Abstract
Sodium polyborate (SPB) has been employed with polyhexamethylene guanidine phosphate (PHMGP) to compose a nitrogen-phosphorus-boron intumescent coating on cotton fabric via a layer-by-layer (LBL) technique for obtaining excellent flame retardancy. The infrared spectra and atomic emission spectra indicate that the PHMGP-SPB multilayer grows gradually during the assembly process. Thermogravimetric analysis results show that the residue char of cotton is greatly enhanced after treatment with the LBL coating, which has a high char forming effect on cellulose during testing. The micro-calorimeter test finds that the assembly coating drastically decreases the peak heat release rate and total heat release of cotton fabric as a result of the catalytic charring effect. Cotton’s burning behavior is monitored by the flammability test, and the results show that ten bilayer (BL) coating with only 7.5 wt% can completely extinguish the flame on cotton samples. The oxygen index test reveals that the limiting oxygen index (LOI) of cotton samples is substantially increased by introducing the LBL coating. When the BL increases to 20, the LOI value reaches up to 41. The residual char after burning is analyzed by scanning electron microscope and infrared spectroscopy, and the results show the the LBL coating has an intumescent flame-retardant effect on cotton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton has excellent characteristics of softness, breathability and hygroscopicity, etc., so it has been widely applied to clothing and household and industrial supplies (Wakelyn et al. 2007). However, cotton belongs to the flammable fibers that might cause fire, which could gravely damage the security of people’s life and property. To prevent fire disasters and further protect people’s lives and wealth, researchers have made great efforts to develop flame-retardant cotton fabric (Alongi et al. 2014a; Alongi and Malucelli 2015a, b). Until now, various approaches such as nanoparticle adsorption (Alongi et al. 2011b; Liu et al. 2008), the sol-gel process (Alongi et al. 2011a, c) and plasma treatment (Lam et al. 2011; Shahidi 2014) have been exploited to confer flame retardancy to textiles by depositing nanocoatings on the fabric surface (Malucelli et al. 2014). However, nanoparticle adsorption has a limited flame-retardant effect, and other methods always involve complex physical or chemical steps, which significantly limit their application.
Recently, a layer-by-layer (LBL) assembly technique, as a frontier of flame-retardant treatment methods (Malucelli 2016), has attracted extensive attention in both academic research and industrial applications. As an evolution of nanoparticle adsorption, the LBL technique involves alternate physical adsorption of oppositely charged polyelectrolytes or nanoparticles to form thin multilayered films on given substrates. This technique can freely control the nanocoating’s structural composition and the resulting properties by adjusting the deposition conditions (pH and concentration, etc.). Apart from flame retardance, varying deposition materials have led to the exploitation of LBL assembly nanocoating with electrical conductivity, anti-ultraviolet, oxygen barrier and antimicrobial properties. In 2009, Grunlan et al. first applied the technique to the flame-retardant treatment of cotton fabric by preparing an LBL thin film of branched polyethylenimine and Laponite clay platelets (Li et al. 2009). This assembly significantly improved the thermal stability of cotton fabric. Since then, the LBL flame-retardant coating has evolved into various species such as an organic-inorganic hybrid coating (Alongi et al. 2012b; Carosio et al. 2011; Huang et al. 2012; Li et al. 2010; Zhang et al. 2013), all-inorganic coating (Patra et al. 2014) and phosphorus-based intumescent coating (Alongi et al. 2012a; Apaydin et al. 2014; Cain et al. 2014; Carosio et al. 2013; Fang et al. 2015a; Laufer et al. 2012; Yang et al. 2015). Among the above coatings, the phosphorus-based intumescent system has the highest efficacy for the suppression of the flame spread on cotton fabric, attributed to the powerful combination of a blowing agent, acid and carbon sources in LBL thin film. When exposed to fire, these ingredients are transformed into an expanded cellular thermal insulating layer that separates the internal materials from heat and flame. The first LBL intumescent nanocoating was demonstrated using LBL assembly of poly(sodium phosphate) and poly(allylamine) (Li et al. 2011), which served as an acid source and blowing agent, respectively. A 20-BL assembly coating (17.5 wt% weight relative to the untreated sample) could completely extinguish the flame on cotton fabric. Afterwards, various phosphorous polyelectrolytes were introduced into the current intumescent coating, such as ammonium polyphosphate (APP) (Alongi et al. 2012a; Carosio et al. 2012; Fang et al. 2015b), phytic acid (Laufer et al. 2012), deoxyribonucleic acid (Carosio et al. 2013), poly(vinylphosphonic acid) (Wang et al. 2014), poly(phosphoric acid) (Carosio et al. 2015), phosphorylated cellulose (Pan et al. 2014) and phosphorylated chitin (Pan et al. 2015). Our group has constructed an intumescent coating of polyhexamethylene guanidine phosphate (PHMGP) and APP, achieving an enhanced flame-retardant effect on cotton fabric (Fang et al. 2015a). However, organic phosphorus could lead to problems of color change for fabrics and odor release and toxicity for humans, and the intumescent flame-retardant effect on cotton fabric needs to be further improved.
