; Zhou et al. 2012). Therefore, in this research, two modified guar samples, namely: hydroxypropyl guar (HPG) and carboxymethyl guar (CMG) were used to study their effect on properties of guar/TOCN composite films. Special attention was paid to the water vapor barrier property. The hypothesis was that the presence of more hydrogen bonding capacities in the modified guar will lead to more compact structures of the resultant composite films, thus exhibiting positive effect on the water vapor barrier property. Furthermore, due to the fact that carboxyl groups in CMG have stronger hydrogen bonding capacity than the hydroxyl groups in HPG, it would be expected that the CMG-based films will have more enhanced properties than the HPG-based films. The composite films were characterized by tensile strength, oxygen barrier, water vapor barrier, and other fundamental properties, e.g., thermal stability and elongation of the films were also investigated.
Experimental
Materials
A commercial bleached softwood kraft pulp was kindly provided by a local pulp mill. Hydroxypropyl guar (HPG) and carboxymethyl guar (CMG) (properties are shown in Table 1) were from Wuxi Jinxin Group Co., Ltd. (Wuxi, China). All other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without purification.
Preparation of TOCNs
The pulp was pretreated with 5 % (v/v) formic acid aqueous solution to improve the efficiency of TEMPO-mediated oxidation (Dai et al. 2014). Afterwards, 5 g pretreated pulp was dispersed in a solution containing 0.10 g TEMPO, 1.00 g NaBr, 3.71 g Na2CO3 and 1.26 g NaHCO3. 22.5 mmol NaClO (pH was adjusted to 10.5 with dilute HCl solution) was added to the suspension to start the TEMPO-mediated oxidation. Specifically, Na2CO3 and NaHCO3 were used to prepare a buffer solution to control the pH value of the system during the oxidation treatment. After 4 h, the reaction was quenched by adding 10 mL ethanol and the resultant oxidized cellulose was thoroughly washed. Subsequently, the TEMPO-oxidized cellulose with carboxylate content of 1.34 mmol/g was further homogenized to obtain the TOCNs sample. The obtained TOCNs possessed the diameters of 5–10 nm and lengths of 400–700 nm.
Preparation of guar/TOCNs films
10 g HPG powder was dispersed in 1 L deionized water at room temperature with constant stirring (300 rpm) for 8 h to get a 1 % (w/w) HPG solution. Then, the HPG solution was mixed with TOCNs to prepare HPG/TOCNs mixtures with different mass ratios. The weight ratios of HPG in the consequent blends were 100, 70, 50 and 30 %. Thereafter, each mixture was continually stirred for 3 h and then poured into polytetrafluoroethylene petri dishes to be dried at 50 °C for 3 days. The CMG/TOCN composite films were prepared in the same way as the HPG/TOCNs films except that CMG, instead of HPG, was used. All these films were conditioned at 23 °C and 50 % relative humidity (RH) for 2 days before characterization. The experimental procedures of film preparation are shown in Scheme 1.
UV–Vis transmittance
The transparency of films was determined by following light transmittance measurements in the range of 200 to 800 nm using a UV–Vis spectrometer (Shimadzu UV-1800, Japan), in accordance with a literature procedure (Hayaka Fukuzumi 2009).
SEM characterization
The cross-sections of the films were observed with a SEM (HITACHI SU1510, Japan), operating at 5 kV. All specimens were sputter coated with gold before imaging.
Gas permeability tests
The oxygen permeability (OP) of the films was measured with a Labthink VAC-V1 apparatus (Labthink, China) at 23 °C and a RH of 40 ± 1 %. The sample size was 38.48 cm2 and the partial pressure of oxygen was 0.1 MPa.
The water vapor permeability (WVP) of the films was determined according to a literature procedure (Das et al. 2011; Sharma et al. 2014). Briefly, a sample film with an area of 19.64 cm2 was sealed on a cup containing 10 g of fresh oven-dried CaCl2 as a desiccant. The covered cups were accurately weighed and placed in a desiccator cabinet containing saturated NaCl solution to generate 75 % RH gradient. Each covered cup was reweighted every 24 h until a constant weight was obtained. All measurements were repeated three times and the average was reported.
