Abstract
In this study, the effects of helium or a helium/oxygen mixture atmospheric pressure plasma treatment on the adsorption of chitosan onto the cotton fabric were investigated. Fabrics were treated with plasma prior to a chitosan finishing process, whereby fabrics were surface coated using a pad/dry/cure method. Fourier transform infrared spectroscopy, scanning electron microscopy, X-ray photoelectron spectroscopy, surface energy analyser and contact angle measurements were used to investigate the changes on the cotton surface. Furthermore, antimicrobial activity of the cotton fabric was evaluated. The results showed that plasma pre-treatment enhanced the chitosan adsorption to the cotton surface through physical bonding and there was weak evidence of chemical bonding interactions. A combination of plasma and chitosan treatment did not show any significant differences on the antimicrobial properties compared to chitosan only treated fabric. Plasma treatment changed the fibres physically and enhanced the surface energy and thickness of chitosan distributed on the fibres.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitosan is modified chitin, a natural polymer derived from the exoskeletons of crustaceans and arthropods, which can be used in biotechnology (Costa et al. 2014), wastewater treatment (Kyzas et al. 2013), pharmaceuticals (Ilium 1998), agriculture (El-Sawy et al. 2010), food science (Suseno et al. 2014), and textiles (El-tahlawy et al. 2005; Enescu 2008; Krishnaveni and Thambidurai 2013; Li et al. 1997). It is a polysaccharide that is a copolymer of glucosamine and N-acetyl-glucosamine (Li et al. 1997). The structure of chitosan is similar to cellulose structure except that the hydroxyl group in the C2 position of cellulose is substituted with an amino group in chitosan (Fig. 1).
Chitosan can be applied to cotton and regenerated cellulose textiles as a finishing agent for processes such as antimicrobial treatment and crease recovery (Chattopadhyay and Inamdar 2013; El-tahlawy et al. 2005; Gupta and Haile 2007; Ye et al. 2006). Since chitosan is nontoxic and biodegradable, its application in finishing can be prefered over traditional treatments (Enescu 2008). The bonding forces between cellulose and chitosan are not strong, therefore chitosan coating on cotton has poor durability. To provide efficient and irreversible adsorption of chitosan on a cotton substrate, it is necessary to construct appropriate binding sites and to activate the substrate material properly. An appropriate activation of cellulose is introducing aldehyde and carboxyl groups on the cellulose structure by oxidation of the hydroxyl groups (Potthast and Kosma 2006).
For this purpose, researchers have attempted to modify chitosan by graft copolymerization and crosslinking agents. Different chemical processes have been used that often require toxic chemicals, to form ester crosslinks and uniform adsorption between cotton and chitosan. In the presence of chitosan cotton fabric were treated with two different crosslinking agents, butanetetracarboxylic acid (BTCA) and arcofix NEC (El-tahlawy et al. 2005). Type, concentration and treatment conditions of crosslinking agent enhanced the antimicrobial activity and improved the performance of cotton fabrics. Water soluble carboxymethyl chitosan derivative (CMCH) was used by Gupta and Haile (2007), where treatment with the modified chitosan significantly reduced the antimicrobial activity of treated cotton samples after dyeing with anionic dyes. Washing fastness was slightly improved.
The fibre-reactive chitosan derivative O-acrylamidomethyl-HTCC (NMA–HTCC) was applied to cotton. It was synthesized from the water-soluble chitosan derivative N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride (HTCC). While the durability of the treatment was not reported, an excellent antimicrobial activity was reported for treated cotton fabric (Lim and Hudson 2003).
Using these chemicals is ecologically undesirable and there is a chance for deterioration of the fibre mechanical properties (El-tahlawy et al. 2005; Liu et al. 2008; Wenzel 1936). Therefore, more environmentally friendly methods are still needed to reduce or eliminate the use of toxic chemicals without adversely affecting the properties of the fibre.
An environmentally friendly option involves the application of plasma treatments, which can introduce different functional groups on the fibre surface, without using chemicals or solvents. It improves surface bonding and changes the hydrophilicity and roughness of the surface. The effectiveness of plasma treatments can be controlled by plasmas parameters; pressure, discharge power, treatment times, and the type of the gas (Graham 2007; Naebe et al. 2011).
