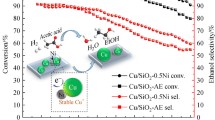
Abstract
The selective hydrogenation of acetylene is of industrially indispensable in the production of polymer-grade ethylene. The design of non-precious metal catalysts with outstanding performance is of pivotal importance in order to replace the supported Pd–Ag catalysts. Our previous work showed that a copper carbide (CuxC)-containing catalyst exhibited high hydrogenation activity and selectivity under mild conditions. In the present work, Mg(OH)2 was used to modify the CuxC-containing catalyst in order to improve its catalytic performance. Mg(OH)2-modified CuxC-containing catalyst was prepared from a coprecipitate of Cu(OH)2 and Mg(OH)2, which was obtained by precipitation of Cu(NO3)2 and Mg(NO3)2 solution with dropwise addition of NaOH solution, by thermal treatment in C2H2/Ar (0.5%) at 120 ℃ followed by H2 reduction at 150 ℃. The introduction of Mg(OH)2 led to reduced CuxC crystalline size and to increased amount of CuxC crystallites. In addition, the basic nature of Mg(OH)2 is favorable to suppress the undesired oligomerization. The prepared catalyst showed excellent catalytic performance with complete acetylene conversion, low selectivity to unwanted ethane (24%), and high stability at 100 °C and atmospheric pressure in the presence of large excess ethylene in 100 h.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ethylene is industrially used in the production of polymers, and the ethylene produced by cracking of naphtha contains about 1% acetylene impurity. A trace amount of acetylene in the ethylene feedback results in poisoning the Ziegler–Natta catalyst in the downstream ethylene polymerization, degrading the polymer product. As a consequence, the acetylene content in ethylene must be reduced to less than 5 ppm. Selective hydrogenation of acetylene to ethylene is regarded as an efficient, economical, and environmentally friendly method to remove acetylene impurity in industry [1,2,3,4,5]. Supported Pd–Ag catalysts are widely used for selective hydrogenation of acetylene due to their high activity and reasonable selectivity [6,7,8,9,10]. However, at near complete conversion of acetylene, an ethylene loss frequently occurs without co-feeding CO, which adsorbs stronger than ethylene but weaker than acetylene, because of the over-hydrogenation of ethylene to yield ethane. Moreover, supported Pd–Ag catalyst often produces green oil, which is generated by oligomerization, leading to gradual deactivation of the catalysts [8, 11,12,13,14,15]. Therefore, it is highly desirable to develop high-performance catalysts with reduced selectivity to undesired ethane and green oil.
To improve the performance of Pd-based catalysts, promoters such as Ag [16,17,18,19], Cu [20,21,22], Mg [23,24,25,26], Zn [27, 28], Ga [29, 30], Co [31, 32], and In [33, 34] are often added. Most of them tune the catalytic performance by forming intermetallic alloys with Pd, which have ordered crystal structures and provide unique and uniform active sites [16,17,18,19,20, 22, 27, 28, 30,31,32,33]. As for Mg species, they usually act as supports in the form of MgO [23,24,25]. Yuan et al. [23] prepared a 3D-Al-Pd/MMO catalyst derived from an Al-LDH containing Mg species, which demonstrated high activity, excellent selectivity as well as long-term stability for selective hydrogenation of acetylene to ethylene. Qin et al. [24] prepared a Pd/MgO catalyst with low loading of Pd (7.8 ppm) for selective hydrogenation of acetylene to ethylene, and the catalyst showed outstanding performance. Guo et al. [25] developed a single-atom Pd catalyst supported by MgO using a ball-milling method, and the prepared catalyst showed good catalytic performance for selective hydrogenation of acetylene to ethylene in excess ethylene. He et al. [30] prepared Pd–Ga/MgO-Al2O3 catalysts with high activity and high selectivity in selective hydrogenation of acetylene, owing to high dispersion and synergistic effect of bimetallic nanoalloys. Lomonosov et al. [26] synthesized bimetallic Pd-Mg nanoparticles by partial galvanic replacement of plasmonic Mg nano-particles, which exhibited a much higher ethylene selectivity than Pd alone in selective hydrogenation of acetylene. The improved catalytic behavior was attributed to the well-separated Pd nanoparticles on Mg, due to the ability of suppressing Pd aggregation. However, since Pd is scarce and expensive, there is ample room for improving the cost-effectiveness in catalyst design by finding a non-precious metal, such as Ni [35, 36] and Cu [37,38,39], to replace Pd-based catalysts [40].