Remarkably, boron-containing flame retardants (boric acid and borates, etc.) have a good flame-retardant effect on cellulosic materials such as cotton (Mostashari and Fayyaz 2008) and wood (Sogutlu et al. 2011; Yuksel et al. 2014) products. In addition, boron-containing flame retardants have also drawn great researcher attention, owing to their characteristics of outstanding flame resistance and low smoke suppression (Armitage et al. 1996). Actually, when exposed to heat, borate is melted, forming a glassy protective layer that insulates the underlying material from heat and oxygen (Dogan 2014). Additionally, boron-containing compounds can promote the formation of char during burning (Mansour 2012; Xie et al. 2013), performing a similar flame-retardant mechanism with phosphate in the condensed phase. What is more, boron and its cmpounds are widely found in salina, leafy vegetables, beans, fruits and nuts (Davis et al. 2015). Thus, it can be seen that boron-containing compounds can be promising candidates for organic phosphorus flame retardants. Since 1735, borax has been used as a flame-resistant finishing for textile fabrics. Apart from textiles, borates (such as ammonium pentaborate, sodium metaborate, barium metaborate and zinc borate, etc.) have been applied in plastics such as polypropylene (Yin et al. 2013), polycarbonate (Yang et al. 2013), polyamides (Dogan and Bayramli 2014; Mohaddes et al. 2014), etc. Importantly, to further reach excellent flame-retardant performance, researchers are beginning to focus on the development of boron-containing intumescent flame-retardant systems by combining borates with nitrogenous and phosphorous compounds (Dogan and Bayramli 2011; Dogan et al. 2010). This intumescent system transforms into multicellular graphitized char isolating the underlying substrate from outside heat and oxygen when exposed to fire. The introduction of boron compounds in intumescent flame-retardant systems can enhance the flame retardancy.
In this article, sodium polyborate (SPB) is introduced to combine with a nitrogenous and phosphorous ingredient PHMGP in the LBL coating on cotton fabric to obtain an outstanding flame-retardant property. SPB is highly water soluble and able to interact with cationic polyelectrolytes (e.g., PHMGP), which is very appropriate for LBL assembly. Remarkably, SPB-based coatings have a pronounced, strong fire-retarding effect on polyethylene terephthalate and polypropylene nonwoven and rigid polyurethane foam (Tsuyumoto et al. 2010, 2011a, b) as a result of the synergy effect of the polyborate foam layer and the resultant char layer. Infrared spectroscopy and atomic emission spectrometry were conducted to monitor the element contents on the fabric samples. Thermogravimetric analysis (TGA) in nitrogen atmosphere was performed to assess the thermal stability of the treated samples, respectively. Micro-scale combustion calorimetry (MCC) was carried out to evaluate the combustion behavior of the fabric samples. The flame resistance of the fabric samples was measured by the limiting oxygen index (LOI) and flammability test.
Experimental
Materials
Cotton woven fabric (230 g/m2, white) was purchased from an online Taobao store. PHMGP [(C7H15N3·H3PO4)n, degree of polymerization > 60] aqueous solution (25 wt%) was bought from Shanghai Scunder Industry Co., Ltd. (China). SPB (0.22Na2O·B2O3·nH2O) was obtained by Qingdao Minerals Co., Ltd. (Shandong, China).
Woven cotton was soaked in deionized water for 24 h to remove impurities, and then it was dried at 60 °C (40 min) for further LBL deposition. All reagents were prepared in 0.5 wt% aqueous solutions.
LBL deposition
Cotton fabric was alternately immersed into 0.5 wt% cationic PHMGP and anionic SPB solutions (5 min) to activate the fabric surface. After that, the cotton sample was dipped into cationic and anionic solutions (1 min) until the designated number of BLs was achieved. All immersions were followed by 1 min washing and 40 min drying at 60 °C. The coating weight was 3.6, 7.5 and 12.9 wt% for 5, 10 and 20 BLs, respectively.