There are very few studies where the effect of plasma treatment on adsorption of chitosan only on the cotton surface have been reported; Fras Zemljič et al. (2009) showed the effect of vacuum oxygen plasma treatment on the functionalization of viscose fibre with 1 % chitosan solution using XPS and conductometric titration. The influence of plasma treatment on antimicrobial activity of chitosan treated viscose fibre was investigated. Compared to the untreated sample, higher chitosan adsorption and higher antimicrobial activity as results of an increase in amino groups responsible for the antimicrobial activity were reported on plasma treated viscose.
Using atmospheric pressure helium/nitrogen plasma (Zhou and Kan 2014) improved the chitosan adsorption onto cotton fabric. Based on the FTIR results, these authors suggested that N2 plasma treatment added amide groups on the surface of cotton fabric and functionalized the substrate with C≡N (nitrile), and improved the coating rate of chitosan on cotton fabric.
However, there is a gap in understanding what chemical and physical changes plasma produces at the surface of the cotton and how these changes promote adhesion between chitosan and cotton. In addition there are very few, if any, report on the application of atmospheric helium/oxygen plasma pre-treatment on chitosan adsorption onto cotton fabric. This work is therefore aimed at investigating the effect of atmospheric helium/oxygen pressure plasma treatment of cotton on the adsorption of chitosan onto cotton fabric, where the results have been compared with helium plasma pre-treatment.
Changes on the surface were characterised using SEM, FTIR, and XPS to understand how plasma treatments have affected the adsorption and/or adhesion of chitosan. Surface energies of cotton fabrics before and after surface treatment have also been determined using Inverse Gas Chromatography (IGC). We have investigated the extent to which chitosan adsorption, and in turn the antimicrobial activity of chitosan treated fabrics could be improved by plasma treatment.
Experimental
Materials and methods
A plain weave pure cotton fabric of 140 g/m2 [54 ends per inch, 54 picks per inch, 62 inches wide, made of 2/40 Ne (R2/30 tex)], was used. To obtain a well-defined reference or control fabric, the fabrics were scoured with a non-ionic detergent (Lissapol N450) using a Fong’s SDM2-12-140 laboratory dyeing machine (Fong’s National Engineering Co. Ltd., Hong Kong). Fabric samples were wetted out in the scouring bath at room temperature until fully saturated. Temperature was then raised to 60 °C at 1 °C/min, held for 60 min, and cooled to room temperature. Finally fabrics were washed with distilled water and were left to dry in the conditioning laboratory at 25 ± 2 °C and 65 ± 2 % relative humidity (RH).
Fabric were then bleached with a 1 % (v/v) hydrogen peroxide (H2O2) solution by the exhaustion method for 40 min at 85 °C using the same dyeing machine, where the pH of 10 was obtained using Na2CO3 (100 g/L aqueous solution). After cooling, fabrics were rinsed 3 times with distilled water at room temperature and then dried at 40 °C. Fabrics were conditioned under standard conditions (25 °C/65 % RH) for 24 h before use.
Preparation of chitosan finishing solution
A 2 % (w/v) chitosan finishing solution was prepared by dissolving chitosan (with bulk density of 0.15–0.3 g/cm3 and 75–85 % deacetylated, insoluble in water and organic solvent from Sigma-Aldrich) in 0.1 M acetic acid at 70 °C with stirring for 8 h.
Plasma treatment
An atmospheric pressure, ambient temperature plasma machine (APC 2000) manufactured by Sigma Technologies International (Tucson, AZ, USA) was used (Naebe et al. 2010). This device contains three electrodes, two upper and one lower. The lower electrode is in the form of a roller and is ceramic-coated. The operating frequency and maximum power input were 90 kHz and 5 kW, respectively. The voltage range and the roller speed were 1.8–2.2 kV and 25 rpm, respectively.
Helium (Helium 5.0, Coregas, Australia) with a flow rate of 14 L/min was used as the plasma gas. The reactive gas was oxygen (5 % v/v). Fabrics were attached to the roller with the face side up and treated with only helium (He) or the helium and oxygen mixture (He/O2) for 30 s plasma exposure time, where the exposure time was controlled by the rotation of the roller electrode.
Treatment with chitosan
Control and plasma treated fabric samples were impregnated in a freshly made chitosan solution at 80 °C for 1 h prior to padding. The fabric samples were padded (100 % pick-up) and then dried on a pin frame in a laboratory drier (Rapid Labortex Co. Ltd., Taiwan) for 5 min at 100 °C and then were cured at 160 °C for 2 min. Samples were then conditioned for 24 h (25 °C, 65 % RH) prior to testing. Table 1 shows the notation of samples used in this study.
