Abstract
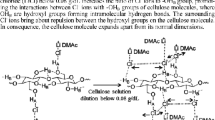
Four sources of cellulose with different molecular weights were dissolved in the ionic liquid 1-ethyl-3-methylimidazolium acetate at 100 °C over a 10 h period. The solution densities were determined and these results were subsequently utilised to access the influence of dissolved cellulose on surface tension properties of cellulose/ionic liquid solutions. Surface tension measurements revealed increasing molecular weight and concentration reduced surface tension while temperature increases showed the opposite effect. These results are consistent with that of repulsive polymer-wall interactions near the interface in good solvent conditions. The semi-flexible nature of this carbohydrate in solution can help explain deviations of these results when compared to ideal flexible chains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In contrast to the viscosity of cellulose/IL solutions, the density and surface tension of concentrated cellulose solutions remain largely unexplored, although pure ILs have been thoroughly characterised (Le et al. 2012; Sescousse et al. 2010). The surface tension of solutions impacts both the contact angle and wetting ability of a solution, thus representing valuable information for expanding the current applications of both cellulose and ILs in an industrial context, with applications as diverse as solution extrusion and electrospinning (Viswanathan et al. 2006). A linear density-temperature relationship is often observed for many ILs, with an increase in temperature reducing the density (Gardas et al. 2008; Jacquemin et al. 2006; Sescousse et al. 2010). The surface tension of ILs is much lower than water, varying between 30 and 50 mN/m at room temperature, depending on the anion, cation and side chain used, with the value decreasing as the temperature is raised (Sánchez et al. 2009).
The IL 1-ethyl-3-methylimidazolium acetate (EmimAc) is known to be capable of dissolving high concentrations of cellulose (Kosan et al. 2008), thus allowing synthesis of solutions of increasing cellulose concentrations. While for engineering based applications shorter dissolution times are preferable, the chosen dissolution time of 10 h and dissolution temperature of 100 °C represents a practical timeframe over which large amounts of cellulose can be dissolved (Pinkert et al. 2009). The foremost goal of the current work is to provide surface tension data for cellulose/IL solutions. Hence, the effects of cellulose concentration and DP on surface tension were analysed across temperatures commonly used for cellulose processing in IL.
Experimental procedures
Materials
Four different pure cellulose materials were used as received: (1) Sigmacell Type 20 microcrystalline cellulose (MCC) powder (Sigma-Aldrich, St. Louis, USA); (2) Arbocel BC 200 consisting of powdered cellulose pulp (J. Rettenmaier & Söhne GmbH, Rosenberg, Germany); (4) continuous rayon fibre (Cordenka GmbH, Obernburg, Germany); and (5) wood pulp (Cordenka). The moisture content of the Type 1 celluloses was in the region of 5 wt%, whereas the regenerated rayon fibre contained 11 wt%. The solvent and all celluloses were dried under vacuum at 80 °C for a 24 h period prior to use which removed all moisture from the cellulose samples. DP of as received and regenerated cellulose was determined according to ISO 5351/1-198 as outlined in Table 1. EmimAc was purchased from Sigma-Aldrich (Lot no. 51053) and used throughout. The Sigmacell and Arbocel powders were used as received, while the Cordenka pulp was manually frayed to loosen the fibres and the continuous Cordenka rayon fibres were cut into smaller sections of 3–5 mm to promote dissolution.
Preparation of cellulose/IL solutions
The various celluloses were added in 1 % increments and completely dissolved in EmimAc at 100 °C until a clear solution was obtained. The solution was heated using an IKA C-MAG HS7 hotplate (IKA Werke GmbH, Staufen, Germany) with temperature being continuously monitored. Dissolution was aided by the use of an IKA Eurostar overhead stirrer. The highest dissolution capacity of the various cellulose sources in the IL was determined over 10 h. Complete cellulose dissolution was initially accessed visually and was subsequently confirmed by polarised optical microscopy using a Leica DM IRM microscope (Leica Microsystems GmbH, Wetzlar, Germany). Sample names were designated according to the type and amount of cellulose fully dissolved in the IL. For example, 13.0A refers to a solution in which 13.0 wt% Arbocel was completely dissolved in EmimAc (Table 1).
Density measurement
Solution densities were measured by recording the mass of 10 ml of the solutions in volumetric flasks. Temperatures were set at 25, 80, 100 and 120 °C as these also correspond to the surface tension. The density values recorded were used in surface tension analysis.