In our previous work, it was found that a novel phase, copper carbide (CuxC), derived from copper acetylide showed high hydrogenation activity at low temperatures, comparable to Pd-based catalysts [41,42,43,44,45,46,47]. The CuxC-containing catalysts were prepared from a variety of copper precursors, including Cu2O [41], Cu(OH)2CO3 [42], Cu(OH)2 [43] and CuO [44], by thermal treatment with acetylene-containing gas followed by hydrogen reduction. Cu-based catalysts are highly selective in hydrogenation of acetylene because the adsorption of acetylene is significantly stronger than that of ethylene [38, 48]. The precious-metal-like hydrogenation activity is attributed to the interstitial CuxC, whereas the porous carbonaceous shell layer, generated from the thermal decomposition of intermediate copper acetylide, blocked the chain growth of linear oligomers, thus suppressing the formation of green oil [41,42,43,44].
In the present work, Mg(OH)2 species was used to modify the CuxC-containing catalyst. The prepared Mg(OH)2-modified catalysts were tested in selective hydrogenation of acetylene with enhanced performance. The characterization results confirmed that the introduction of Mg(OH)2 resulted in increased amount and reduced size of CuxC crystallites. In addition, the presence of basic Mg(OH)2 decreased the production of C4=, among which 1,3-butadiene is regarded as the precursor of green oil.
2 Experimental
2.1 Chemical Reagents
Cu(NO3)2·3H2O was purchased from Guangdong Guanghua Sci-Tech CO., Ltd, China. Mg(NO3)2·6H2O was purchased from Tianjin Damao Chemical Reagent Factory, China. NaOH was purchased from Tianjin Kemiou Chemical Reagent CO., Ltd, China. All reagents were of analytical grade and used without further purification. Hydrogen gas was purchased from Dalian Kena Science and Technology Co., Ltd, China. The reaction gas (0.8% CH4, 0.5% C2H2, and 98.7% C2H4) and the treatment gas (0.5% C2H2, 99.5% Ar) were purchased from Dalian Guangming Chemical Research Institute, China.
2.2 Synthesis of Catalyst Precursors
The catalyst precursors were prepared by coprecipitation. Cu(NO3)2·3H2O and Mg(NO3)2·6H2O (the total amount of Cu2+ and Mg2+ was 0.02 mol, and Mg2+/Cu2+ = n/(10-n), n = 0–4) were dissolved in 200 mL deionized water and stirred for 30 min in an ice-water bath. Then 20 mL NaOH solution (2 mol/L) was added dropwise to the above solution. The resultant suspension was stirred for 30 min. The resultant blue suspension was filtered, and the solid was washed with deionized water and dried in vacuum at room temperature for 12 h to obtain the catalyst precursor, which is denoted as Cu10-nMgn(OH) (n = 0–4).
2.3 Catalyst Preparation
0.1 g Cu10-nMgn(OH) precursor was treated in a flow of acetylene-containing gas (0.5% C2H2/99.5% Ar, 30 mL/min) at 100–180 °C for 2 h, and then cooled down to room temperature. The obtained material was denoted as Cu10-nMgn(OH)(Tx), where x represents the treatment temperature. Subsequently, Cu10-nMgn(OH)(Tx) was reduced in H2 (10 mL/min) at 110–190 °C for 3 h to obtain the CuxC-containing catalyst, which is denoted as Cu10-nMgn(OH)(Tx-Ry), where y represents the reduction temperature.
2.4 Catalyst Characterization
All of the catalysts for characterization were passivated in an 0.50 vol% O2/N2 flow (10 mL⋅min−1) at ambient temperature for at least 2 h before exposure to air.
X-ray diffraction (XRD) patterns were acquired on a Rigaku SmartLab diffractometer with Cu-Kα radiation source (λ = 0.154 nm) at 200 mA and 45 kV. The XRD patterns were collected in the range of 20–80° with a scanning speed of 8°/min.
Surface morphology was observed by means of scanning electron microscopy (SEM) on an SU8220 instrument with a test voltage of 5.0 kV. Transmission electron microscopy (TEM) images were taken on an FEI Tecnai G2 F30 model with an accelerating voltage of 300 kV.
X-ray photoelectron spectroscopy (XPS) measurement was conducted on an Escalab 250 X-ray photoelectron spectrometer equipped with Al-Kα as the excitation source (1486.6 eV). The binding energy was calibrated using the binding energy of C 1 s at 284.6 eV.
The adsorption/desorption isotherms of nitrogen were measured on a Micromeritics Tristar II 3020 instrument at -196 °C. The specific surface area was calculated from the isotherms according to Brunauer–Emmett–Teller (BET) equation. Thermal gravimetric analysis and differential scanning calorimetry (TG-DSC) were carried out on an instrument (SDT Q600, TA) in a N2 flow (50 mL/min) from room temperature to 500 °C at 3 °C/min.