Scanning electron microscopy (SEM)
Morphological changes on the surface of the fabrics were observed using a Zeiss Supra 55VP SEM. Fabric samples were gold coated using a Bal-tec SCD50 sputter coater.
Assessment of wettability
The changes in hydrophobic and hydrophilic properties of the fabrics were observed with a contact angle measurement using a KSV CAM 200, KSV Instruments Ltd, Finland. A liquid drop of 3.65 µL was placed on the surface of each fabric sample, while the image of each drop was immediately captured. The average of 5 measurements is reported.
Fourier transform infrared spectroscopy (FTIR)
FTIR-ATR spectroscopy was used to analyse the chemical composition and functional groups on the surface of the fabrics. Infrared spectra were recorded with a PerkinElmer Spectrum 100 (USA) in ATR mode, with an accumulation of 32 scans at 4 cm−1 resolution. Each result was the average of 2 different measurements. Prior to analysis the spectra were ATR corrected then normalised on the maximum near 1120 cm−1 with the minimum set at 1075 cm−1 using the Spectrum software.
For further investigation of chemical composition of the surface of chitosan coated fabric samples, FTIR spectra of the normalised control fabric was subtracted from the normalised spectra of the treated fabric.
X-ray photoelectron spectroscopy (XPS)
Treated and untreated fabrics were analyzed using an AXIS Nova spectrometer (Kratos Analytical Ltd., UK). The device is equipped with a monochromatic X-ray source (Al Kα, hν = 1486.6 eV). Pass energies of 160 and 20 eV were used to acquire the survey spectra and high resolution spectra, respectively. The C 1s signal (Eb = 284.8 eV) was used as a reference to charge correct the spectra. The estimated errors were ±10 %..
Surface energy analyser (SEA)
The surface energy of samples were determined using an IGC-Surface Energy Analyzer (SEA) (Surface Measurement Systems, Alperton, Middlesex, UK). For an IGC experiment approximately 1.0 g of each cotton fabric sample was packed into silanised glass columns (30 cm long, 4 mm inner diameter). Prior to measurement the samples were pre-treated (conditioning) at 30 °C for 2 h in situ with an 8 standard cubic centimetres per minute (sccm) total flow rate of helium carrier gas.
A series of surface coverage with n-alkanes (n-hexane, n-heptane, n-octane and n-nonane) and polar probes (chloroform, ethyl acetate, 1, 4-Dioxane, ethanol and dichloromethane) were chosen to measure the dispersive surface energy (\(\gamma_S^D\)) as well as the specific free energies of adsorption (\(\Delta G_{SP}^0\)), respectively. All probes were of chromatographic grade and were used as received (Sigma–Aldrich). Methane was used as a reference probe for dead volume corrections. The retention times of the chosen probes at specific fractional surface coverage was measured, and converted into retention volumes. The values of dispersive surface energy and specific free energy of adsorption were determined using the standard method described by Jones (Jones et al. 2012). Finally, the profile of the surface energy was obtained by fitting the data to an exponential decay function; y = y0 + Ae−x/t, where y0 is the value of the function at infinity, A is the determination of the range of energies and t is the decay constant, both important parameters in assessing the heterogeneity of the surface.
Antimicrobial test
The antimicrobial efficiency of fabrics was evaluated in accordance with a modified “ASTM E2149-13a (2014)” test method. Fabric sample is shaken into a phosphate buffer solution containing a known concentration of bacteria. After a specified time, the bacterial count in the solution is determined to estimate the survived bacterial count. The antibacterial performance is then calculated as a percentage reduction of bacterial count over the original count. A gram negative species Escherichia coli (ATCC 11229) was used for the test. A single colony of the bacteria was grown in a sterile Tryptic Soy Broth for 18 h at 37 ± 2 °C in a shaker incubator to obtain a bacterial culture. The culture was then diluted with sterile 0.3 mM phosphate buffer solution and adjusted to an absorbance of 0.28 ± 0.02 at 475 nm. This absorbance corresponds to a concentration of 1.5 − 3.0 × 108 colony forming units (CFU)/mL. The culture was further diluted 1000 times to obtain the “working bacterial solution” with concentration in the range of 1.5 − 3.0 × 105 CFU/ml.
