Abstract
Fabricating an aqueous ionic liquid (IL) for deconstruction and dissolution of lignocellulose is attractive because addition of water could reduce the cost and viscosity of the solvent and improve the biomass processing, but the solvating power of the IL is usually depressed in the presence of water. In the present study, an aqueous IL consisting of 1-butyl-3-methylimidazolium chloride (BmimCl), water, and lithium chloride was fabricated for efficient deconstruction and dissolution of lignocellulose (bamboo). The dissolution of cell wall components (cellulose, lignin, and hemicelluloses) in the aqueous IL was investigated. The results indicated that the presence of water significantly reduced the solvating power of BmimCl; For example, 11.5 % water decreased the dissolution of bamboo in BmimCl from ~97 to ~53 %. Dissolution of cellulose and lignin was specifically depressed. However, addition of lithium chloride was able to improve the tolerance of BmimCl to water and enhance the deconstruction and dissolution of biomass in BmimCl with high water content. It was found that approximately 80 % bamboo could be dissolved in solvent consisting of 45 wt% BmimCl and 55 wt% LiCl·2H2O (25 wt% overall water content in the solvent). In particular, lignin and hemicelluloses were selectively dissolved by 96 and 92 %, respectively. The undissolved residue was predominantly composed of cellulose (~86 %) with a small amount of lignin (<5 %). BmimCl-LiCl-H2O is a promising and effective solvent system with low cost and viscosity for biomass processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Concerns about dwindling petroleum reserves and environmental deterioration are the driving forces behind the current boom in biofuel and biochemical production from lignocellulose, a sustainable, biodegradable, renewable, and carbon-neutral natural resource (Huber et al. 2006; Carlson et al. 2008). However, the recalcitrance of lignocellulose, primarily originating from the crystallinity of cellulose, the presence of lignin (Cosgrove 2005), and the tough structure of biomass (Iiyama et al. 1994), hinders economical and efficient processing and conversion of biomass to desired products (Himmel et al. 2007). Many technologies have been developed to remove or reduce the recalcitrance of lignocellulose, such as mechanical comminution, dissolution by novel solvents such as ionic liquids (ILs), steam explosion, acid pretreatment, and ammonia fiber expansion (AFEX), to break down the compact plant cell wall, increase the pore size of substrates, and remove hemicellulose and/or lignin (Wen et al. 2014).
Ionic liquids (ILs) are considered to be green solvents for processing lignocellulose, having great solvating power for lignocellulose and tunable properties. Ionic liquids refer to a group of molten salts with low melting point (<100 °C), possessing various unique properties (Rogers and Seddon 2003; Kulkarni et al. 2007). Many ILs with various ion combinations have been demonstrated to be good solvents for cellulose, lignin, and even whole lignocellulose (Liu et al. 2012); For example, 1-allyl-3-methylimidazolium (AmimCl), 1-butyl-3-methylimidazolium chloride (BmimCl), and 1-ethyl-3-methylimidazolium acetate (EmimOAc) exhibit excellent solvating power and are even able to dissolve whole lignocellulose (Kilpeläinen et al. 2007).
The performance of ILs for dissolving lignocellulose is affected by many factors, such as processing conditions, cosolvents, and impurities. In particular, the presence of water can substantially impair the solvating power of ILs; For example, Swatloski et al. (2002) observed that as little as 1.0 wt% water could significantly inhibit dissolution of cellulose in IL. Martin et al. (2011) found that addition of water depressed dissolution of cellulose in BmimCl, AmimCl, and EmimOAc. Essentially, water competes with ILs to form hydrogen bonds with lignocellulose and markedly reduces the dissolving power of ILs (Cammarata et al. 2001; Pang et al. 2014). On the other hand, addition of water to IL has the advantages of lowering the IL loading (and thereby cost), reducing the viscosity, improving mass transfer, and simplifying recovery of ILs. ILs generally have high viscosity because of strong interactions between their cations and anions, but small amounts of water can disrupt such interactions and thus remarkably reduce the viscosity, because water molecules preferably associate with the anion of ILs (Cammarata et al. 2001) and destroy network linkages among the ions (Sebastian et al. 2011). Low viscosity would improve mass transfer, facilitate operation, and reduce energy consumption for mixing and transport. In addition, use of aqueous ILs could eliminate the need for water removal during IL recovery or regeneration; this process is generally conducted through distillation, which is energy intensive, particularly at low water content, because of the strong affinity of water to the anions of ILs. Furthermore, considering that both ILs and lignocellulose naturally exhibit strong hydroscopicity and are inclined to absorb water from the atmosphere, it is quite difficult to process lignocellulose in ILs in the complete absence of water. Aqueous ILs are therefore promising and attractive for processing lignocellulose.
Understanding and improving the performance of aqueous ILs for processing lignocellulose have been explored but need further investigation. Mazza et al. (2009) found that the tolerance of BmimCl to water depended on both temperature and cellulose concentration, and precipitation occurred at approximately 0.2 g water per g BmimCl in a solution of 1 g cellulose/100 g BmimCl. Several groups have investigated pretreatment of lignocellulose in aqueous ILs to enhance cellulose digestibility and lignin/cellulose fractionation (Agnieszka et al. 2011; Fu and Maz 2011a, b), but such processes still suffer from low effectiveness. In addition, cosolvents and metal salts can substantially alter the properties and performance of ILs in processing biomass (Andanson et al. 2014); For example, salts can enhance biomass dissolution and deconstruction in ILs (Binder and Raines 2009). Some metal salt hydrates, such as zinc(II) chloride and lithium salts, were found to be able to swell and dissolve cellulose (Amarasekara and Ebede 2009; Yang et al. 2014).