Surface tension
All surface tension measurements were performed in a Krüss DSA 100 drop shape analysis system (Krüss GmbH, Hamburg, Germany) using the pendant drop method. Measurements were performed at controlled temperatures using the high temperature chamber Krüss TC21 in combination with the Krüss DO3241 high temperature dosing system with a syringe diameter of 2.1 mm. The instrument was controlled using Krüss drop shape analysis software DSA3, and the surface tension was calculated according to the implemented method devised by Song and co-workers (Song and Springer 1996). This study mainly focused on temperatures at 80, 100 and 120 °C, covering the range of temperatures typical for the dissolution of cellulose in ILs (Pinkert et al. 2009; Stark 2011; Sun et al. 2011). The surface tension was also recorded at 25 °C where possible.
Results and discussion
Influence of cellulose source and DP on dissolution capability
The dissolution of cellulose in IL is known to be governed by a number of parameters, namely: cellulose DP and physical particle size, IL type, moisture, temperature and time. While cellulose related dissolution is discussed in the following paragraph, the dissolution time and temperature, along with the solvent are the key parameters determining the amount of degradation experienced by the cellulose during the dissolution process. EmimAc is known to be a highly efficient solvent for dissolving cellulose (Kosan et al. 2008), although the addition of only 1 % of water has been shown to impair the dissolution capability of ILs (Pinkert et al. 2009; Swatloski et al. 2002). It can therefore be concluded that the removal of any moisture, either within the cellulose or IL, was successful as large quantities of cellulose were dissolvable (Table 1). A higher dissolution temperature and a longer dissolution time will increase the amount of cellulose that can be dissolved, albeit at the risk of severely degrading the cellulose (Bentivoglio et al. 2006; Pinkert et al. 2009; Vitz et al. 2009). While some cellulose degradation is inevitable, the dissolution time and temperature chosen here are both at medial values commonly used for cellulose dissolution, as shown by an extensive overview given by a review by Pinkert et al. (Pinkert et al. 2009). Hence it can be concluded that the conditions chosen give a balance between efficient dissolution and excessive cellulose degradation.
The as-received cellulose samples exhibited a broad range of DP values from 130 (Sigmacell) to ~1500 (Arbocel) as shown in Table 1. MCC is generally considered to have a DP in the range of 160–200 (Duchemin et al. 2009; Song et al. 2011). Thus, the DP of Sigmacell was on the low side of this range due to increased grinding required to produce a 20 μm powder as reported elsewhere (Terinte et al. 2011; Virtanen et al. 2012). The two different pulps show quite different DP values, with the wood pulp sheets having a lower DP of 880 in contrast to the DP of 1540 of the Arbocel. The DP of pulp is known to vary depending on the cellulose source, purification steps and treatment time involved, however both values fall well into the DP range common for pulps (Hallac and Ragauskas 2011; Vizárová et al. 2012). Regenerated rayon fibres usually have a DP ranging between 350 and 600 (Krässig et al. 2004; Ruan et al. 2006), which compares well with the rayon fibres measured here showing a DP of 440. The dissolution procedure generally reduces the DP of cellulose, with extended dissolution time and elevated temperature having a negative effect on DP, although different cellulose sources are not all affected to the same extent (Cao et al. 2010; Duchemin et al. 2009; Kosan et al. 2008; Pinkert et al. 2009). Here, the wood pulp shows the highest DP loss (>50 %) following dissolution in EmimAc. Rayon fibres have the lowest DP reduction, with a decrease <20 % during dissolution. However, it is important to remember that the as-received rayon fibre consists of regenerated cellulose II such that its DP has already been reduced by the viscose process.
The viscosity of the IL, and thus solution viscosity, is understood to govern the dissolution kinetics implying a higher viscosity solution will diffuse and dissolve the remaining cellulose at a slower rate (Gericke et al. 2009; Ries et al. 2014). Thus, an increase in DP reduces the rate of cellulose dissolution in IL. During the dissolution procedure the transparency of the solution increased as cellulose dissolves, with complete dissolution yielding a clear solution As expected, the low DP Sigmacell has the highest dissolution capacity in EmimAc (25.9 wt%), despite the limited dissolution time (Table 1). EmimAc can therefore be considered an effective solvent for cellulose dissolution, capable of dissolving large quantities of cellulose. The highest fully dissolved cellulose concentrations obtained here are in line with other studies where methylimidazolium based ILs were employed using similar dissolution procedures (Pinkert et al. 2009). Higher Arbocel concentrations were dissolvable when compared to either the wood pulp or rayon fibres, despite the Arbocel having the highest DP of these celluloses. However, Arbocel BC 200 is supplied in powder form that promotes dissolution due to its smaller fibre diameter enabling the cellulose particles to be more thoroughly contacted by the IL. The wood pulp was received in the form of pressed sheets, which despite being manually frayed prior to dissolution had larger fibre diameters which hindered the dissolution process. Despite the fineness of the rayon fibres and being cut into shorter sections before dissolution, fibre entanglement during stirring could be observed reducing the ability of the IL to dissolve the fibres efficiently. Additionally, the relative length of these short-cut fibres additionally slowed the dissolution process. Based on these observations, the physical form of the pulp and accessibility of the fibres appears to have a greater influence on the dissolution efficiency than the DP of the cellulose. This is in agreement with findings by Sun and co-workers, where the effect of wood particle size on dissolution capacity in EmimAc was examined, finding that smaller particles dissolve more completely than larger particles over a 16 h timeframe (Sun et al. 2009).