Characterization
Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectra were recorded by a Nexus infrared spectrometer (Thermo Nicolet, USA) using 64 scans at a 4 cm−1 resolution. The FTIR spectra of residue chars after burning were recorded by a Nicolet infrared spectrometer. The B and P element contents on the fabric samples were evaluated with an Optima 7300 DV inductive coupling plasma atomic emission spectrometer (ICP-AES) (PerkinElmer, USA). The thermal stability of the fabric samples was assessed using a Pyris 1 thermogravimetric balance (PerkinElmer, USA). All samples were heated from 50 to 600 °C at the rate of 10 °C/min in nitrogen and air, respectively. MCC was conducted on an FAA Micro Calorimeter (FTT, Ltd., USA) from 50 to 600 °C (heating rate of 60 °C/min) in nitrogen (flowing rate of 80 ml/min). The burning behaviors in both vertical and horizontal directions were investigated using an AG5100A combustion apparatus (Zhuhai Angui Testing Instrument Co., Ltd., China) according to ASTM D6413 and D5132, respectively. The LOIs of fabric samples were measured by a JF-3 oxygen index tester (Jiangning Nanjing Analytical Instrument Corp., Ltd., China) according to the ASTM D2863 standard. The fabric samples and their residue chars were observed by a Sirion 200 field emission scanning electron microscope (SEM) (FEI Corp., USA; beam voltage: 10 kV). An energy-dispersive X-ray detector was used to perform element mapping.
Results and discussion
Coating structures
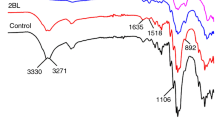
The surface structures of cotton samples were analyzed by ATR-FTIR spectroscopy, and the corresponding spectra are shown in Fig. 1. Untreated cotton presents the characteristic absorption bands of cellulose. The broad O–H and C–H stretching appear from the 3100 to 3550 cm−1 and 2800 to 2980 cm−1 region, respectively. The characteristic absorption bands at 1429, 1370 and 1202 cm−1 are due to the C–H wagging, bending and deformation stretch (Chung et al. 2004). A small peak at 1202 cm−1 corresponds to the OH in-plane bending. The broad typical characteristic bands from 1000 to 1160 cm−1 and absorption peak at 898 cm−1 are attributed to the C–O stretching in the cellulose backbone (Zhang et al. 2006). The coated cotton samples also show the typical characteristic bands of cellulose. However, the characteristic peaks become weaker after introducing the assembly coating, and the peak intensity decreases with the increase of the BL number. Spectra of the coated fabrics show extra peaks at 1635 cm−1 belonging to the NH bending vibration of PHMGP (Zhang et al. 1999). However, the characteristic peaks of SPB cannot be distinguished from the IR spectra because they show overlapping signals with cellulose.
To further monitor the LBL growth of the assembly coating on cotton, the absorption intensity of C–O–C at 1057 cm−1 for the cotton substrate was selected as the reference; the relative intensity ratio (N–H/C–O–C: A 1635 cm−1/A 1057 cm−1) is calculated as a function of the BL number. As shown in the inset, the ratio increases gradually with the growth of the BL number, suggesting that the PHMGP amount grows gradually in the LBL assembly process. The surface compositions of the coated fabrics were also evaluated by ICP-AES, and the corresponding results are shown in Fig. 1b. It is noteworthy that the B and P contents increase gradually with the growth of the BL number, indicating that the PHMGP and SPB amounts grow gradually in the LBL assembly process.
SEM analysis was performed to observe the untreated and coated fabrics. As shown in Fig. 2, the untreated sample exhibits a wave structure. At higher magnification, it can be found that cotton fiber shows a smooth surface. By introducing the PHMGP–SPB coating, the cotton fiber surface becomes a little rough. Even so, the coated samples show a similar appearance to that of the untreated sample. In addition, the coating constituents PHMGP and SPB are uniformly distributed on the surface of the cotton fiber, as evidenced by the B and P element mapping, as shown in Fig. 2. It can be observed visually that the amounts of PHMGP and SPB increase with the growth of BL. Notably, there is no bonding phenomenon among cotton fibers for all coated samples, so the coated fabrics basically maintain the softness of the untreated sample.
Thermal stabilities
The thermal degradation and decomposition processes of untreated and LBL-coated fabrics were monitored by TGA in nitrogen and air, respectively. Figure 3 reports the TG and DTG curves of untreated and coated samples in nitrogen, and the corresponding data are collected in Table 1. As shown in Fig. 3, cotton begins to break down at 359 °C (T onset) and performs a one-step thermal degradation behavior around 377 °C as a result of two competitive chemical processes. Cellulose depolymerizes to some volatile products (mainly levoglucosan, furan and furan derivatives); meanwhile it is also dehydrated to form thermally stable aromatic char (Alongi et al. 2014b, 2015).
LBL-coated fabrics still show one-step thermal degradation processes. However, the PHMGP–SPB coating anticipates the thermal degradation, which can be confirmed by the decreased initial degradation temperature (T onset) and the temperature of the maximum weight loss (T max). What is more, the T onset and T max values gradually decrease with the increasing number of BLs. The anticipation can be attributed to the catalytic effect of the PHMGP–SPB coating on the thermal degradation of cotton, which can be considered as a favorable phenomenon. The assembly coating catalyzes the dehydration of cellulose and promotes the formation of thermally stable char, thus leading to the production of less volatile materials. As a result, the coated fabrics leave more residue chars than the untreated sample at T max, and the residue mass increases with the growth of the PHMGP–SPB BL number (see Table 1). Moreover, the residue chars maintain higher thermal stability for the coated fabrics after 400 °C. After testing, the coated fabrics leave extremely high residues (29.9, 33.4 and 38.6 wt% for 5, 10 and 20 BLs, respectively) at 600 °C, and only 6.6 wt% residue char is remnant for the untreated fabric, as listed in Table 1. Additionally, the residue weight is much higher than the coating increment, further confirming that the PHMGP–SPB coating can effectively protect the internal cellulose substrate from fire.