Against this background, the present study aims to investigate the deconstruction and dissolution of lignocellulose (bamboo) in ionic liquid (BmimCl) with high water content, including the disruption (disintegration) of the bamboo matrix, the depolymerization/hydrolysis of the bamboo cell wall components (cellulose, hemicelluloses, and lignin), and the dissolution of the components and whole bamboo. Metal salts such as lithium chloride were used to improve the tolerance of BmimCl to water and enhance the dissolution of the biomass in aqueous BmimCl. The dissolution and changes of individual cell wall components (cellulose, lignin, and hemicelluloses) were examined.
Experimental
Materials
All reagents used in experiments were of analytical grade and used without any further purification. BmimCl with purity of 99.0 % was purchased from Shanghai Chengjie Chemicals, China. N,N-Dimethylformamide (DMF), lithium chloride, nickel(II) chloride, lithium bromide, lithium iodide, and zinc chloride were purchased from VWR. Bamboo as lignocellulosic feedstock was harvested from the southern USA and ground using a Wiley mill (IKA MF10 Basic). The ground bamboo was fractionated using a series of US standard sieves, and the fractions with size of 20–30, 30–50, and 50–100 mesh were collected for this study. Unless otherwise noted, the bamboo fraction with size of 30–50 mesh was used in most experiments. The samples were dried at 50 °C for 48 h to remove moisture prior to dissolution in ILs. The bamboo contained 51.3 % cellulose, 20.2 % hemicelluloses, 23.4 % lignin (21.6 % acid-insoluble lignin and 1.8 % acid-soluble lignin), 1.78 % ash, and 2.56 % extractives.
Deconstruction and dissolution of lignocellulose
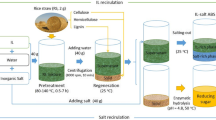
Deconstruction and dissolution of lignocellulose (bamboo) in ILs was conducted in a 25-mL round-bottomed flask, which was placed into an oil bath with vigorous magnetic stirring (800 rpm) under nitrogen gas atmosphere. The flask was equipped with a condenser to avoid solvent loss during treatment. Prior to addition of bamboo powder (0.5 g), BmimCl, metal salt, and water (10 g in total) were successively added into the flask and mixed homogeneously for 30 min at a specified treatment temperature. After treatment, the reaction mixture was diluted with DMF to biomass consistency of approximately 1.5 wt%, then vigorously stirred (1500 rpm) for 30 min at room temperature. Because DMF is an aprotic solvent and can be used as cosolvent with ILs, this dilution will not result in precipitation of dissolved biomass. Subsequently, the solution was filtered through ash-free filter paper (Whatman no. 1442 070) on a Büchner funnel. The undissolved lignocellulose was thoroughly washed with DMF (5 × 10 mL) to remove dissolved substances that were absorbed or trapped within the solid. The residual solid was further washed with deionized water (5 × 10 mL) and then dried at 105 ± 3 °C for 6 h. The dissolubility of lignocellulose was calculated according to Eq. (1).
where m i is the initial weight of lignocellulose and m r is the residual weight of lignocellulose after filtration.
Regeneration of dissolved lignocellulose
After the undissolved biomass had been removed by filtration, the filtrate (10 mL) was mixed with water (40 mL) by vigorous stirring (1500 rpm) at room temperature for 30 min to regenerate the biomass. The regenerated (precipitated) lignocellulose was collected by filtration through ash-free filter paper (Whatman no. 1442 070) on a Büchner funnel, washed with water, and dried at 105 ± 3 °C for 6 h. The regeneration yield was calculated as the percentage of the weight of regenerated lignocellulose to the weight of the dissolved lignocellulose.
FT-IR analysis
IR spectra were collected on a Fourier-transform infrared (FT-IR) spectrometer (PerkinElmer Spectrum 100) with attenuated total reflectance (ATR) accessory at 2-cm−1 resolution with 16 scans within 4000 to 600 cm−1. The crystallinity index (CrI) of cellulose was estimated using Eq. (2) (O’Connor et al. 1958).
where \(A_{{1428\,{\text{cm}}^{ - 1} }}\) and \(A_{{898\,{\text{cm}}^{ - 1} }}\) are the absorbance at 1428 and 898 cm−1, respectively.
Determination of structural carbohydrates and lignin in starting bamboo and bamboo undissolved in ILs
The structural carbohydrates (cellulose and hemicelluloses) and lignin [acid-insoluble lignin (AIL) and acid-soluble lignin (ASL)] of bamboo sample and the undissolved bamboo were determined using a scaled-down (ca. 0.1 g sample) National Renewable Energy Laboratory analytical procedure (NREL/TP-510-42618). Acid-soluble lignin was determined by using an ultraviolet–visible (UV–Vis) spectrophotometer (Thermo Scientific Genesys 10S) at 205 nm using an extinction coefficient of 110 L g−1 cm−1.
Determination of 5-hydroxymethylfurfural (HMF), furfural, and levulinic acid (LA)
Dehydration products of monosaccharides generated during the deconstruction treatment, including furfural, levulinic acid, and HMF, were analyzed using high-performance liquid chromatography (HPLC, Dionex ICS-3000) equipped with a Supelcogel C-610H column at temperature of 30 °C and an UV detector at 210 nm. The eluent was 0.1 % phosphoric acid at flow rate of 0.7 mL/min.