Density of cellulose/EmimAc solutions
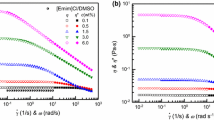
A linear reduction in the density of all cellulose/IL solutions is evident with increasing temperature (Fig. 1). Previous studies related to temperature induced density changes in pure IL have found similar results (Gardas et al. 2008; Ghani et al. 2014; Jacquemin et al. 2006; Sescousse et al. 2010). Indeed, in further analysis, Yusoff and coworkers evaluated the temperature/density correlations using linear, exponential, power and polynomial expressions and concluded that a linear relationship most accurately described the relationship (Ghani et al. 2014). The present work demonstrates that the density of the cellulose/EmimAc solutions decreases with temperature at a similar rate as that found for pure EmimAc (Fig. 1). The density-temperature relationship of EmimAc has been previously characterised as ρEmimAc = −0.0006T + 1.1314 where T is in °C (Sescousse et al. 2010). In the present work, it is found that ρEmimAc = −0.0005T + 1.1104 (using Fig. 1) which corresponds closely to previous findings. The slope of ρEmimAc was found to range between −0.0005 and −0.0006 for all solutions tested, indicating that dissolved cellulose does not influence the expansion of the solution with increasing temperature and only increases the absolute solution density.
Surface tension of dissolved cellulose solutions
Measurement of the surface tension at room temperature of dissolved cellulose concentrations exceeding 4.8 wt% proved unreliable despite the use of the closed temperature chamber and have not been included in Table 2. Indeed, strong drop distortion and asymmetry were observed, probably due to moisture uptake and excessive viscosity (Le et al. 2012; Vitz et al. 2009). The asymmetry renders the implemented axisymmetric drop shape analysis routine useless. Within the studied concentration range, an increase in cellulose concentration is shown to decrease surface tension (Table 2), with the reduction in surface tension becoming more pronounced at higher concentrations. These observations indicate that cellulose acts as a polymeric surfactant when dissolved in EmimAc, with concentration and chain lengths acting as the determining variables. Indeed, most polymers act as surfactants when dissolved in a solvent (Kim and Cao 1993; Oosawa and Asakura 1954; Stubenrauch et al. 2000).
The surface tension of pure ILs generally decreases slowly with increasing temperatures (Dzyuba and Bartsch 2002; Sánchez et al. 2009). For the cellulose solutions studied in this work, an increase in temperature induces an increase in surface tension, with the effect becoming more apparent at higher cellulose concentrations (Table 2). Unfortunately it was not possibly to establish a clear relationship linking the cellulose DP, cellulose concentration and solution temperature with the observed surface tension, most likely due to the temperature related dissolved chain migration within the sessile drop discussed below. The temperature related surface tension increase observed here appears somewhat counterintuitive, however not totally uncommon for polymer solutions. Indeed, it is known that a depletion layer can be created near the surface of solvent/gas wall (Allain et al. 1982; Di Meglio et al. 1983; Redon et al. 1992). This depletion layer translates in what is often called a “repulsive wall”. This solvent-rich zone is the result of a reduction of the entropic contribution of the macromolecular coil, resulting in an increased steric repulsion between the polymer and the liquid/vapour interface. Starting from the wall where it equals zero, the polymer concentration increases continuously until a thickness of about one radius of gyration Rg (true in the dilute and semi-dilute cases) is reached, at which point the polymer concentration Φ(z) reaches Φbulk, where Φbulk is the polymer concentration in the bulk of the polymer solution (Di Meglio et al. 1983). Following Cahn’s approach and using the expression of the Flory mean-field free energy for a polymer in solution, the same authors showed that the introduction of a depletion layer created a linear temperature dependence of the surface tension on temperature. In the case of semi-flexible polymers like xanthan or cellulose, the picture appeared slightly more complicated and the concentration Φ(z = 0) cannot be considered null anymore. Instead, the concentration profile would show a finite number of monomers directly linked to Kühn’s length at the surface and Φ(z = 0) would scale as ~Φbulk.R −2g (Ausserre et al. 1985). This result is also in accordance with the observed phenomenon that shows that an increase of Φbulk can decrease the surface tension by locally increasing the amount of polymer adsorbed against the wall. However, this result fails to explain the decrease of surface tension with increasing molecular weight. This could be explained by the dilute nature of the solutions for which this theory was devised instead of the semi-dilute or entangled one studied in this work for which the confinement tube would necessarily be confined itself against the wall. Further work needs to be undertaken to explain the effect on surface tension of semi-dilute or entangled solutions of semi-flexible chains in a good solvent near a repulsive wall. Strictly speaking, it is not possible to discard the influence of the degradation products on the surface tension behaviour either.