Figure 4 reports the TG and DTG curves of untreated and coated samples in air, and the corresponding data are collected in Table 1. Unlike the thermal degradation in nitrogen, cotton’s thermal decomposition under air occurs in two stages. In the first stage (300–400 °C), cotton begins to decompose at 358 °C to some combustible volatiles; meanwhile, it is also dehydrated and forms aliphatic carbon. In the second stage (400–600 °C), aliphatic carbon transforms into aromatic carbon or decomposes to water, methane, CO and CO2.
Similar to the thermal degradation, the initial thermal decomposition of cotton was also accelerated by introducing the PHMGP–SPB coating. It can be seen from the reduced T onset and T max1 as the BL number increases. Moreover, the coated fabric presents a proactive second thermal decomposition stage, indicating the earlier formation of thermally stable char in the presence of the PHMGP–SPB coating. The PHMGP–SPB system can act as a condensed phase flame-retardant, affecting cotton’s thermal decomposition stage. In this case, cotton tends to dehydrate to aliphatic char in the first stage, and it is further carbonized to aromatic char. Consequently, the volatile formation decreases and the residue mass increases. As expected, the coated fabric leaves more residue than virgin cotton after the TGA test, and the residue mass increases with the BL number.
In order to confirm the catalytic charring effect of the PHMGP–SPB coating on cotton, TGA measurement was further compared with the MCC test (Alongi et al. 2014c, 2015). The MCC curves of untreated and coated samples are shown in Fig. 5, and the corresponding data are listed in Table 2. Cotton goes through a one-step pyrolysis process. It starts to degrade at 326 °C (T onset) and presents the peak heat release rate (PHRR) at 375 °C. After testing, the total heat release (THR) is 11.6 kJ/g. However, the pyrolysis proceeds in two steps for the coated samples. As listed in Table 2, the coated fabrics show decreased values of T onset and T p1 relative to the untreated ones, which is similar to the results in TGA (T onset and T max1, see Table 1). This confirms that the PHMGP–SPB coating plays a catalytic role in the thermal degradation of cotton. As expected, the coated samples display a smaller PHRR1 than virgin cotton, and the value decreases with increasing BL numbers (see Table 2), showing that the LBL coating promotes char formation and inhibits the release of burnable volatile substances. For the second pyrolysis step, the value of T p2 increases with the growth of the coating, suggesting that the PHMGP–SPB coating with higher BL numbers leads to the residue char with higher thermal stability. In addition, the PHRR2 value increases with the number of BLs as a result of more residues being left for the sample coated with more BLs. The samples coated with the PHMGP–SPB multilayer show decreased THR, and the value decreases with the increase of BL number, as listed in Table 2. For example, for the 20-BL-coated sample, the PHRR and THR decreased to 32 W/g and 3.5 kJ/g and were reduced by 87.7 and 69.8 % with respect to those of the untreated sample, respectively. The PHRR and THR values, as important parameters that are closely related to the flammability of materials, are significantly decreased for cotton fabric after being coated with a PHMGP–SPB multilayer. All these results indicate that the PHMGP–SPB multilayer with high catalytic charring action would have an excellent flame-retardant effect on cotton fabric.