Each treated fabric sample was tested in duplicate. Fabric samples (1.0 ± 0.1 g) were cut into small pieces (10 mm × 10 mm) then transferred to a sterile 250 mL glass bottle. To this, 50 mL of “working bacterial solution” was added. In each test batch, two control specimen were included: one was untreated cotton fabric and the other was a blank containing only 50 ml working bacterial solution (referred as “inoculum only”).
The bottles were capped tightly and transferred into a shaker incubator. The shaker was rotated for 1 h ± 5 min at 155 rpm at room temperature (22 ± 2 °C). This ensures contact of fabric sample with the bacterial solution. At the end of 1 h, all bottles were taken out from the shaker. 1 mL of the solution from each bottle (including “inoculum only” bottle) was serially diluted in buffer solution to 10−1 and 10−2 dilutions. A 0.1 mL aliquot from of each dilution (100, 10−1 and 10−2) was then spread on plate count agar in duplicates. The plates were then transferred in an incubator and stored at 37 °C for 24 h. The number of colonies grown on each plate were counted manually. Finally, the cells were quantified by taking into account the serial dilutions and expressed as CFU per millilitre (CFU/mL). Photographs of the plates were taken for visual representation of the antimicrobial performance. The antibacterial activity of each sample was expressed as percent bacterial reduction (R%) and calculated as follows:
A = CFU/mL for the treated sample after 1 h contact time, B = CFU/mL for the “inoculum only” bottle after the 1 h contact time.
Results and discussion
SEM analysis
Figure 2 shows the surface morphology of the control and treated fabric samples. While the control sample (Fig. 2a) showed a smooth and even surface, all treated fabrics show some changes to the surface morphology. The distribution of chitosan was observed on all samples treated with chitosan, showing enhanced surface roughness. Some signs of surface damage including transverse cracks and grooves as results of etching by the plasma treatments were observed on the surfaces of both plasma treated fabric samples. Helium/oxygen plasma treated sample showed slightly more surface damage compared to helium plasma treated samples, suggesting that helium/oxygen plasma was harsher than helium alone plasma treatment, though similar plasma exposure time was applied.
In addition, chitosan coated onto the surface of helium/oxygen plasma treated fabrics appeared thicker compared to helium only plasma treated samples, where there is evidence of chitosan bridging between fibres. The distribution of chitosan on the control fabric (CHT/Cure), was much lower than those samples pre treated with plasma, where no bridging was observed, yet the surface roughness was enhanced. Since cracks on the surface makes the coating of materials easier (Zhou and Kan 2014), this suggested that plasma pre-treatment enhanced the distribution of chitosan coating and provids a good surface for the two polysaccharides to physically bonds together and apparently helium/oxygen plasma is more effective than helium plasma. Obviously, SEM does not provide any evidence of chemical bonding which will be discussed further in this paper.
Assessment of wettability
The results of the wettability assessments of control and treated cotton fabrics are summarised in Table 2. While the control and plasma treated fabric samples were hydrophilic, all fabrics coated with chitosan, regardless of plasma pre-treatments, showed a reduction in hydrophilicity. This can be explained based on the superhydrophilicity principals, where it has been shown that enhancement in surface roughness increased the water contact angle and reduced wettability (Wenzel 1936). The results are also is in agreement with the previous study (Liu et al. 2008), where superhydrophilic cotton fabric became hydrophobic with a water contact angle of 130° after coating with chitosan and creating a rough surface on cotton.
Fabric samples treated with helium plasma and then coated with chitosan (PTHe/CHT/Cure) were more hydrophobic in comparison to the helium/oxygen plasma pre-treated fabric (PTO/CHT/Cure) as indicated by the shorter water absorption time. The changing of the wetting time between these samples shows the chitosan film rearranges and surface energy increases with oxygen presented in the plasma. Helium/oxygen plasma has the potential to oxidize the fibre surface more than helium only plasma.
FTIR-ATR analysis
The FTIR-ATR spectra of control fabric and all treated fabrics are shown in Fig. 3. As expected FTIR spectra show very few differences between two plasma treated fabrics as the effect of plasma treatment is confined to 2.5–10 nm of the surface and would be difficult to detect by FTIR. Plasma treated fabrics (PTHe and PTO) showed small changes in absorbance near 1650 cm−1 and between 1160 and 950 cm−1. The peaks near 1650 cm−1 corresponds to the C=O stretching vibration, which is produced by oxidation of the hydroxyl groups.