Tensile test
Tensile strength and elongation were determined on a BZ2.5/TNIS Zwick Material Tester (Zwick, Germany) at room temperature. The initial grip separation of the machine was set at 50 mm and the specimens were loaded at a constant cross-head speed of 50 mm/min. All the film samples were cut into rectangular strips with the size of 10 mm × 100 mm. At least 5 specimens of each film were tested, and the average was reported.
Thermogravimetric analysis (TGA)
TGA of films was performed on a TGA/SDTA 851e thermogravimetric analyzer (Mettler Toledo, Switzerland). The specimens were heated from 25 to 600 °C at a heating rate of 10 °C/min. A nitrogen gas stream was continuously passed into the furnace at the speed of 10 mL/min and the gradual weight loss of the sample was recorded.
Results and discussion
Justification of using HPG and CMG to improve the properties of guar/TOCN composite films
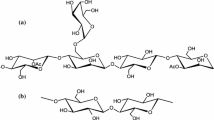
Introducing more hydrogen bonding capacity in the polymer network can lead to the improvements of both tensile strength and gas barrier property. Carboxyl groups in CMG will have stronger hydrogen bonding capacity in the films than hydroxyl groups in HPG. Additionally, for HPG-based films, hydrogen bonds could only be formed between alcoholic hydroxyl groups, while protonated carboxyl groups in the CMG-based films could form hydrogen bonds with both adjacent carboxyl groups and hydroxyl groups. Thus, CMG-based films would have stronger hydrogen bonding than HPG-based ones, which would lead to more compact and denser film structure of the CMG-based films. The schematic representations of hydrogen bonds in guar derivatives/TOCN composite films are depicted in Scheme 2 and 3.
Based on the above analyses, it is expected that CMG-based films would have better mechanical and gas barrier properties than their HPG-based counterparts. Moreover, the enhancement in hydrogen bonding could give rise to higher stiffness, thus lower elongation.
Film transparency
Film transparency can be a factor of great importance to packaging materials because customers are interested in visualizing the inside contents of the packaging. The film transparency results are shown in Fig. 1. It can be seen that either 100 % HPG or 100 % CMG film had a low light transmittance. The addition of TOCNs increased the transparency of both HPG and CMG-based films, and the more TOCNs added, the higher the transparency of the films. The improvement is attributed to the presence of nano-sized TOCNs, because nano-sized particles do not scatter light (Yano et al. 2005). In the literature, Rodionova et al. (2012) studied the properties of films prepared from fibrillated TEMPO-oxidized pulps and found that films with high contents of cellulose nanofibers were highly transparent.
Furthermore, as shown in Fig. 1, the CMG-based films had lower transparency than their HPG-based counterparts. The reason may be due to the denser structure resulting from the stronger hydrogen bonding capacity in CMG-based films, which can increase the light scattering. In preparing nanocellulose films, Yang et al. (2012) found that the transparency of films decreased as the sodium form of the carboxyl groups on cellulose nanofibers was changed to acid form, as a result of increased hydrogen bonding, which will favor interactions of nanocellulose.
Film structures
Figure 2 presents the cross-section pictures of 100 % HPG, and 100 % CMG films, as well as their 50 % composite films. As shown, the polymer matrices are rather uniform, indicating that both of the two guar derivatives were compatible with TOCNs, thanks to the abundant hydrogen bonds. It was also reported by Woehl et al. (2014) that guar gum can spread evenly on the surface of nanocellulose in their study of bioactive films consisting of bacterial cellulose and hydrocolloids (guar gum and hyaluronic acid). In another study of tuning cellulose nanocrystal gelation with polysaccharides, Hu et al. (2014) also reported that HPG can be well adsorbed onto cellulose nanocrystal.