Flame-retardant properties
To further evaluate the flame-retardant property, the untreated and LBL-coated fabrics were subjected to a flammability test in both vertical and horizontal configuration, respectively . Figure 6 reports the flame scenes at 15 and 30 s after ignition in vertical flammability test (VFT), and the corresponding data are collected in Table 3. Untreated cotton burns vigorously with high red flame for 32 s. After the flame goes out, the residue continues to smolder for 14 s and have been completely consumed after testing. As shown in Fig. 6, five-BL-coated fabric burns with a pale blaze after encountering the direct flame. Although the after-flame and afterglow times are 34 and 23 s, respectively, which are longer than those of the untreated sample, the five-BL-treated sample leaves considerable residue (54.8 wt%) after testing. Besides that, the damaged length of five-BL-treated sample is equal to the smaple length (SL). As shown in Fig. 6, the ten-BL-coated samples with only 7.5 wt% coating increment can self-extinguish once the fire source moves away. This result confirms that the fabric coated with the PHMGP–SPB multilayer has outstanding flame retardancy. Additionally, only 105 mm fabric (see Fig. 7) is damaged in the vertical direction, and 97.2 wt% residue (see Table 3) is left after testing. As the BL number grows to 20, the damaged length decreases to 85 mm and the residue mass increases to 98.4 wt%, indicating that the flame-retardant effect on cotton increases with the increase of BL number. The flame-spreading speeds for the untreated and coated samples were measured with horizontal flammability test (HFT). Figure 7 reports the image of the residues of all samples after HFT, and the corresponding data are listed in Table 3. Untreated cotton burns at a rate of 1.47 mm/s (burning time: 174 s) and have been completely consumed after testing. Surprisingly, by introducing a PHMGP–SPB multilayer, cotton becomes self-extinguishing and leaves a larger portion of fabric undamaged in the horizontal direction. Moreover, the damaged length decreases and the residue left increases with the growth of BL number, respectively. This confirms that the flame-retardant effect increases with the growth of the PHMGP–SPB assembly coating. Beyond the burning test, the oxygen index test was executed to further characterize the combustibility of cotton fabric. Pure cotton shows LOI values of 18.5 %. The LOI values increase to 24.5, 29.0 % for the five- and ten-BL samples, respectively. As the number of BLs grows to 20, the LOI value increases to 41.0 %, which is extremely high for cotton fabric. Actually, the LOI has been considered a good flammability index of a material in both academia and industry (Alongi et al. 2015). All these results confirm that the PHMGP–SPB multilayer has an excellent flame-retardant effect on cotton fabric.
To investigate the flame-retardant mechanism of the PHMGP–SPB assembly coating, the untreated and coated fabrics were observed by SEM after the flammability test, and Fig. 8 displays the corresponding SEM images. As mentioned before, the pure cotton had been completely consumed after the flammability test. However, the coated fabrics had left the wave structures intact after testing, as shown in Fig. 8. At higher magnification, it can be found that cotton fibers still maintain their original structures after burning, confirming that the PHMGP–SPB coating effectively protects the cotton fiber during the flammability test. What is more, some bubbles appear after burning as a result of intumescence effect. In this system, the released boracic acid from SPB and phosphoric acid from PHMGP as acid sources promote the dehydration and carbonization of cellulose, forming thermally stable graphitized char. The elemental mapping indicates the presence of the B and P elements in the residue, probably owing to the further formation of complex boron and phosphorus compounds derived from PHMGP and SPB, respectively. Meanwhile, the generated water vapor can dilute the air atmosphere in the vicinity of the burning substrate. Then, the released non-flammable gas from PHMGP as a blowing agent foams the graphitized char, forming intumescent char, as shown in Fig. 8.
FTIR analysis of residue chars
FTIR analysis was performed to investigate the chemical compositions of the residue chars after burning. As shown in Fig. 9, the five-BL-coated sample presents a weak absorption band from 1450 to 1650 cm−1, corresponding to the benzene ring. However, strong absorption peaks of carbohydrate at 1337 and 1068 cm−1 appear, which are assigned to the in-plane bending of OH and in-plane ring stretch of C–O–C (Chung et al. 2004), respectively. This suggests that the cellulose of the five BLs is only partly graphitized during burning. For the ten-BL-treated sample, the absorption peak at 1068 cm−1 still exists. Even so, new apparent absorption signals appear at 1692 and 1596 cm−1, attributed to the stretch vibration of C=O and skeletal vibration of benzene. This result indicates that most of the cellulose is catalytically dehydrated to form graphitized char, which can be confirmed by the strong characteristic peaks at 753, 699 and 668 cm−1 due to the out-of-plane bending of the aromatic ring. The appearing absorbing peaks at 1430, 876 and 811 cm−1 belong to the vibrations of the boron-oxygen triangular and tetrahedral groups (Abdelghany et al. 2014; Mansour 2012). This shows that borate serves as a main acid source. As the BL number increases to 20, the absorption peak of carbohydrate disappears, indicating that the cotton residue after burning is completely graphitized char. The new signals at 1250 and 1100 cm−1 are assigned to P=O and P–O–C (Ke et al. 2011), respectively, suggesting the presence of phosphorus groups in the residue char. The other appearing absorption peaks at 1364, 1027 and 928 cm−1 are attributed to the vibrations of the borate groups (Abdelghany et al. 2014). Because of the excellent catalytic charring of phosphate and borate (mainly), cotton is carbonized into thermally stable graphitized char, which swells further to form heat-insulating intumescent char, which can effectively inhibit the fire propagation.
Conclusions
Boron-containing intumescent nanocoating of the PHMGP–SPB multilayer has been successfully constructed on cotton fabric via the LBL assembly technique. The results of ATR-FTIR and ICP-AES spectroscopy suggest that the LBL coating grows gradually in the assembly process. This LBL coating has a strong catalytic charring effect on cotton fabric. As a consequence, the treated samples leave greatly enhanced residue char with respect to pure cotton after TGA. MCC reveals that the PHRR and THR of cotton are significantly decreased after the introduction of the flame-retardant coating. The fabric treated with ten BLs is able to self-extinguish during the flammability test. The PHMGP–SPB coating can dramatically raise the value of the LOI. SEM images display that obvious bubbles appear on the residue char as a result of the intumescent effect. FTIR analysis finds that the PHMGP–SPB multilayer graphitizes the cotton fabric, forming thermally stable residue char.