Normalised FTIR spectrum of; a control fabric, b control fabric treated with chitosan (CHT/Cure), c helium plasma treated fabric (PTHe), d helium plasma treated coated with chitosan (PTHe/CHT/Cure), e helium/oxygen plasma treated fabric (PTO), f helium/oxygen plasma treated coated with chitosan (PTO/CHT/Cure)
After chitosan coating, the absorbance due to amide I at 1630 cm−1 increased for all fabrics, which confirmed the presence of chitosan on both plasma pre-treated and untreated fabrics. It is difficult to determine if plasma enhanced the adsorption on the surface of cotton fabric compared to untreated fabrics. In order to show the effect of plasma, the normalised FTIR spectrum of the fabric coated with chitosan was subtracted from the equivalent uncoated fabric. The results are shown in Fig. 4.
Subtracted FTIR spectrum of different treated fabrics from corresponding untreated fabric samples: a chitosan treated fabric (CHT/Cure)—control fabric, b helium plasma treated coated with chitosan (PTHe/CHT/Cure)—helium treated fabric (PTHe), c helium/oxygen plasma treated coated with chitosan (PTO/CHT/Cure)—helium/oxygen plasma treated (PTO)
The main absorbance bands of chitosan are the amide I (C=O stretching vibration, 1680–1630 cm−1), amide II (N–H bending vibration, 1640–1550 cm−1) and amide III (C–N stretching vibration, 1315–1400 cm−1) (Leceta et al. 2015; Yuen et al. 2012). In comparison with the chitosan only coated fabric (CHT/Cure) very slight changes was observed on plasma pre-treated samples. No evidence of chemical bonding between two polysacharids was observed.
XPS analysis
Table 3 shows the relative atomic concentrations of carbon, oxygen and nitrogen on the surface of the untreated and treated cotton fabrics. In order to further characterize the changes in surface chemistry resulting from the plasma and chitosan treatments, different components have been fitted to the high resolution C 1s region spectra (Fig. 5) and these are also detailed in Table 3.
High resolution XPS C 1s spectra of a control cotton fabric, b chitosan treated fabric (CHT/Cure), c helium plasma treated fabric (PTHe), d helium/oxygen plasma treated fabric (PTO), e chitosan treated helium plasma pre-treated fabric (PTHe/CHT/Cure), f chitosan treated helium/oxygen plasma pre-treated fabric (PTO/CHT/Cure)
A significant increase in the oxygen and a decrease in the carbon concentration was observed for all treated fabrics (chitosan treatment and plasma treatment) compared to the control fabric. Treatment with chitosan increases the nitrogen content of all samples and a higher nitrogen content on fabrics that were pre-treated with He/O2 plasma, indicating greater coverage of the fibre by chitosan.
The C 1s peaks (Fig. 5) showed three component for cotton fabrics; near 284.8 eV typical of carbon without oxygen bonds and only bound to carbon and hydrogen C1 (C–C, C–Hx); near 286.3 eV, typical of carbon single bond to oxygen or nitrogen C2 (C–O, C–O–C) and near 287.8 eV carbon with two oxygen and/or nitrogen bonds C3 (O–C–O, N–C=O) typical of acetal and amide (Dufresne 2012; Maachou et al. 2013).
A reduction in C–C and enhancement in carbon bounded to oxygen (C–O and O–C–O) was observed on both plasma treated cotton fabrics, compared to the control sample. This suggests that plasma treatment leads to an increase in oxidised cellulose (aldehyde) group on C2, C3 and C6 glycoside atoms (Potthast and Kosma 2006). As expected, this is greater on He/O2 plasma treated sample with higher concentration of C–O and O–C–O and also a higher O/C ratio compared to the helium only plasma as a results of oxygen in gas mixture. The emergence of a fourth peak typical of carbon with three bonds to oxygen (O–C=O) (i.e., carboxyls) was not observed on plasma treated samples as reported in previous research conducted by Fras et al. on viscose fabrics (Fras Zemljič et al. 2009). This might be due the fact that the concentration of O–C=O was less than the XPS detection limit.