Figure 2 also demonstrates that the voids and cracks in the 50 % HPG/50 % TOCN composite film are much less than those in the 100 % HPG film (the same is true for the 50 % CMG/50 % TOCNs film versus 100 % CMG film), indicating that the addition of TOCNs decreased the formation of voids and cracks in the composite films. Furthermore, the film thickness of the 50 % HPG/50 % TOCNs and the 50 % CMG/50 % TOCNs film were thinner than those of the 100 % HPG or 100 % CMG film. These results indicated that the presence of TOCNs caused denser inner structures of films and it is due to the differences in shrinkage during the drying processes of the composite films: (1) linear polymers, such as cellulose, have more compact structures than the nonlinear polymers with large branches, such as guar (Mikkonen et al. 2010); (2) the addition of TOCNs can result in uniform shrinkage of guar since localized TOCNs can help distribute the stress derived from the evaporation of water. Seantier et al. (2016) studied aerogels made of bleached cellulose fibers and TOCNs, and they found that TOCNs can nucleate on the impurities embedded on the TOCNs. Additionally, the formation of TOCN networks reduced macropores. Cheng et al. (2015) prepared transparent oxygen barrier guar-based films incorporated with nanocrystalline cellulose (NCC) and they also found that the voids in films became smaller upon the addition of NCC.
Figure 2 also indicates that HPG-based films, including both 100 % HPG and 50 % HPG/50 % TOCN composite film, had more and larger pores and cracks than their CMG counterparts. The enhanced molecular interactions of the CMG films were partially responsible for these results. The carboxyl groups in CMG had better hydrogen bonding capacity (between CMG molecules and also between CMG and TOCNs) than HPG, leading to more compact film structures. The –COOH groups in CMG can form stronger hydrogen bonds than –OH groups in HPG, causing thinner CMG-based films and less voids and cracks in comparison with their HPG films. Shimizu et al. (2013) reported that the film densities increased due to the improved hydrogen bonding after heating the TOCN-COONH4 films.
Gas barrier properties
As noted above, the increased hydrogen bonding capacity in guar will lead to more compact structures of the guar/TOCN composite films, thus causing a positive effect on the barrier property. Furthermore, due to the fact that carboxyl groups in CMG have stronger hydrogen bonding capacity than the hydroxyl groups in HPG, it would be expected that the CMG-based films will have better barrier properties than the HPG-based films. Shown in Fig. 3a is the oxygen permeability (OP) data. The OP of the 100 % HPG and CMG films was 8.03 and 7.58 cm3/(m2 day 0.1 MPa), respectively. With the incorporation of 70 % (w/w) TOCNs, the OP of HPG/TOCN and CMG/TOCN films reduced to 5.67 and 5.50 cm3/(m2 day 0.1 MPa), respectively. These results support the conclusion that increasing the TOCN content in guar/TOCN composite films resulted in decreased OP. The OP results were in agreement with the film structure that the addition of TOCNs led to more compact films (Fig. 2). In the literature, it was reported that the galactose side groups in guar molecules can loosen the film structure (Mikkonen et al. 2010), which was responsible for the high OP of the 100 % HPG or CMG films. With the addition of TOCNs, the formed films had more compact structure (Fig. 2); moreover, the presence of TOCNs can lead to a uniform shrinkage of guar during the drying process. Song et al. (2013) reported that the addition of TOCNs to poly(vinyl alcohol) matrix enhanced the interactions of the network by hydrogen bonding, thus improving the gas barrier property.
At a low mass ratio of TOCNs, HPG-based films had higher OP values than their CMG-based films, which was consistent with more porous structures of the HPG films (Fig. 2). Compared with hydroxyl groups in HPG-based films, protonated carboxyl groups in CMG-based films can give rise to stronger hydrogen bonding with other CMG molecules and/or cellulose molecules; in addition, the CMG-based films will undergo a more uniform drying process, resulting in fewer voids and cracks (Fig. 2). The above is especially true for the 100 % HPG and CMG films. The big difference in their inner structures can be credited to the role of carboxyl groups in the CMG film structures. As discussed above, the CMG-based films had a higher density than that of the corresponding HPG-based films. A higher film density can lead to a better oxygen barrier performance (Shimizu et al. 2016).