Conclusion
Unlike the surface tension of pure EmimAc which decreases with increasing temperature, the surface tension of cellulose solutions showed an opposite trend. The addition of dissolved cellulose was found to reduce the surface tension of a solution with the reduction becoming more pronounced with increasing cellulose amounts, increasing cellulose DP and lowering temperature. It was inferred that cellulose/EmimAc mixture is a system in which the concentration regime, the backbone stiffness as well as the repulsive nature of the interface on the polymer chains play an important role. The information obtained within this work is expected to promote the use of cellulose/IL solutions as viscosity and surface tension dictate capillary forces. The assessment of capillary forces behaviour is the key to success in applications such as continuous coating, wet-spinning, extrusion, electrospinning and all-cellulose composite processing.
References
Allain C, Ausserre D, Rondelez F (1982) Direct optical observation of interfacial depletion layers in polymer solutions. Phys Rev Lett 49(23):1694–1697
Ausserre D, Hervet H, Rondelez F (1985) Depletion layers in polymer solutions: influence of the chain persistence length. J Phys Lett 46(19):929–934
Bentivoglio G, Röder T, Fasching M, Buchberger M, Schottenberger H, Sixta H (2006) Cellulose processing with chloride-based ionic liquids. Lenzinger Berichte 86:154–161
Cao Y, Li H, Zhang Y, Zhang J, He J (2010) Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids. J Appl Polym Sci 116(1):547–554
Di Meglio JM, Ober R, Paz L, Taupin C, Pincus P, Boileau S (1983) Study of the surface tension of polymer solutions: theory and experiments in theta solvent conditions. J Phys 44(9):1035–1040
Duchemin BJC, Mathew AP, Oksman K (2009) All-cellulose composites by partial dissolution in the ionic liquid 1-butyl-3-methylimidazolium chloride: composites part A. Appl Sci Manufact 40(12):2031–2037
Dzyuba SV, Bartsch RA (2002) Influence of structural variations in 1-alkyl (aralkyl)-3-methylimidazolium hexafluorophosphates and bis (trifluoromethylsulfonyl) imides on physical properties of the ionic liquids. Chem Phys Chem 3(2):161–166
Gardas RL, Dagade DH, Coutinho JAP, Patil KJ (2008) Thermodynamic studies of ionic interactions in aqueous solutions of imidazolium-based ionic liquids [Emim][Br] and [Bmim][Cl]. J Phys Chem B 112(11):3380–3389
Gericke M, Schlufter K, Liebert T, Heinze T, Budtova T (2009) Rheological properties of cellulose/ionic liquid solutions: from dilute to concentrated states. Biomacromolecules 10(5):1188–1194
Ghani NA, Sairi NA, Aroua MK, Alias Y, Yusoff R (2014) Density, surface tension, and viscosity of ionic liquids (1-ethyl-3-methylimidazolium diethylphosphate and 1, 3-dimethylimidazolium dimethylphosphate) aqueous ternary mixtures with MDEA. J Chem Eng Data 59(6):1737–1746
Hallac BB, Ragauskas AJ (2011) Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod Biorefin 5(2):215–225
Jacquemin J, Husson P, Padua AAH, Majer V (2006) Density and viscosity of several pure and water-saturated ionic liquids. Green Chem 8(2):172–180
Kim MW, Cao BH (1993) Additional reduction of surface tension of aqueous polyethylene oxide (PEO) solution at high polymer concentration. EPL (Europhys Lett) 24(3):229
Kosan B, Michels C, Meister F (2008) Dissolution and forming of cellulose with ionic liquids. Cellulose 15(1):59–66
Krässig H, Schurz J, Steadman RG, Schliefer K, Albrecht W, Mohring M et al (2004) Cellulose. Ullmann’s Encycl Ind Chem 7:279–330
Le KA, Sescousse R, Budtova T (2012) Influence of water on cellulose-EMIMAc solution properties: a viscometric study. Cellulose 19:45–54
Oosawa F, Asakura S (1954) Surface tension of high-polymer solutions. J Chem Phys 22(7):1255