It has been reported that binding chitosan onto oxidised cellulose fibre reduces the aldehyde groups on the fibre surface due to the formation of a Schiff’s base between the chitosan and oxidised cellulose (Fras Zemljič et al. 2009). In this study, application of He/O2 plasma prior to chitosan treatments resulted in a reduction of the O–C–O peak, indicating a reduction in the aldehyde concentration from 12.6 % on plasma treated (PTO) to 9.2 % on plasma chitosan treated fabric (PTO/CHT/Cure). However, this reduction was not observed with helium plasma treatment. Overall it can be concluded that, the plasma treatment resulted in a slightly higher degree of chitosan adsorption onto cotton fabric and the He/O2 plasma treatment is more effective than helium plasma treatment. This suggests that both etching and oxidation by plasma treatment have been effective and these two effects were more severe on He/O2 plasma, as confirmed by SEM and XPS. This effect was also observed by higher wettability on He/O2 plasma treated fabrics.
Surface energy analysis
Typically, adhesion and cohesion phenomena depend on the surface energies of the materials. For IGC, the probe molecule at finite concentration conditions preferentially attach to the most reactive sites of tested materials. The measured surface energy as a function of fractional surface coverage reveals information about the range of the surface energies of the different sites on the sample (Buckton and Gill 2007; Grimsey et al. 2002; Jones et al. 2012). An energetically homogeneous surface is indicated by a completely flat line. However, the more commonly observed profile is an exponentially decaying surface energy function which is indicative of surface heterogeneity. The extent of heterogeneity can be assessed in terms of the range of energies (the pre-exponential factor, A) as well as the fraction of the surface over which the decline occurs (proportional to the decay constant, t). From the exponential equation of fit the maximum (y0 + A) as well as y (1), the average surface energy of the whole sample (fractional coverage of 1), are also obtained (Buckton and Gill 2007; Grimsey et al. 2002; Jones et al. 2012).
The total surface energy (\(\gamma_S^T\)) profiles for the fibres before and after surface treatments are shown in Fig. 6. The range, average and decay constants are given in Table 4. It can be seen that plasma treatments, regardless of the kind of gas used, significantly affected the surface energy of cotton fabrics. The surface energy of the control cotton fabric varies from a minimum of 64 mJ/m2 to a maximum of 103 mJ/m2. After plasma treatment, the average surface energy increased to 67 mJ/m2 after the helium plasma treatment and to 70 mJ/m2 after He/O2 plasma treatment, indicating the plasma treated fabrics are more hydrophilic. Figure 6 also shows differences in the rate of decay of the curves, as reflected by the decay constants in Table 4. The surface energy for the control fabric and fabric treated by helium plasma exhibit surface heterogeneity over 30 % of the fibre surface. This means that more than 30 % of the sites have surface energy values significantly different from the rest. By comparison, the surface energy of the He/O2 plasma treated fabric drops relatively rapidly and then stabilises at a relatively constant value after a fractional surface coverage of approximately 15 %, indicating the surface properties of the fibre are more uniform after He/O2 plasma treatment.
The total surface energy is the sum of the dispersive (non-polar) interactions, \(\gamma_S^D,\) and the specific (acid–base or polar) contributions, \(\gamma_S^{AB}\). Figure 7, shows that the helium plasma treatment mainly contributed to an increase of dispersive surface energy, while He/O2 plasma treatment shows increased dispersive surface energy as well as specific surface energy, indicating the roughness, surface area and chemical activity of fibre have been increased. More importantly, He/O2 plasma treatment has enhanced the homogeneity of the fibre surface energy.
Figure 8 shows the surface energy profile of plasma treated fabrics coated with chitosan. It is worth noting that He/O2 plasma treated fabric has the highest value at every surface coverage, indicating fabric treated with this method adsorbed more chitosan. These results are in agreement with the SEM observations, as treatment with He/O2 plasma and coating with chitosan showed a relatively higher adsorption of chitosan on the surface of the fabric compared to the helium plasma treated and only chitosan treated fabrics.
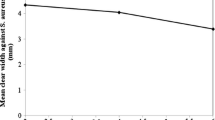
Antimicrobial activity
The results of antimicrobial activity of different fabric samples are summarised in Table 5. Figure 9 shows the agar plates and visual representation of the antimicrobial performance of inoculum only (no-fabric), control and treated samples and the growth of colonies. The agar plate photos corresponding to the treated samples show fewer colonies at 10−1 dilutions which indicates good antimicrobial activity.