Due to the hydrophilic nature of polysaccharide-based films, water vapor permeability (WVP) is a challenge issue. During the WVP testing, the adsorption of moisture can cause the partial cleavage of internal hydrogen bonds, increasing WVP. For the HPG-based films (Fig. 3b), the addition of TOCNs increased the WVP from 5.33 × 10−6 g m/(m2 day Pa) for the 100 % HPG film to 8.69 × 10−6 g m/(m2 day Pa) for the 30 % HPG/70 % TOCN composite film, an increase of about 63 %. For the CMG-based films it increased from 5.56 × 10−6 (100 % CMG film) to 7.75 × 10−6 g m/(m2 day Pa) (30 % CMG/70 % TOCNs), an increase of about 39 %. The increased WVP results of the guar/TOCN composite films with the increasing TOCN content can be explained as follows: guar films will swell upon the water vapor adsorption, a higher TOCN content means a lower guar presence in the film, which will result in less swelling, in turn, less plugging of the pores in the film, thus a higher WVP.
As depicted in Fig. 3b, the WVP of the 100 % HPG film was 5.33 × 10−6 g m/(m2 day Pa), which was lower than the 100 % CMG film’s 5.56 × 10−6 g m/(m2 day Pa). The explanation is: the stronger interactions of molecular chains in the CMG film will render it less accessible to water, thus less guar swelling, less plugging, thus higher WVP. The adsorption of water is affected by several factors, such as presence of hydrophilic groups and pore size (Abdel-Halim and Al-Deyab 2014), a denser CMG film structure will lead to less intermolecular space inside the film to accommodate water molecules, thus less swelling.
It is noted in Fig. 3 that with more TOCNs in the composite films, their WVP increased for both HPG and CMG-based films, but it was less pronounced for the CMG films. The explanations are: (1) the compact structure as a result of the improved hydrogen bonding from CMG came into play. Due to the fact that stronger hydrogen bonding capacity of CMG, the CMG/TOCN composite films showed a better water vapor barrier performance than the HPG/TOCNs films. (2) The decreased network voids of the CMG/TOCNs films (Fig. 2) in comparison with the HPG/TOCNs films can be another factor. (3) The enhanced intermolecular associations in CMG/TOCN composite films will limit the molecular mobility, which is another reason for the lower WVP of the CMG/TOCN composite films, in comparison with the HPG/TOCN composite films. This is in accordance with the study by Mikkonen et al. (2010) that a lower molecular mobility in the film matrix would lead to lower WVP.
Mechanical properties
As shown in Fig. 4a, the tensile strength of the films increased with the addition of TOCNs, and this improvement is more pronounced at a higher weight ratio of TOCNs. For instance, in the case of HPG-based films, the addition of 30 % (w/w) TOCNs doubled the tensile strength in reference to the 100 % HPG film. For CMG-based films, the 100 % CMG film had a tensile strength of 51.17 MPa while the 70 % CMG/30 % TOCN composite film showed a much higher tensile strength of 75.88 MPa. Furthermore, at the weight ratio of 70 % TOCNs, the tensile strength of the 30 % CMG/70 %TOCN composite film reached 96.45 MPa. This enhancement in tensile strength benefits from the reinforcing effect of cellulose nanofibers, which have high tensile strength and large aspect ratio. In fact, nanocellulose has been widely researched as a reinforcing element (Deepa et al. 2011; Liu et al. 2015; Wen et al. 2015).