References
Abdelghany AM, ElBatal FH, ElBatal HA, EzzElDin FM (2014) Optical and FTIR structural studies of CoO-doped sodium borate, sodium silicate and sodium phosphate glasses and effects of gamma irradiation—a comparative study. J Mol Struct 1074:503–510. doi:10.1016/j.molstruc.2014.06.011
Alongi J, Malucelli G (2015a) Cotton flame retardancy: state of the art and future perspectives. RSC Adv 5:24239–24263. doi:10.1039/C5ra01176k
Alongi J, Malucelli G (2015b) Thermal degradation of cellulose and cellulosic substrates. In: Tiwari A, Raj B (eds) Reactions and mechanisms in thermal analysis of advanced materials. Wiley, Hoboken. doi:10.1002/9781119117711.ch14
Alongi J, Ciobanu M, Malucelli G (2011a) Novel flame retardant finishing systems for cotton fabrics based on phosphorus-containing compounds and silica derived from sol–gel processes. Carbohydr Polym 85:599–608. doi:10.1016/j.carbpol.2011.03.024
Alongi J, Tata J, Frache A (2011b) Hydrotalcite and nanometric silica as finishing additives to enhance the thermal stability and flame retardancy of cotton. Cellulose 18:179–190. doi:10.1007/s10570-010-9473-z
Alongi J, Carosio F, Malucelli G (2012a) Influence of ammonium polyphosphate-/poly(acrylic acid)-based layer by layer architectures on the char formation in cotton, polyester and their blends. Polym Degrad Stab 97:1644–1653. doi:10.1016/j.polymdegradstab.2012.06.025
Alongi J, Carosio F, Malucelli G (2012b) Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: flammability and combustion behaviour. Cellulose 19:1041–1050. doi:10.1007/s10570-012-9682-8
Alongi J, Colleoni C, Malucelli G, Rosace G (2012c) Hybrid phosphorus-doped silica architectures derived from a multistep sol–gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym Degrad Stab 97:1334–1344. doi:10.1016/j.polymdegradstab.2012.05.030
Alongi J, Carosio F, Malucelli G (2014a) Current emerging techniques to impart flame retardancy to fabrics: an overview. Polym Degrad Stab 106:138–149. doi:10.1016/j.polymdegradstab.2013.07.012
Alongi J, Colleoni C, Rosace G, Malucelli G (2014b) Sol–gel derived architectures for enhancing cotton flame retardancy: effect of pure and phosphorus-doped silica phases. Polym Degrad Stab 99:92–98. doi:10.1016/j.polymdegradstab.2013.11.020
Alongi J, Milnes J, Malucelli G, Bourbigot S, Kandola B (2014c) Thermal degradation of DNA-treated cotton fabrics under different heating conditions. J Anal Appl Pyrol 108:212–221. doi:10.1016/j.jaap.2014.04.014
Alongi J, Cuttica F, Carosio F, Bourbigot S (2015) How much the fabric grammage may affect cotton combustion? Cellulose 22:3477–3489. doi:10.1007/s10570-015-0717-9
Apaydin K, Laachachi A, Ball V, Jimenez M, Bourbigot S, Toniazzo V, Ruch D (2014) Intumescent coating of (polyallylamine-polyphosphates) deposited on polyamide fabrics via layer-by-layer technique. Polym Degrad Stab 106:158–164. doi:10.1016/j.polymdegradstab.2014.01.006
Armitage P, Ebdon JR, Hunt BJ, Jones MS, Thorpe FG (1996) Chemical modification of polymers to improve flame retardance.1. The influence of boron-containing groups. Polym Degrad Stab 54:387–393. doi:10.1016/S0141-3910(96)00069-9
Cain AA, Murray S, Holder KM, Nolen CR, Grunlan JC (2014) Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in single step. Macromol Mater Eng 299:1180–1187. doi:10.1002/mame.201400022
Carosio F, Alongi J, Malucelli G (2011) alpha-Zirconium phosphate-based nanoarchitectures on polyester fabrics through layer-by-layer assembly. J Mater Chem 21:10370–10376. doi:10.1039/C1jm11287b
Carosio F, Alongi J, Malucelli G (2012) Layer by layer ammonium polyphosphate-based coatings for flame retardancy of polyester–cotton blends. Carbohydr Polym 88:1460–1469. doi:10.1016/j.carbpol.2012.02.049
Carosio F, Di Blasio A, Alongi J, Malucelli G (2013) Green DNA-based flame retardant coatings assembled through layer by layer. Polymer 54:5148–5153. doi:10.1016/j.polymer.2013.07.029