Antibacterial activity of: a inoculum only after 0 h, b inoculum only after 1 h, c control cotton fabric, d chitosan treated fabric (CHT/Cure), e helium plasma treated fabric (PTHe), f chitosan treated helium plasma pre-treated fabric (PTHe/CHT/Cure), g helium/oxygen plasma treated fabric, h chitosan treated helium/oxygen plasma pre-treated fabric (PTO/CHT/Cure)
As mentioned the “inoculum only” sample was included to ensure any reduction is not due to either the mechanical motion or poor viability of the colonies. The results showed that untreated cotton fabric did not reduce bacterial growth, whereas all of the chitosan coated fabrics showed the same high degree of anti-bacterial activity. Compared to control fabric samples, the results show that plasma treatments with no chitosan coating also reduced bacterial activity. This might be due to the presence of active agents; OH, O and He*, produced by helium plasma which are three candidate agent responsible for the observed bactericidal effect. Fabric samples treated with helium/oxygen plasma showed higher antibacterial efficiency than helium plasma treated fabrics, consistent with Richardson et al. (2000) study. They observed that oxygen added to helium plasma was more effective in the sterilization and killing of bacteria (Richardson et al. 2000).
Chitosan is an antimicrobial agent and the fabrics on which chitosan is adsorbed are expected to provide an antimicrobial activity due to the presence of more amino groups (Lim and Hudson 2004). Helium/oxygen plasma increased the adsorption of chitosan, which in turn, added more amino groups and slightly reduced the degree of bacterial growth. Though plasma treatment slightly enhanced the adsorption of the chitosan on the surface of the cotton fabric, no changing in antibacterial activity was observed compared to chitosan only treated fabric.
Conclusion
The effect of plasma treatment prior to chitosan coating and its impact on the adsorption of chitosan onto cotton fabric, was investigated using different analytical techniques. The FTIR results and morphology observations confirmed the presence of chitosan on the surface of both plasma pre-treated and untreated fabrics, however no evidence of chemical bonding was observed.
Plasma treatment leads to greater spread of chitosan onto cotton fabric regardless of the type of plasma gas used. However, higher homogeneity of fibre surface energy was observed with helium oxygen plasma, which was in agreement with the wetting times of the corresponding fabrics. The total surface energy showed a higher dispersive surface energy on helium plasma treatment, while helium/oxygen plasma treatment increased both dispersive and specific surface energy, as a result of increased damage, surface area and chemical activity of fibres.
Fabric samples treated with plasma did not show significant changes on antibacterial efficiency compared to chitosan only treated fabrics. As assessed by XPS, helium oxygen plasma may enhance the binding between chitosan and oxidised cellulose fibre. Overall, it seems that any enhanced adhesion of chitosan on the surface of plasma treated fabrics, is mostly due to physical adsorption rather than chemical binding between two polysaccharides.
References
ASTM E2149-13a (2014) Standard test method for determining the antimicrobial activity of antimicrobial agents under dynamic contact conditions
Buckton G, Gill H (2007) The importance of surface energetics of powders for drug delivery and the establishment of inverse gas chromatography. Adv Drug Deliv Rev 59:1474–1479
Chattopadhyay D, Inamdar MS (2013) Improvement in properties of cotton fabric through synthesized nano-chitosan application. Indian J Fibre Text Res 38:14–21
Costa EM, Silva S, Madureira AR, Cardelle-Cobas A, Tavaria FK, Pintado MM (2014) A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr Polym 101:1081–1086
Dufresne A (2012) Chemical modification of nanocellulose. In: Dufresne A (ed) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter GmbH, Berlin, pp 147–192
El-Sawy NM, Abd El-Rehim HA, Elbarbary AM, Hegazy E-SA (2010) Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr Polym 79:555–562
El-tahlawy KF, El-bendary MA, Elhendawy AG, Hudson SM (2005) The antimicrobial activity of cotton fabrics treated with different crosslinking agents and chitosan. Carbohydr Polym 60:421–430