CMG-based films had a significantly higher tensile strength than that of the HPG-based films, the tensile strength of 100 % HPG film was 19.10 MPa while that of the 100 % CMG film was 51.17 MPa. Such a difference can be credited to the enhanced hydrogen bonding in CMG-based films. Hydrogen bonds like COOH···COOH and COOH···OH in CMG-based films were stronger than those OH···OH hydrogen bonds in HPG-based films. Similar results were obtained in the comparative study of TOCN–COONa and TOCN–COOH films (Fujisawa et al. 2011), the latter had better mechanical properties because of the improved hydrogen bonding induced by protonated carboxyl groups. In addition, the lower moisture content of the CMG-based films, which was due to fewer adsorption sites for water molecules, is another factor contributing to the higher tensile strength since water can be a plasticizer in the films. Besides, as shown in Fig. 2, the CMG-based films had fewer voids and cracks than the HPG-based films, which is partially responsible for the higher tensile strength. Strength is extremely sensitive to the presence of voids because voids can start failure (Svagan et al. 2007).
As to the elongation results, incorporation of TOCNs into the guar matrix had a negative effect. In the case of HPG-based films, the elongation reduced from 7.23 to 4.13 % as the weight ratio of TOCNs increased from 0 to 70 %, while it decreased from 5.23 to 2.48 % for the CMG-based films. On one hand, the addition of TOCNs increased the stiffness of films. On the other hand, the decreased voids/cracks in films were another reason. It was reported (Benitez et al. 2013) that the voids in films played an important role in elongation. Specifically, the small-sized voids could lead to strong capillary forces to hold moisture inside the material, and films with a higher moisture content often exhibit an improved elongation due to water-induced plasticization (Shimizu et al. 2016). As shown in Fig. 2, due to the incorporation of TOCNs, guar/TOCN composite films showed decreased voids and cracks. Meanwhile, the moisture adsorption (not provided) of guar/TOCN composite films also reduced with the addition of TOCNs. An earlier publication also showed an increase in the toughness of cellulose nanofibers film with the addition of guar (Lucenius et al. 2014).
The CMG-based films had consistently lower elongation than the HPG-based films, which is partly attributed to the increased stiffness of the molecular chains in CMG-based films, due to their stronger hydrogen bonding capacity, as discussed earlier. What is more, the higher water content in HPG-based films can increase the elongation.
Thermal stability
As shown in Fig. 5, the samples had similar TG characteristics. The first stage of weight loss within the temperature range of 50–110 °C was due to the moisture evaporation. The weight loss data indicated that HPG-based films had higher moisture content. These results were consistent with the results on WVP and tensile, all of which were in accord with the proposed mechanism that the improved hydrogen bonding capacity in CMG-based films would lead to lower water adsorption. The DTG data showed that the 100 % HPG and 50 % HPG/50 % TOCN composite films had peak decomposition temperature of 270 and 258 °C, respectively, and those for 100 % CMG and 50 % CMG/50 % TOCN composite films were 281 and 276 °C, respectively. Therefore, one can conclude that the CMG-based films had higher thermal stability than the HPG films. Again, these results are in agreement with the fact that CMG-based films had better interactions in the polymer matrices via hydrogen bonding, which will always result in an improved thermal stability of films.
Conclusions
Influence of different guar products, i.e. hydroxypropyl guar (HPG) and carboxymethyl guar (CMG), on the properties of their respective composite films containing various amount of nanocellulose was investigated. The results showed that the interactions of the modified guar molecules with nanocellulose are key factors in determining the properties/characteristics of the resultant films. Due to their stronger hydrogen bonding capacity, the CMG-based films showed better gas barrier, tensile strength and thermal properties than the HPG-based films. The 30 % HPG/70 % TOCN composite film had the WVP value of 8.69 × 10−6 g m/(m2 day Pa) while the 30 % CMG/70 % TOCNs film had the WVP of 7.75 × 10−6 g m/(m2 day Pa). Moreover, the tensile strength of 30 % HPG/70 % TOCN composite film was 51.60 MPa, whereas it was 96.45 MPa for the 30 % CMG/70 % TOCN composite film. The addition of nanocellulose in these guar films improved the transparency, barrier property, tensile and thermal stability. These biodegradable composite films have great potential for food or medicine packaging, and for this purpose CMG has advantages over HPG.