Determination of monosaccharides
Monosaccharides were quantitated using a high-performance ion chromatography (HPIC, Dionex ICS-3000) system equipped with an integrated amperometric detector and a Carbopac™ PA1 guard column and an analytical column at 30 °C. Eluent was provided at flow rate of 0.7 mL/min, according to the following gradient: 0–25 min, 100 % water; 25.1–35 min, 30 % water and 70 % 0.1 M NaOH; 35.1–40 min, 100 % water. Postcolumn eluent of 0.5 M NaOH at flow rate of 0.3 mL/min was used to ensure baseline stability and detector sensitivity.
Determination of carbohydrate loss after deconstruction treatment in ILs
Lignocellulose (bamboo) was fractionated into dissolved and undissolved portions after deconstruction treatment in ILs, which were separated by filtration. The carbohydrates in the dissolved portion (filtrate) were determined after acid-catalyzed hydrolysis of oligosaccharides to monosaccharides. Briefly, 72 wt% sulfuric acid (0.59 g) was added into the filtrate (10 g); the mixture was stirred for 30 min at 30 °C; then the mixture was autoclaved at 121 °C for 60 min to complete the hydrolysis. The hydrolysate was cooled down to room temperature, neutralized with sodium carbonate to pH 5–6, then filtrated on filter paper (Whatman no. 1442-090). The monosaccharides in the hydrolysate were quantitated using HPIC as described above. Meanwhile, the mixture of standard sugars d-glucose, d-xylose, d-mannose, d-galactose, and l-arabinose was autoclaved to calibrate the sugar loss during the autoclaving. The carbohydrates in the undissolved portion were determined according to the procedure described above. The carbohydrate loss after deconstruction was calculated according to Eq. (3).
where CL is the percentage carbohydrate loss after deconstruction treatment, ∑MD is the total molar percent of monosaccharides relative to the feedstock in the dissolved portion, and ∑MU is the total molar percent of monosaccharides relative to the feedstock in the undissolved portion.
Results and discussion
Deconstruction and dissolution of bamboo in BmimCl
Processing lignocellulose in ILs has been reported in many studies, mainly focusing on condition optimization, synthesis of novel ILs, and catalytic conversion of biomass (Zhang 2013; Vo et al. 2011; Xu et al. 2010). However, the dissolving abilities of ILs for individual components of lignocellulose, namely cellulose, lignin, and hemicelluloses, have not been sufficiently investigated. We studied the deconstruction and dissolution of bamboo in BmimCl (Table 1). When the treatment was conducted at lower temperature (130 °C), the solvating power of BmimCl was weak. Only 66.8 % of bamboo was dissolved in 8 h (entry A1), while the dissolubility increased to 75.5 % when the treatment was extended to 16 h (entry A3). The composition of the undissolved bamboo showed that the majority of lignin was retained, whereas most of the cellulose and hemicelluloses were dissolved; For example, the percentage dissolution of lignin remained nearly unchanged when the treatment time was extended from 8 h (38.0 %) to 16 h (39.7 %), whereas the lignin content in the undissolved bamboo increased from 43.7 to 57.5 %, implying that more carbohydrates were dissolved. In addition, the CrI of the undissolved bamboo increased with the treatment time. Generally, amorphous cellulose can be more easily dissolved out, leading to enrichment of crystalline cellulose in the undissolved portion. The results suggest that lignin and crystalline cellulose should be the predominant recalcitrant factors impeding efficient deconstruction of lignocellulose in BmimCl.
When the deconstruction treatment was conducted at higher temperature (140 °C), high dissolubility (91.6 %) of bamboo could be readily achieved (entry A4). Compared with lower temperature, the percentage dissolution of lignin and hemicelluloses at higher temperature significantly increased from 38.0 to 94.3 % and from 63.7 to 93.2 %, respectively, whereas the percentage dissolution of cellulose only increased from 79.3 to 89.4 %. Generally, dissolution of hemicelluloses was believed to be easier because of low polymerization degree and amorphous structure. Additionally, hemicelluloses and lignin were tangled and covalently cross-linked as carbohydrate–lignin complexes (LCC), thus lignin dissolution simultaneously facilitated hemicellulose dissolution. Lignin dissolution was preferable at high temperature, which might be due to enhanced cleavage of covalent bonds among lignin units (Lasse et al. 2013). Approximately complete dissolution of whole biomass was achieved after 12-h treatment (dissolubility of 96.7 %); the composition of the undissolved residual was not analyzed due to the small quantity (entry A5).
ILs tend to adsorb moisture from air, and feedstock lignocellulose brings water into the system as well. Water molecules entering the system will strongly associate with anions of ILs, which reduces the propensity of ILs to interact with lignocellulose and consequently weakens the solvating power of ILs. Thus, the effect of the water content in BmimCl on the deconstruction and dissolution of bamboo was investigated. The deconstruction treatment was conducted at 140 °C for 14 h with 5.0 wt% biomass consistency. As shown in Fig. 1, the solvating power of BmimCl decreased significantly in the presence of water. The dissolubility of bamboo decreased from 96.7 % at no water to 87.5 % at 5.0 % water and then to 53.4 % at 11.5 % water. In addition, the regeneration yield of the dissolved bamboo decreased with increasing water content in BmimCl. This is because the dissolution of high-molecular-weight components was reduced in the presence of water, being easier to precipitate in an antisolvent. Therefore, the regeneration yield could serve as an indicator of the deconstruction and dissolution power of the IL system. High regeneration yield means that more cellulose and lignin with relatively high molecular weight are dissolved after appropriate deconstruction (depolymerization). The results above suggest that the deconstruction performance of BmimCl was reduced with addition of water.