Enescu D (2008) Use of chitosan in surface modification of textile materials. Roum Biotechnol Lett 13:4037–4048
Fras Zemljič L, Peršin Z, Stenius P (2009) Improvement of chitosan adsorption onto cellulosic fabrics by plasma treatment. Biomacromolecules 10:1181–1187
Graham WG (2007) The physics and chemistry of plasma for processing textiles and other materials. In: Shishoo R (ed) Plasma technologies for textiles. Woodhead Publishing, Cambridge, pp 3–24
Grimsey IM, Feeley JC, York P (2002) Analysis of the surface energy of pharmaceutical powders by inverse gas chromatography. J Pharm Sci 91:571–583
Gupta D, Haile A (2007) Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr Polym 69:164–171
Ilium L (1998) Chitosan and Its use as a pharmaceutical excipient. Pharm Res 15:1326–1331
Jones MD, Young P, Traini D (2012) The use of inverse gas chromatography for the study of lactose and pharmaceutical materials used in dry powder inhalers. Adv Drug Del Rev 64:285–293
Krishnaveni R, Thambidurai S (2013) Industrial method of cotton fabric finishing with chitosan-ZnO composite for anti-bacterial and thermal stability. Ind Crops Prod 47:160–167
Kyzas GZ, Kostoglou M, Lazaridis NK, Lambropoulou DA, Bikiaris DN (2013) Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem Eng J 222:248–258
Leceta I, Peñalba M, Arana P, Guerrero P, de la Caba K (2015) Ageing of chitosan films: effect of storage time on structure and optical, barrier and mechanical properties. Eur Polym J 66:170–179
Li Q, Dunn ET, Grandmaison EW, Goosen MF (1997) Applications and properties of chitosan. In: Goosen MFA (ed) Applications of chitin and chitosan. Technomic Publishing, Lancaster, pp 3–29
Lim SH, Hudson SM (2004) Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res 339:313–319
Lim SH, Hudson SM (2003) Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J Macromol Sci Part C Polym Rev 43:223–269
Liu Y, Chen X, Xin JH (2008) Hydrophobic duck feathers and their simulation on textile substrates for water repellent treatment. Bioinspir Biomim 3:46007–46015
Maachou H, Genet MJ, Aliouche D, Dupont-Gillain CC, Rouxhet PG (2013) XPS analysis of chitosan-hydroxyapatite biomaterials: from elements to compounds. Surf Interface Anal 45:1088–1097
Naebe M, Cookson PG, Denning R, Wang X (2011) Use of low-level plasma for enhancing the shrink resistance of wool fabric treated with a silicone polymer. J Text Inst 102:948–956
Naebe M, Cookson PG, Rippon J, Brady RP, Wang X, Brack N, Gv Riessen (2010) Effects of plasma treatment of wool on the uptake of sulfonated dyes with different hydrophobic properties. Text Res J 80:312–324
Potthast ART, Kosma P (2006) Analysis of oxidized functionalities in cellulose. Adv Polym Sci 205:1–48
Richardson JPDF, Dobbs FC, Alexeff I, Laroussi M (2000) On the use of the resistive barrier discharge to kill bacteria: Recent results In 27th IEEE International Conference on Plasma Science, ICOPS2000, 109. New Orleans, USA
Suseno N, Savitri E, Sapei L, Padmawijaya KS (2014) Improving shelf-life of cavendish banana using chitosan edible coating. Procedia Chem 9:113–120
Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28:988–994
Ye W, Xin JH, Li P, Lee K-LD, Kwong T-L (2006) Durable antibacterial finish on cotton fabric by using chitosan-based polymeric core-shell particles. J Appl Polym Sci 102:1787–1793
Yuen C-WM, Yip J, Liu L, Cheuk K, Kan C-W, Cheung H-C, Cheng S-Y (2012) Chitosan microcapsules loaded with either miconazole nitrate or clotrimazole, prepared via emulsion technique. Carbohydr Polym 89:795–801
Zhou C-E, Kan C-W (2014) Plasma-assisted regenerable chitosan antimicrobial finishing for cotton. Cellulose 21:2951–2962
Acknowledgments
Funding for this study was provided by the Central Research Grants Scheme, Deakin University. The authors express their gratitude to Dr. Robert Jones (Centre for Materials and Surface Science, La Trobe University) for running XPS experiments and Mr. Andrew Jones (CSIRO Manufacturing) for providing the cotton fabric.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naebe, M., Li, Q., Onur, A. et al. Investigation of chitosan adsorption onto cotton fabric with atmospheric helium/oxygen plasma pre-treatment. Cellulose 23, 2129–2142 (2016). https://doi.org/10.1007/s10570-016-0915-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0915-0