References
Abdel-Halim ES, Al-Deyab SS (2014) Electrically conducting silver/guar gum/poly(acrylic acid) nanocomposite. Int J Biol Macromol 69:456–463
Benitez AJ, Torres-Rendon J, Poutanen M, Walther A (2013) Humidity and multiscale structure govern mechanical properties and deformation modes in films of native cellulose nanofibrils. Biomacromolecules 14(12):4497–4506
Cheng S, Zhang Y, Cha R, Yang J, Jiang X (2015) Water-soluble nanocrystalline cellulose films with highly transparent and oxygen barrier properties. Nanoscale 8(2):973–978
Dai L, Long Z, Lv Y, Peng Q-C (2014) The role of formic acid pretreatment in improving the carboxyl content of TEMPO-oxidized cellulose. Cell Chem Technol 48(5–6):469–475
Dai L, Wang B, Long Z, Chen L, Zhang D, Guo S (2015) Properties of hydroxypropyl guar/TEMPO-oxidized cellulose nanofibrils composite films. Cellulose 22(5):3117–3126
Das D, Ara T, Dutta S, Mukherjee A (2011) New water resistant biomaterial biocide film based on guar gum. Bioresour Technol 102(10):5878–5883
Deepa B, Abraham E, Cherian BM, Bismarck A, Blaker JJ, Pothan LA, Leao AL, de Souza SF, Kottaisamy M (2011) Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour Technol 102(2):1988–1997
Fujisawa S, Okita Y, Fukuzumi H, Saito T, Isogai A (2011) Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr Polym 84(1):579–583
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 10(1):162–165
Ghanbarzadeh B, Oleyaei SA, Almasi H (2015) Nanostructured materials utilized in biopolymer-based plastics for food packaging applications. Crit Rev Food Sci 55(12):1699–1723
Grzadka E (2013) Influence of surfactants on the adsorption and elektrokinetic properties of the system: guar gum/manganese dioxide. Cellulose 20(3):1313–1328
Grzadka E (2014) Stability of manganese dioxide by guar gum in the absence or presence of surfactants. Cellulose 21(3):1641–1654
Hosseinidoust Z, Alam MN, Sim G, Tufenkji N, van de Ven TGM (2015) Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 7(40):16647–16657
Hu Z, Cranston ED, Ng R, Pelton R (2014) Tuning cellulose nanocrystal gelation with polysaccharides and surfactants. Langmuir 30(10):2684–2692
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50(24):5438–5466
Kumar AP, Singh RP (2008) Biocomposites of cellulose reinforced starch: improvement of properties by photo-induced crosslinking. Bioresour Technol 99(18):8803–8809
Liu K, Nasrallah J, Chen L, Huang L, Ni Y (2015) Preparation of CNC-dispersed Fe3O4 nanoparticles and their application in conductive paper. Carbohydr Polym 126:175–178
Lucenius J, Parikka K, Osterberg M (2014) Nanocomposite films based on cellulose nanofibrils and water-soluble polysaccharides. React Funct Polym 85:167–174
Mao L, Law K, Claude D, Francois B (2008) Effects of carboxyl content on the characteristics of TMP long fibers. Ind Eng Chem Res 47(11):3809–3812
Mikkonen KS, Heikkila MI, Helen H, Hyvonen L, Tenkanen M (2010) Spruce galactoglucomannan films show promising barrier properties. Carbohydr Polym 79(4):1107–1112
Osterberg M, Vartiainen J, Lucenius J, Hippi U, Seppala J, Serimaa R, Laine J (2013) A fast method to produce strong NFC films as a platform for barrier and functional materials. ACS Appl Mater Interfaces 5(11):4640–4647
Pan Y, Xiao H, Song Z (2013) Hydrophobic modification of cellulose fibres by cationic-modified polyacrylate latex with core–shell structure. Cellulose 20(1):485–494
Rodionova G, Saito T, Lenes M, Eriksen Ø, Gregersen Ø, Fukuzumi H, Isogai A (2012) Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps. Cellulose 19(3):705–711
Rosiaux Y, Muschert S, Chokshi R, Leclercq B, Siepmann F, Siepmann J (2013) Ethanol-resistant polymeric film coatings for controlled drug delivery. J Control Release 169(1–2):1–9