Deconstruction performance with varying water content in BmimCl: a dissolubility of lignocellulose, regeneration yield, and CrI of the undissolved portion, b lignin content in the undissolved portion and percentage dissolution of lignin, c cellulose content in the undissolved portion and percentage dissolution of cellulose, d hemicelluloses content in the undissolved portion and percentage dissolution of hemicelluloses
The percentage dissolution of lignin, cellulose, and hemicelluloses in aqueous BmimCl all gradually decreased as the water content was increased (Fig. 1). Whereas the percentage dissolution of lignin was only slightly affected by the water content, the percentage dissolution of cellulose and hemicelluloses were significantly affected; For example, the percentage dissolution of cellulose decreased from 89.8 to 39.1 % when the water content was increased from 5.0 to 11.5 %. Meanwhile, the CrI of the undissolved lignocellulose decreased from 74.9 to 56.1 %. The low percentage dissolution of cellulose and reduced CrI of the undissolved lignocellulose suggest that even the dissolution of amorphous cellulose with low crystallinity was depressed when water was added. Thus, the solvating power of BmimCl for lignin, cellulose, and hemicelluloses were all impaired with water addition, especially on cellulose dissolution. Therefore, it is important practically to know how to improve and enhance the deconstruction of lignocellulose in ILs at high water content.
Deconstruction and dissolution of bamboo in BmimCl with addition of different metal salt hydrates
Nonderivatizing dissolution of cellulose involves disruption of the hydrogen-bond network extensively present in cellulose, while lignin dissolution is largely related to n–π and π–π conjugation, dispersive, and ion–dipole interactions. Solvents of cellulose typically possess cation or anion that can strongly interact with hydroxyl group via hydrogen bond. As discussed above, BmimCl was able to efficiently dissolve cellulose, lignin, and lignocellulose, but addition of water negatively affected the dissolution of biomass components in BmimCl. Many metal salts, such as lithium bromide, magnesium chloride, and chromium chloride, have been found to be able to enhance the dissolution and conversion of biomass (Zhao et al. 2007; Binder and Raines 2009). Thus, whether addition of metal salts could improve the deconstruction of lignocellulose in BmimCl with water was investigated. The results are summarized in Table 2. Water addition was calculated as molar ratio to metal salt. The number in subscript is the weight percentage of the metal salt hydrate (salt plus water) in a solvent system; For example, BmimCl[NiCl2·2H2O]25 is a solvent system consisting of 75 wt% BmimCl and 25 wt% NiCl2·2H2O.
The BmimCl–metal salt–water systems investigated can be divided into three groups based on their performance in dissolving/deconstructing lignocellulose. The first group had extremely low ability to dissolve lignocellulose; addition of the salt hydrate significantly impaired the solvating power of BmimCl, leaving a large portion of the lignocellulose undissolved. This group includes nickel(II) chloride (entry B1) and zinc(II) chloride (entry B2). After treatment in BmimCl with addition of nickel(II) chloride hydrate, the macro appearance of the lignocellulose remained nearly unchanged. It was reported that nickel(II) can coordinate with chloride to form NiCl n (n > 2) coordination complexes (Peter et al. 1993). Therefore, when NiCl2 was added in BmimCl, formation of NiCl n (n > 2) resulted in a significant decrease in the concentration of free chloride ions in the system. It is believed that the chloride ion plays an important role in dissolving cellulose via formation of hydrogen bonds with cellulose hydroxyls. Therefore, the reduction of free chloride ions substantially impaired the dissolving power of BmimCl. As shown in Table 2, BmimCl[NiCl2·2H2O]25 only dissolved 7.4 % of the biomass, including 1.6 % lignin, 2.9 % cellulose, and 11.6 % hemicelluloses. In addition, the dissolved portion could not be regenerated, implying that the dissolved portion was of low molecular weight and unable to precipitate in the antisolvent. Compared with nickel(II) chloride, the solvating power of BmimCl with addition of zinc(II) chloride was slightly better; however, the regeneration yield (23.1 %) was fairly low because not much high-molecular-weight components were dissolved.
The second group is copper(II) chloride (entry B3). This system was able to almost completely dissolve lignocellulose with high dissolubility (99.0 %), but the overall carbohydrate recovery was very low. Only negligible monosaccharides were detected in the regeneration filtrate, and small amounts of levulinic acid (1.23 g/100 g biomass) and HMF (3.98 g/100 g biomass) were produced. Furthermore, the carbohydrate loss was up to 46.2 %, which might have been converted to HMF, levulinic acid, and humins. Considering the low yields of HMF and levulinic acid mentioned above, the majority of the lost carbohydrates were presumably transformed into humins, as indirectly confirmed by the analysis below. When the regenerated biomass was analyzed for lignin content, the acid-insoluble fraction was as high as 77.5 %. Since the maximum possible insoluble fraction is 29.5 % (calculated as the acid-insoluble lignin content in bamboo of 21.6 % divided by the regeneration yield of 73.3 %) even if all the lignin were regenerated, it is reasonable to believe that the rest of the insoluble fraction was humins formed during the treatment.