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109(12):6712–6728
Redon C, Ausserre D, Rondelez F (1992) Concentration dependence of the interfacial tension of polymer solutions near repulsive walls and in good solvent. Macromolecules 25(22):5965–5969
Ries ME, Radhi A, Keating AS, Parker O, Budtova T (2014) Diffusion of 1-ethyl-3-methyl-imidazolium acetate in glucose, cellobiose, and cellulose solutions. Biomacromolecules 15(2):609–617
Ruan D, Zhang L, Lue A, Zhou J, Chen H, Chen X et al (2006) A rapid process for producing cellulose multi-filament fibers from a NaOH/thiourea solvent system. Macromol Rapid Commun 27(17):1495–1500
Sánchez LG, Espel JR, Onink F, Meindersma GW, Haan AB (2009) Density, viscosity, and surface tension of synthesis grade imidazolium, pyridinium, and pyrrolidinium based room temperature ionic liquids. J Chem Eng Data 54(10):2803–2812
Sescousse R, Le KA, Ries ME, Budtova T (2010) Viscosity of cellulose-imidazolium-based ionic liquid solutions. J Phys Chem B 114(21):7222–7228
Song B, Springer J (1996) Determination of interfacial tension from the profile of a pendant drop using computer-aided image processing: 1: theoretical. J Colloid Interface Sci 184(1):64–76
Song H, Niu Y, Wang Z, Zhang J (2011) Liquid crystalline phase and gel-sol transitions for concentrated microcrystalline cellulose (MCC)/1-ethyl-3-methylimidazolium acetate (EMIMAc) solutions. Biomacromolecules 12:1087–1096
Stark A (2011) Ionic liquids in the biorefinery: a critical assessment of their potential. Energy Environ Sci 4(1):19–32
Stubenrauch C, Albouy PA, Klitzing RV, Langevin D (2000) Polymer/surfactant complexes at the water/air interface: a surface tension and X-ray reflectivity study. Langmuir 16(7):3206–3213
Sun N, Rahman M, Qin Y, Maxim ML, Rodriguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11(5):646–655
Sun N, Rodriguez H, Rahman M, Rogers RD (2011) Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem Commun 47(5):1405–1421
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124(18):4974–4975
Terinte N, Ibbett R, Schuster KC (2011) Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): comparison between measurement techniques. Lenzinger Berichte 89:118–131
Virtanen T, Svedström K, Andersson S, Tervala L, Torkkeli M, Knaapila M et al (2012) A physico-chemical characterisation of new raw materials for microcrystalline cellulose manufacturing. Cellulose 19:219–235
Viswanathan G, Murugesan S, Pushparaj V, Nalamasu O, Ajayan PM, Linhardt RJ (2006) Preparation of biopolymer fibers by electrospinning from room temperature ionic liquids. Biomacromolecules 7(2):415–418
Vitz J, Erdmenger T, Haensch C, Schubert US (2009) Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem 11(3):417–424
Vizárová K, Kirschnerová S, Kacik F, Brivskárová A, Suty S, Katuscák S (2012) Relationship between the decrease of degree of polymerisation of cellulose and the loss of groundwood pulp paper mechanical properties during accelerated ageing. Chem Pap 66(12):1124–1129
Acknowledgments
The authors would like to acknowledge the Dumont D’Urville S&T programme and would like to thank D. Dallerac, K. Janel, A. Dufresne, J. Bréard, L. Bizet and J. Dormanns for assistance. The authors are very grateful to Cordenka GmbH and J. Rettenmaier & Söhne GmbH for the supply of materials. J.S. would like to acknowledge the financial assistance provided by a UC Doctoral Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schuermann, J., Huber, T., LeCorre, D. et al. Surface tension of concentrated cellulose solutions in 1-ethyl-3-methylimidazolium acetate. Cellulose 23, 1043–1050 (2016). https://doi.org/10.1007/s10570-015-0850-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0850-5