Saito T, Nishiyama Y, Putaux J-L, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7(6):1687–1691
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 10(7):1992–1996
Seantier B, Bendahou D, Bendahou A, Grohens Y, Kaddami H (2016) Multi-scale cellulose based new bio-aerogel composites with thermal super-insulating and tunable mechanical properties. Carbohydr Polym 138:335–348
Shah SWA, Jahangir M, Qaisar M, Khan SA, Mahmood T, Saeed M, Farid A, Liaquat M (2015) Storage stability of kinnow fruit (Citrus reticulata) as affected by CMC and guar gum-based silver nanoparticle coatings. Molecules 20(12):22645–22661
Sharma S, Zhang XD, Nair SS, Ragauskas A, Zhu JY, Deng YL (2014) Thermally enhanced high performance cellulose nano fibril barrier membranes. RSC Adv 4(85):45136–45142
Shimizu M, Fukuzumi H, Saito T, Isogai A (2013) Preparation and characterization of TEMPO-oxidized cellulose nanofibrils with ammonium carboxylate groups. Int J Biol Macromol 59:99–104
Shimizu M, Saito T, Isogai A (2016) Water-resistant and high oxygen-barrier nanocellulose films with interfibrillar cross-linkages formed through multivalent metal ions. J Membr Sci 500:1–7
Song J, Tang A, Liu T, Wang J (2013) Fast and continuous preparation of high polymerization degree cellulose nanofibrils and their three-dimensional macroporous scaffold fabrication. Nanoscale 5(6):2482–2490
Svagan AJ, Samir MASA, Berglund LA (2007) Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 8(8):2556–2563
Way AE, Hsu L, Shanmuganathan K, Weder C, Rowan SJ (2012) pH-responsive cellulose nanocrystal gels and nanocomposites. ACS Macro Lett 1(8):1001–1006
Wen Y, Zhu X, Gauthier DE, An X, Cheng D, Ni Y, Yin L (2015) Development of poly(acrylic acid)/nanofibrillated cellulose superabsorbent composites by ultraviolet light induced polymerization. Cellulose 22(4):2499–2506
Woehl MA, Ono L, Riegel Vidotti IC, Wypych F, Schreiner WH, Sierakowski MR (2014) Bioactive nanocomposites of bacterial cellulose and natural hydrocolloids. J Mater Chem B 2(40):7034–7044
Wu C-N, Yang Q, Takeuchi M, Saito T, Isogai A (2014) Highly tough and transparent layered composites of nanocellulose and synthetic silicate. Nanoscale 6(1):392–399
Yang H, Tejado A, Alam N, Antal M, van de Ven TGM (2012) Films prepared from electrosterically stabilized nanocrystalline cellulose. Langmuir 28(20):7834–7842
Yano H, Sugiyama J, Nakagaito AN, Nogi M, Matsuura T, Hikita M, Handa K (2005) Optically transparent composites reinforced with networks of bacterial nanofibers. Adv Mater 17(2):153–155
Zhou W, Chen Z, Oshima N, Ito K, O’Rourke BE, Kuroda R, Suzuki R, Yanagishita H, Tsutsui T, Uedono A, Hayashizaki N (2012) In-situ characterization of free-volume holes in polymer thin films under controlled humidity conditions with an atmospheric positron probe microanalyzer. Appl Phys Lett 101(1):014102–014104
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (31270633), State Key Laboratory of Pulp and Paper Engineering (201512), Creative Fund of Combination of Industry, Academia and Research of Jiangsu Province, China-Prospective Joint Research Project (BY2013015-03) and Top-notch Academic Programs Project of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the coauthors of this work are included in this manuscript and they all endorse this submission. There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dai, L., Long, Z., Zhao, Y. et al. Comparison of hydroxypropyl and carboxymethyl guar for the preparation of nanocellulose composite films. Cellulose 23, 2989–2999 (2016). https://doi.org/10.1007/s10570-016-0998-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0998-7