The third group is lithium salts (entries B4–B6), which could enhance deconstruction and dissolution of lignocellulose in BmimCl with water; For example, for the same water content (11.5 %), the dissolubility of lignocellulose in aqueous BmimCl without LiCl was 53.4 % (Fig. 1a), while this increased to 68.7 % in BmimCl[LiCl·2H2O]25. Addition of the salt enhanced the solvating power of aqueous BmimCl for all three components, especially lignin and hemicelluloses. The enhanced deconstruction and dissolution of lignocellulose with the lithium salts can be attributed to interactions between lithium cation and hydroxyls in lignocellulose, which could aid swelling and dissolution of lignocellulose. With the high water content (11.5 %) in BmimCl and the good dissolubility of lignocellulose (68.7 %), LiCl·2H2O seemed to be superior to LiBr·2H2O and LiI·2H2O in terms of dissolving and deconstructing lignocellulose, which is probably related to the higher density of lithium cation per unit weight for LiCl. In addition, the undissolved lignocellulose had low CrI and reduced lignin content. Therefore, in subsequent studies, lithium chloride was chosen to enhance deconstruction of lignocellulose in BmimCl with higher water content.
Effect of process variables on deconstruction of lignocellulose in aqueous BmimCl with LiCl
The effects of process variables, including the water content in the LiCl hydrate, residence time, biomass particle size, and ratio of LiCl hydrate in the BmimCl solvent system, on the deconstruction and dissolution of bamboo in aqueous BmimCl were investigated to understand the function of LiCl and optimize the deconstruction process.
Effect of molar ratio of water to LiCl
The effect of the water content in the solvent, defined as the molar ratio of water to LiCl, on the dissolution and deconstruction of bamboo in aqueous BmimCl was investigated; the results are presented in Fig. 2. In all experiments, the ratio of BmimCl in the solvent was kept constant (75 wt%). At molar ratio of 1, the water was not sufficient to dissolve all LiCl, and therefore this point was not included in this study. As expected, the solvating power of the solvent system decreased with increasing water content. Specifically, the dissolution of overall biomass decreased linearly with the water content. In particular, the dissolution of lignin and cellulose decreased sharply with increased water content; For example, the dissolution of cellulose drastically decreased from 49.8 to 14.0 % when the water-to-LiCl ratio was increased from 2 (absolute water content 11.5 % in the solvent system) to 5 (absolute water content 16.3 %). The CrI of the undissolved bamboo increased due to the reduced dissolution of cellulose. Meanwhile, the dissolution of lignin dropped from 90.0 to 80.9 %. However, the dissolution of hemicelluloses changed little over the water-to-LiCl ratios from 2 to 5. As a result, the undissolved portion increased at high water content because of retention of more lignin and cellulose. Specifically, the power of deconstructing lignocellulose of the solvent was significantly lost at water-to-LiCl ratio of 4, and the CrI of the undissolved portion was close to the untreated counterpart (54.3 %).
Deconstruction of lignocellulose in aqueous BmimCl with varying molar ratio of water to LiCl: a effect on absolute water content in solvent, dissolubility of lignocellulose, regeneration yield, and CrI of the undissolved portion, b effect on lignin content in the undissolved portion and percentage dissolution of lignin, c effect on cellulose content in the undissolved portion and percentage dissolution of cellulose, d effect on hemicelluloses content in the undissolved portion and percentage dissolution of hemicelluloses; treatment conducted at 140 °C for 14 h with 5.0 wt% biomass consistency
Effect of residence time
The effect of the residence time on the deconstruction and dissolution of bamboo was investigated in the solvent system with 25.0 % LiCl·2H2O and 75 % BmimCl at 140 °C and biomass concentration of 5.0 wt%. As shown in Fig. 3, the dissolubility of whole lignocellulose increased with the treatment time, but the increase became slow after 12 h. The dissolution of lignin and hemicelluloses sharply increased with residence time. These results indicate that complete dissolution of bamboo was quite difficult by just extending the residence time, because dissolution of cellulose increased very little after 12 h. Since 96.2 % of lignin and 91.3 % of hemicelluloses were dissolved within 15 h, the majority (~85 %) of the undissolved residue was cellulose. The CrI of the residue decreased with time, probably due to swelling of cellulose by the solvent. In addition, extensive degradation and further transformation of the lignocellulose were observed at residence time of 18 h, which led to low regeneration yield (43.8 %) and high loss of carbohydrates (14.2 %). Also, levulinic acid was detected, but not HMF. The latter might be further converted to levulinic acid and humins due to the long residence time. Therefore, overlong residence time should be avoided.