Carosio F, Fontaine G, Alongi J, Bourbigot S (2015) Starch-based layer by layer assembly: efficient and sustainable approach to cotton fire protection. ACS Appl Mater Interfaces 7:12158–12167. doi:10.1021/acsami.5b02507
Chung C, Lee M, Choe E (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr Polym 58:417–420. doi:10.1016/j.carbpol.2004.08.005
Davis R, Li YC, Gervasio M, Luu J, Kim YS (2015) One-pot, bioinspired coatings to reduce the flammability of flexible polyurethane foams. ACS Appl Mater Interfaces 7:6082–6092. doi:10.1021/acsami.5b01105
Dogan M (2014) Thermal stability and flame retardancy of guanidinium and imidazolium borate finished cotton fabrics. J Therm Anal Calorim 118:93–98. doi:10.1007/s10973-014-3950-9
Dogan M, Bayramli E (2011) Synergistic effect of boron containing substances on flame retardancy and thermal stability of clay containing intumescent polypropylene nanoclay composites. Polym Adv Technol 22:1628–1632. doi:10.1002/Pat.1650
Dogan M, Bayramli E (2014) The flame retardant effect of aluminum phosphinate in combination with zinc borate, borophosphate, and nanoclay in polyamide-6. Fire Mater 38:92–99. doi:10.1002/Fam.2165
Dogan M, Yilmaz A, Bayramli E (2010) Synergistic effect of boron containing substances on flame retardancy and thermal stability of intumescent polypropylene composites. Polym Degrad Stab 95:2584–2588. doi:10.1016/j.polymdegradstab.2010.07.033
Fang F et al (2015a) Construction of intumescent flame retardant and antimicrobial coating on cotton fabric via layer-by-layer assembly technology. Surf Coat Technol 276:726–734. doi:10.1016/j.surfcoat.2015.05.023
Fang F et al (2015b) Intumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf Coat Technol 262:9–14. doi:10.1016/j.surfcoat.2014.11.011
Huang GB, Liang HD, Wang X, Gao JR (2012) Poly(acrylic acid)/clay thin films assembled by layer-by-layer deposition for improving the flame retardancy properties of cotton. Ind Eng Chem Res 51:12299–12309. doi:10.1021/ie300820k
Ke C, Li J, Fang K, Zhu Q, Zhu J, Yan Q (2011) Enhancement of a hyperbranched charring and foaming agent on flame retardancy of polyamide 6. Polym Adv Technol 22:2237–2243. doi:10.1002/pat.1751
Lam YL, Kan CW, Yuen CWM (2011) Effect of zinc oxide on flame retardant finishing of plasma pre-treated cotton fabric. Cellulose 18:151–165. doi:10.1007/s10570-010-9466-y
Laufer G, Kirkland C, Morgan AB, Grunlan JC (2012) Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 13:2843–2848. doi:10.1021/bm300873b
Li YC, Schulz J, Grunlan JC (2009) Polyelectrolyte/nanosilicate thin-film assemblies: influence of pH on growth, mechanical behavior, and flammability. ACS Appl Mater Interfaces 1:2338–2347. doi:10.1021/am900484q
Li YC et al (2010) Flame retardant behavior of polyelectrolyte-clay thin film assemblies on cotton fabric. ACS Nano 4:3325–3337. doi:10.1021/nn100467e
Li Y-C, Mannen S, Morgan AB, Chang S, Yang Y-H, Condon B, Grunlan JC (2011) Intumescent all-polymer multilayer nanocoating capable of extinguishing flame on fabric. Adv Mater 23:3926. doi:10.1002/adma.201101871
Liu YY, Wang XW, Qi KH, Xin JH (2008) Functionalization of cotton with carbon nanotubes. J Mater Chem 18:3454–3460. doi:10.1039/B801849a
Malucelli G (2016) Layer-by-Layer nanostructured assemblies for the fire protection of fabrics. Mater Lett 166:339–342. doi:10.1016/j.matlet.2015.12.103
Malucelli G, Carosio F, Alongi J, Fina A, Frache A, Camino G (2014) Materials engineering for surface-confined flame retardancy. Mat Sci Eng R 84:1–20. doi:10.1016/j.mser.2014.08.001
Mansour E (2012) FTIR spectra of pseudo-binary sodium borate glasses containing TeO2. J Mol Struct 1014:1–6. doi:10.1016/j.molstruc.2012.01.034
Mohaddes F, Wang LJ, Shanks RA, Fergusson SM (2014) Elevation of charring level of polyamide-6,6 films via ionic introduction of phosphoric acid and boric acid esters. Green Chem Lett Rev 7:184–190. doi:10.1080/17518253.2014.922624
Mostashari SM, Fayyaz F (2008) TG of a cotton fabric impregnated by sodium borate decahydrate (Na2B4O7.10H(2)O) as a flame-retardant. J Therm Anal Calorim 93:933–936. doi:10.1007/s10973-007-8933-7