Hydrogen temperature-programmed reduction (H2-TPR) was carried out on a Chembet-3000 analyzer equipped with a TCD. The sample (0.2 g) was reduced in a stream of H2-containing gas (10.0% H2 in Ar, 50 mL/min) at 10 °C/min up to 600 °C.
2.5 Catalytic Performance
The catalytic performance in selective hydrogenation of acetylene was conducted in a tubular quartz fixed-bed reactor. In a typical run, 0.1 g Cu10-nMgn(OH) precursor was mixed with quartz sand (0.6 g, 40–80 mesh), and then charged into the middle of the quartz tube reactor (inner diameter: 10 mm). The catalyst bed in the quartz reactor was placed in the constant temperature zone of a tubular electric furnace. Cu10-nMgn(OH)(Tx-Ry) catalyst was prepared by thermal treatment with C2H2/Ar gas followed by H2 reduction. Then, the reaction gas (0.8% CH4, 0.5% C2H2, and 98.7% C2H4) at 10 mL/min and H2 gas at 1.0 mL/min were fed into the reactor. CH4 was used as the internal standard for gas chromatographic analysis. The outlet gas was analyzed by an online chromatography (Panna A60), equipped with a flame ionization detector and an Agilent HP-AL/S capillary column (30 m × 0.535 mm × 15.00 μm). In addition to ethylene and ethane, a small amount of C4= olefins, including trans-2-butene, 1-butylene, cis-2-butene, and 1,3-butadiene, were detected. No green oil was observed downstream of the quartz tube even in the 100-h run.
In the presence of large excess ethylene in the gas feed, it is difficult to accurately measure the selectivity of ethylene. Therefore, the selectivity to undesired ethane was used to determine the selectivity performance of the catalyst, a measure of ethylene gain or loss. The conversion of acetylene and the selectivities to ethane and C4= were calculated as follows:
where [C2H2]inlet and [C2H2]outlet were the mole concentrations of acetylene in the feed and in the effluent, respectively. [C2H6]outlet and [C4=]outlet were the mole concentrations of ethane and that of total C4= olefins, respectively, in the outlet.
In order to accurately determine the selectivity to ethylene, a gas feed of acetylene in N2 (0.5% C2H2/99.5% N2) was used. The selectivity to ethylene was determined by
where [C2H4]outlet was the mole concentration of ethylene in the effluent.
3 Results and Discussion
To investigate the effect of Mg(OH)2 addition on the structure and morphology of the Cu(OH)2 precursor, XRD measurement was conducted, as shown in Fig. 1. In the XRD patterns of Cu10-nMgn(OH), the diffraction peaks at 2θ = 23.8, 34.1, 35.9, 38.0, 38.2, 39.8, and 53.2° corresponded to the (021), (002), (111), (041), (022), (130), and (150) planes of Cu(OH)2 (PDF 13–0420), respectively. It is indicated that the intensity of diffraction peaks ascribed to Cu(OH)2 was decreased as the amount of Mg(OH)2 was increased, indicating reduced crystalline size and lower crystallinity of Cu(OH)2 phase. No distinctive diffraction peaks of Mg(OH)2 species were detected in the patterns of all the samples, suggesting that the size of Mg(OH)2 was below the detection limit.
Figure 2a and b illustrate the SEM images of Cu(OH)2 and Cu7Mg3(OH), respectively. It is demonstrated that the Cu(OH)2 was in bundles of nanowires, whereas the morphology of Cu7Mg3(OH) became a mixture of shorter nanowires and nanosheets, indicating that the introduction of Mg(OH)2 significantly changed the morphology of the obtained catalyst precursor. In the HRTEM images of Cu7Mg3(OH) (Fig. S1), the lattice spacings of 0.250 and 0.225 nm corresponded to the (111) and (130) planes of Cu(OH)2, respectively, whereas that of 0.273 nm corresponded to the (100) plane of Mg(OH)2. It is shown that Cu7Mg3(OH) was composed of Cu(OH)2 and Mg(OH)2 while the introduction of Mg(OH)2 facilitated the dispersion of Cu(OH)2.
Figure 3 presents the SEM images of Cu7Mg3(OH), after thermal treatment with C2H2/Ar at 120 °C, and after subsequent H2 reduction at 150 °C. It is displayed that Cu7Mg3(OH) was in nanosheets, and the nanosheets were covered with cauliflower-shaped materials after the thermal treatment with C2H2/Ar at 120 °C. When Cu7Mg3(OH)(T120) was reduced in H2 at 150 °C, the cauliflower shapes shrank in Cu7Mg3(OH)(T120-R150). It implies that the reaction of Cu(OH)2 with acetylene occurred in the thermal treatment, producing fabrics on the surface. The subsequent hydrogen reduction did not change the morphology significantly but reduced the length of the hydrocarbon fabrics.