Deconstruction of lignocellulose with varying residence time in aqueous BmimCl: a effect on dissolubility of lignocellulose, regeneration yield, and CrI of the undissolved portion, b effect on lignin content in the undissolved portion and percentage dissolution of lignin, c effect on cellulose content in the undissolved portion and percentage dissolution of cellulose, d effect on hemicelluloses content in the undissolved portion and percentage dissolution of hemicelluloses
Effect of bamboo particle size
The complex and compact structure of the lignocellulosic cell wall essentially inhibits penetration and diffusion of solvent into the interior of the cell wall, retarding mass transfer during treatment. In general, processing biomass in ILs requires small particle size of the biomass to improve mass transfer because of the high viscosity of ILs. However, it is well known that size reduction of lignocellulose is very energy and cost intensive, especially when grinding to small particle size. To demonstrate whether the BmimCl[LiCl·2H2O] system could handle large particles, bamboo with different particle sizes (50–100, 30–50, and 20–30 mesh) was treated in the solvent. As shown in Fig. 4, unsurprisingly, the deconstruction and dissolution decreased with increasing lignocellulose size. However, the decrease was not directly proportional to the particle size; For example, there was no significant difference between the 30–50 and 20–30-mesh samples in terms of dissolubility of whole lignocellulose or percentage dissolution of lignin, cellulose, and hemicelluloses. Generally, poor mass transfer in ILs is primarily due to the high viscosity and large molecule size of ILs. Therefore, partial replacement of BmimCl with LiCl and water would be expected to disrupt the strong interactions between anion and cation of BmimCl, thereby reducing the viscosity. On the other hand, LiCl and water have much smaller molecule size than BmimCl, and they can easily penetrate into the interior of the lignocellulose cell wall. The regeneration yield decreased with increasing particle size, which might be due to inhibited transfer of large molecular components out of the biomass matrix. These results suggest that BmimCl[LiCl·2H2O] solvent was not very sensitive to particle size and was able to handle biomass with relatively large particles, which would substantially reduce the complexity and energy consumption of feedstock preparation.
Deconstruction of lignocellulose of different particle size in BmimCl[LiCl·2H2O]25: a effect on dissolubility of lignocellulose, regeneration yield, and CrI of the undissolved portion, b effect on lignin content in the undissolved portion and percentage dissolution of lignin, c effect on cellulose content in the undissolved portion and percentage dissolution of cellulose, d effect on hemicelluloses content in the undissolved portion and percentage dissolution of hemicelluloses; deconstruction treatment conducted at 140 °C for 14 h with 5.0 wt% biomass consistency
Effect of LiCl hydrate ratio in BmimCl[LiCl·2H2O]
High cost is one of the obstacles to large-scale application of ILs for biomass processing. Replacing BmimCl with LiCl hydrate would reduce the cost, in addition to decreasing the viscosity and improving operation, as discussed above. Therefore, the effect of the ratio of LiCl·2H2O to BmimCl on the deconstruction and dissolution of lignocellulose was investigated. It is also important to determine the maximum water content that BmimCl can tolerate without significantly degrading the solvating ability. As shown in Fig. 5, the dissolution of lignocellulose did not decline but rather increased with increasing water content in the solvent until the ratio of LiCl·2H2O reached 55 wt% (absolute water content 25.3 wt% in the system), where the maximum dissolubility of lignocellulose of 79.8 % was reached. Similarly, all three cell wall components (lignin, cellulose, and hemicelluloses) achieved their maximum dissolution (96.0, 66.5, and 92.0 %, respectively) at 55 % LiCl·2H2O. This improved dissolution of the lignocellulose components at higher water content can be attributed to synergetic action of BmimCl and LiCl·2H2O. It was apparent that water addition affected the dissolution of lignin and cellulose more than that of hemicelluloses. As shown in Fig. 5, the dissolution of hemicelluloses (ca. 90 %) changed little when the ratio of LiCl·2H2O was varied between 25 and 70 wt%, while the dissolution of lignin and cellulose were significantly enhanced as the ratio of LiCl·2H2O was increased up to 55 wt%, then dropped sharply. The CrI of the undissolved lignocellulose varied little, implying that amorphous and crystalline cellulose were dissolved synchronously during treatment with combined BmimCl and LiCl·2H2O.
Deconstruction of lignocellulose in aqueous BmimCl with varying ratio of LiCl·2H2O to BmimCl: a effect on water content in solvent, dissolubility of lignocellulose, regeneration yield, and CrI of the undissolved portion, b effect on lignin content in the undissolved portion and percentage dissolution of lignin, c effect on cellulose content in the undissolved portion and percentage dissolution of cellulose, d effect on hemicelluloses content in the undissolved portion and percentage dissolution of hemicelluloses; deconstruction treatment conducted at 140 °C for 14 h with 5.0 wt% biomass consistency
In the BmimCl–LiCl–H2O system, a large portion of the water molecules are tightly bound to the inner sphere of lithium cations due to their high coordination power. Hydrated lithium cations still possess high capability to break hydrogen bonds by interacting with oxygen atoms of hydroxyl groups, as evidenced by the finding that cellulose can be dissolved in LiCl hydrate (Sanghamitra et al. 2013). Additionally, anionic chloride ions in the system can interact with hydrogen atoms of hydroxyl groups of cellulose to disrupt hydrogen bonds in the lignocellulose. Thus, both hydrated lithium cations and chloride anions could simultaneously disrupt hydrogen bonds by forming electron donor–acceptor (EDA) complexes, respectively (Scheme 1). Based on the excellent performance for deconstructing and dissolving lignocellulose, high ratio of LiCl·2H2O would lower the loading of BmimCl and the viscosity of the solvent system, thereby reducing the cost of the solvent and facilitating lignocellulose processing.