Pan HF, Song L, Ma LY, Pan Y, Liew KM, Hu Y (2014) Layer-by-layer assembled thin films based on fully biobased polysaccharides: chitosan and phosphorylated cellulose for flame-retardant cotton fabric. Cellulose 21:2995–3006. doi:10.1007/s10570-014-0276-5
Pan H, Wang W, Pan Y, Song L, Hu Y, Liew KM (2015) Formation of self-extinguishing flame retardant biobased coating on cotton fabrics via Layer-by-Layer assembly of chitin derivatives. Carbohydr Polym 115:516–524. doi:10.1016/j.carbpol.2014.08.084
Patra D, Vangal P, Cain AA, Cho C, Regev O, Grunlan JC (2014) Inorganic nanoparticle thin film that suppresses flammability of polyurethane with only a single electrostatically-assembled bilayer. ACS Appl Mater Interfaces 6:16903–16908. doi:10.1021/am504455k
Shahidi S (2014) Novel method for ultraviolet protection and flame retardancy of cotton fabrics by low-temperature plasma. Cellulose 21:757–768. doi:10.1007/s10570-013-0127-9
Sogutlu C, Demirci Z, Dongel N, Imirzi HO, Doruk S, Yalinkilic AC (2011) The determination of the resistance to burning of some wood types impregnated with sodium borate solution. Wood Res-Slovakia 56:233–244
Tsuyumoto I, Miura Y, Hori Y (2010) Fire-resistant nonwovens of EVOH and PET treated with amorphous sodium polyborate. J Mater Sci 45:2504–2509. doi:10.1007/s10853-010-4222-0
Tsuyumoto I, Miura Y, Nirei M, Ikurumi S, Kumagai T (2011a) Highly flame retardant coating consisting of starch and amorphous sodium polyborate. J Mater Sci 46:5371–5377. doi:10.1007/s10853-011-5475-y
Tsuyumoto I, Onoda Y, Hashizume F, Kinpara E (2011b) Flame-retardant rigid polyurethane foams prepared with amorphous sodium polyborate. J Appl Polym Sci 122:1707–1711. doi:10.1002/app.34025
Wakelyn PJ, Bertoniere NR, French AD, Thibodeaux DP (2007) Cotton fiber chemistry and technology. Taylor & Francis Group, UK
Wang LL, Zhang T, Yan HQ, Peng M, Fang ZP, Li Y, Hao W (2014) Flame-retardant coating by alternate assembly of poly(vinylphosphonic acid) and polyethylenimine for ramie fabrics. Chinese J Polym Sci 32:305–314. doi:10.1007/s10118-014-1408-y
Xie KL, Gao AQ, Zhang YS (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohydr Polym 98:706–710. doi:10.1016/j.carbpol.2013.06.014
Yang SN, Lv GP, Liu Y, Wang Q (2013) Synergism of polysiloxane and zinc borate flame retardant polycarbonate. Polym Degrad Stab 98:2795–2800. doi:10.1016/j.polymdegradstab.2013.10.017
Yang J-C, Cao Z-J, Wang Y-Z, Schiraldi DA (2015) Ammonium polyphosphate-based nanocoating for melamine foam towards high flame retardancy and anti-shrinkage in fire. Polymer 66:86–93. doi:10.1016/j.polymer.2015.04.022
Yin YX, Zhang YH, Zhen ZC, Chu PK, Lv FZ, Ji JH (2013) Thermal degradation and flame retarding characteristics of polypropylene composites incorporated with boron mud. Compos Sci Technol 85:131–135. doi:10.1016/j.compscitech.2013.06.002
Yuksel M, Baysal E, Toker H, Simsek H (2014) Combustion characteristics of oriental beech wood impregnated with commonly used borates. Wood Res-Slovakia 59:39–49
Zhang YM, Jiang JM, Chen YM (1999) Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polymer 40:6189–6198. doi:10.1016/S0032-3861(98)00828-3
Zhang C, Price LM, Daly WH (2006) Synthesis and characterization of a trifunctional aminoamide cellulose derivative. Biomacromolecules 7:139–145. doi:10.1021/bm050465n
Zhang T, Yan HQ, Peng M, Wang LL, Ding HL, Fang ZP (2013) Construction of flame retardant nanocoating on ramie fabric via layer-by-layer assembly of carbon nanotube and ammonium polyphosphate. Nanoscale 5:3013–3021. doi:10.1039/c3nr34020a
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (nos. 51303182 and 51402002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fang, F., Zhang, X., Meng, Y. et al. Boron-containing intumescent multilayer nanocoating for extinguishing flame on cotton fabric. Cellulose 23, 2161–2172 (2016). https://doi.org/10.1007/s10570-016-0928-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0928-8