Comparison of deconstruction and dissolution of bamboo in aqueous BmimCl, BmimCl[LiCl·2H2O]55, and LiCl·2H2O
As discussed above, the lignocellulose solvating power and dissolution ability of BmimCl systems with/without lithium chloride hydrate differed significantly, and BmimCl[LiCl·2H2O]55 was found to be a superior solvent for deconstructing and dissolving lignocellulose. To better understand the dissolution mechanism of the solvents, the deconstruction and dissolution of bamboo in aqueous BmimCl, BmimCl[LiCl·2H2O]55, and LiCl·2H2O was compared. As summarized in Table 3, aqueous BmimCl exhibited the weakest power to dissolve lignocellulose, whereas BmimCl[LiCl·2H2O]55 had the highest power to dissolve lignocellulose. Partial replacement of BmimCl with LiCl could significantly improve the tolerance of the IL to water; For example, BmimCl[H2O]16 (with 16 % water) could only dissolve 44.1 % of the lignocellulose; however, with addition of LiCl, the dissolubility of lignocellulose in BmimCl[LiCl·2H2O]55 (with 25 % water) was substantially increased to 79.8 %. These results indicate that addition of LiCl could alleviate the negative effect of water on the IL and enhance the biomass dissolution in the IL. In addition, the undissolved lignocellulose had significantly lower CrI and lignin content. The lignocellulose could be dissolved by 65.7 % in LiCl·2H2O alone. Almost all cellulose and hemicelluloses were dissolved or removed from the biomass, with only trace amounts detected in the undissolved residue, as further discussed below.
During the deconstruction and dissolution of the lignocellulose in the solvents, a certain amount of carbohydrates (cellulose and hemicelluloses) was not recovered in either the regenerated or undissolved residue. In particular, a large portion of the carbohydrates was lost during treatment in LiCl·2H2O; For example, approximately 4 % carbohydrates were lost when the biomass was treated in BmimCl[H2O]16 and BmimCl[LiCl·2H2O]55, but up to 32 % was not recovered in LiCl·2H2O. Therefore, the filtrates after recovering the regenerated biomass were analyzed, and the results are presented in Table 4.
The monosaccharides formed in dry BmimCl were negligible, which might be because the water available for polysaccharide hydrolysis in this anhydrous system was limited, so the hydrolysis of polysaccharides was halted at the oligosaccharide stage. The high water content in the aqueous BmimCl and BmimCl[LiCl·2H2O]55 was expected to result in more hydrolyzed carbohydrates, but only minor monosaccharides formed in BmimCl[H2O]16 and BmimCl[LiCl·2H2O]55, except for glucose in LiCl·2H2O (Table 4). A plausible reason is the low acidity of the solvent, which limited the hydrolysis of carbohydrates. In addition to the monosaccharides, small amount of HMF (0.92 g/100 g biomass) and levulinic acid (1.34 g/100 g biomass) were detected from dehydration of saccharides. It was found that a large amount of humins was produced; For example, the acid-insoluble fractions of undissolved residue and regenerated lignocellulose were 99.2 and 67.9 %, respectively, based on the NREL acid-insoluble lignin procedure. Obviously, part of the insolubles was from humins. It seemed that the presence of BmimCl in LiCl·2H2O could prevent or reduce the formation of humins.
No lignin was dissolved from the biomass in LiCl·2H2O, while significant lignin (51.6 %) was dissolved in BmimCl[H2O]16. Impressively, with addition of LiCl, almost all lignin (96.0 %) was dissolved in BmimCl[LiCl·2H2O]55, suggesting that lignin was depolymerized during the treatment. Hydrolysis of lignin–carbohydrate complexes was probably another factor.
The mass balance when bamboo was treated in aqueous BmimCl, BmimCl[LiCl·2H2O]55, and LiCl·2H2O is summarized in Fig. 6. It is clear that BmimCl[LiCl·2H2O]55 was able to dissolve more of both the carbohydrates and lignin, with lower carbohydrate loss than for aqueous BmimCl or LiCl·2H2O.
IR spectra of the undissolved lignocellulose residues in the three solvents were recorded (Fig. 7). The broad band at 3020–3600 cm−1 (hydroxyl group stretching), peak at 2894 cm−1 (CH stretching), sharp peak at 1020 cm−1 (C–OH stretching), and shoulder peaks at 1159 (C–O–C asymmetric bridge stretching) and 895 cm−1 (C–H deformation vibration) are associated with cellulose. The intensity of these peaks in the residues treated in BmimCl[H2O]16 and BmimCl[LiCl·2H2O]55 increased significantly, suggesting enriched content of cellulose in the undissolved fraction. In other words, lignin and hemicelluloses were selectively dissolved during these treatments. However, these bands nearly disappeared in the residue treated with LiCl·2H2O, suggesting complete dissolution or removal of cellulose from the biomass. Lignin characteristic peaks at 1590/1509 cm−1 (C=C stretching vibration) and 1459 cm−1 (asymmetric bending in CH3 of lignin methoxyl group) of the residue from BmimCl[LiCl·2H2O]55 were not detected, suggesting significant delignification of the undissolved lignocellulose residue, whereas these peaks were still visible in the residue treated in LiCl·2H2O, implying insignificant delignification. These results of IR analysis are consistent with the compositional analysis discussed above.
Conclusions
This study demonstrated an efficient method to improve the deconstruction and dissolution of bamboo in ionic liquid (BmimCl) with high water content by adding metal salts, such as lithium chloride. At water content of 25 wt%, approximately 80 % of bamboo could be dissolved in BmimCl containing LiCl. In particular, lignin and hemicelluloses were selectively dissolved by 96 and 92 %, respectively. The undissolved residue was predominantly composed of cellulose (~86 %) with small amount of lignin (<5 %). Unlike many existing ionic liquids, this aqueous ionic liquid could treat biomass with relatively large particle size, which would significantly reduce the complexity and energy consumption of feedstock preparation. The BmimCl–LiCl–H2O system has the advantages of low cost, low viscosity, facile implementation, and high efficiency.
References
Agnieszka B, Michael JR, Trang QT, David JL, Richard JM, Tom W (2011) Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid–water mixtures. Green Chem 13:2489–2499
Amarasekara AS, Ebede CC (2009) Zinc chloride mediated degradation of cellulose at 200 degrees C and identification of the products. Bioresour Technol 100:5301–5304
Andanson JM, Bordes E, Devemy J, Leroux F, Padua AAH, Gomes MF (2014) Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem 16:2528–2538
Binder JB, Raines RT (2009) Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc 131:1979–1985
Cammarata L, Kazarian SG, Salter PA, Welton T (2001) Molecular states of water in room temperature ionic liquids. Phys Chem Chem Phys 3:5192–5200
Carlson TR, Vispute TP, Huber GW (2008) Green gasoline by catalytic fast pyrolysis of solid biomass derived compounds. ChemSusChem 1:397–400
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Fu DB, Maz G (2011a) Aqueous ionic liquid pretreatment of straw. Bioresour Technol 102:7008–7011
Fu DB, Maz G (2011b) Optimization of processing conditions for the pretreatment of wheat straw using aqueous ionic liquid. Bioresour Technol 102:8003–8010
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106:4044–4098
Iiyama K, Lam T, Stone BA (1994) Covalent cross-links in the cell wall. Plant Physiol 104:315–320
Kilpeläinen I, Xie HB, King A, Granstrom M, Heikkinen S, Argyropoulos DS (2007) Dissolution of wood in ionic liquids. J Agric Food Chem 55:9142–9148
Kulkarni PS, Branco LC, Crespo JG, Afonso CA (2007) A comparative study on absorption and selectivity of organic vapors by using ionic liquids based on imidazolium, quaternary ammonium, and guanidinium cations. Chem Eur J 13:8470–8477
Lasse K, Arno P, Somdatta D, Martin L, Mikhail G, Ilkka K, Alistair WTK (2013) On the solubility of wood in non-derivatising ionic liquids. Green Chem 15:2374–2378
Liu CZ, Wang F, Stiles AR, Chen G (2012) Ionic liquids for biofuel production: opportunities and challenges. Appl Energy 92:406–414
Martin G, Tim L, Omar AES, Thomas H (2011) Tailored media for homogeneous cellulose chemistry: ionic liquid/co-solvent mixtures. Macromol Mater Eng 296:483–493
Mazza M, Catana DA, Garcia CV, Cecutti C (2009) Influence of water on the dissolution of cellulose in selected ionic liquids. Cellulose 16:207–215
O’Connor RT, DuPre EF, Mitcham D (1958) Applications of infrared absorption spectroscopy to investigations of cotton and modified cottons. Part I: physical and crystalline modifications and oxidation. Text Res J 28:382–392
Pang ZQ, Chen JC, Dong CH, Yang GH (2014) Highly selective and efficient extraction of lignin in kraft pulp by aqueous ionic liquids for enhanced bleaching properties. RSC Adv 4:29897–29900
Peter BH, Kenneth RS, Thomas W (1993) Hydrogen-bond acceptor abilities of tetrachlorometalate (II) complexes in ionic liquids. J Chem Soc Dalton Trans 17:2639–2643
Rogers RD, Seddon KR (2003) Ionic liquids–solvents of the future? Science 302:792–793
Sanghamitra S, James DM, Dimitris SA (2013) Review of cellulose non-derivatizing solvent interactions with emphasis on activity in inorganic molten salt hydrates. ACS Sustain Chem Eng 1:858–870
Sebastian F, Sasisanker P, Harvey WB, John MP (2011) Viscosities of acetate or chloride-based ionic liquids and some of their mixtures with water or other common solvents. J Chem Eng Data 56:31–34
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Vo HT, Kim CS, Ahn BS, Kim HS, Lee H (2011) Study on dissolution and regeneration of poplar wood in imidazolium-based ionic liquids. J Wood Chem Technol 31:89–102
Wen JL, Yuan TQ, Sun SL, Xu F, Sun RC (2014) Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem 16:181–190
Xu AR, Wang JJ, Wang HY (2010) Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem 12:268–275
Yang YJ, Shin JM, Kang TH, Kimura S, Wada M, Kim UJ (2014) Cellulose dissolution in aqueous lithium bromide solutions. Cellulose 21:1175–1181
Zhang ZC (2013) Catalytic transformation of carbohydrates and lignin in ionic liquids. WIREs Energy Environ 2:655–672
Zhao HB, Holladay JE, Brown H, Conrad Zhang ZC (2007) Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316:1597–1600
Acknowledgments
The authors are grateful for financial support from the National Science Foundation of China (31370580), Special Fund of Taishan Scholar Project, Natural Science Foundation of Shandong Province (ZR2015CM006), and Talented Scientist Funding of Shandong Province (BS2013HZ020) to Z.P. and for NSF (CBET 1159561) and WARF Accelerator grants to X.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, Z., Dong, C. & Pan, X. Enhanced deconstruction and dissolution of lignocellulosic biomass in ionic liquid at high water content by lithium chloride. Cellulose 23, 323–338 (2016). https://doi.org/10.1007/s10570-015-0832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0832-7