As shown in Fig. 4a, after the thermal treatment with acetylene-containing gas, the XRD pattern of Cu7Mg3(OH)(T120) remained similar to that of Cu7Mg3(OH), suggesting that the reaction with acetylene took place on the external surface of the precursor. The weaker diffraction peaks characteristic of Cu(OH)2 in the XRD pattern of Cu7Mg3(OH)(T120) than Cu7Mg3(OH) was attributed to reduced particle size of the precursor and to the presence of amorphous hydrocarbon fabrics on the external surface. In the XRD pattern of Cu7Mg3(OH)(T120-R150) (Fig. 4b), the intense peaks at 2θ = 43.3, 50.4, and 74.1° were ascribed to the (111), (200), and (220) planes of metal Cu (PDF 04–0836), indicating that the core of the precursor was reduced to generate metal Cu particles at temperature as low as 150 °C. An additional weak peak at 2θ = 37.2° was indicative of the formation CuxC crystal [41,42,43,44,45,46,47]. The intensity of this characteristic peak attributed to CuxC in Cu7Mg3(OH)(T120-R150) was lower than that of Cu(OH)2(T120-R150) [43], indicating that the crystalline size of CuxC derived from Cu7Mg3(OH) was smaller, probably because of decreased size of Cu(OH)2 particles in the precursor.
In the HRTEM image of Cu7Mg3(OH)(T120-R150) (Fig. 5), three kinds of crystallites were observed. The lattice spacing of 0.208 nm corresponded to the (111) plane of Cu, whereas that of 0.273 nm corresponded to the (100) plane of Mg(OH)2. It implies that Mg species existed in the form of Mg(OH)2 instead of MgO in Cu7Mg3(OH)(T120-R150), which was in agreement with the TG-DSC measurement of Mg(OH)2 (Fig. S2).The lattice spacing of 0.240 nm corresponded to that of CuxC crystallite [41]. Fig. S3 shows the EDS elementary mapping analysis of Cu7Mg3(OH)(T120-R150). It is demonstrated that Cu species, Mg(OH)2 and C species were homogeneously distributed in Cu7Mg3(OH)(T120-R150). Moreover, the specific surface areas, calculated from the N2 adsorption–desorption isotherms (Fig. S4) using Brunauer–Emmett–Teller (BET) methods, indicated that, after treatment with acetylene-containing gas and subsequent hydrogen reduction, the surface area increased from 84, to 117, and to 123 m2/g, respectively (Table S1). The significant increment after thermal treatment with acetylene might be due to the formation of external fabrics and amorphous carbonaceous materials [41]. Therefore, it is concluded that Cu7Mg3(OH)(T120-R150) was composed of CuxC, Cu and Mg(OH)2 nanoparticles, which were embedded in the matrix of amorphous carbonaceous materials.
Figure 6 illustrates the XPS spectra of Cu7Mg3(OH)(T120-R150). The absence of a satellite peak at ~ 943 eV in the Cu 2p3/2 spectrum suggests that there was no Cu2+ species [49], corroborating that the surface Cu(OH)2 was transformed by reacting with C2H2 followed by hydrogen reduction (Fig. 6a). The major peak was centered at binding energy of 932.6 eV (Fig. 6a), which fell between that of Cu2+ and that Cu0/Cu+. Since it is not possible to distinguish Cu0 and Cu+ in the Cu 2p3/2 spectrum, Cu LMM spectrum of Cu7Mg3(OH)(T120-R150) was measured, and is presented in Fig. 6b. It is indicated that the peak was centered at 917.7 eV, which was between that of Cu0 (918.7 eV) [50] and that of Cu+ (916.8 eV) [51]. It implies that the major contribution to this peak came from partially positive species of Cu (Cuδ+), which is characteristic of interstitial copper carbide (CuxC) [41, 52]. In the C 1 s spectrum of Cu7Mg3(OH)(T120-R150) (Fig. 6c), the large peak was tentatively deconvoluted into three peaks at binding energies of 284.9, 286.5, and 284.2 eV. The peak at 284.9 was attributed to the C–C bond [52], whereas the shoulder peak at 286.5 eV corresponded to the C–OH species [53]. Kim et al. [52] concluded that the simultaneous appearance of the two peaks at 932.6 eV in the Cu 2p3/2 spectrum and at 284.2 eV in the C 1 s spectrum was due to the formation of copper carbide. In the XPS spectrum of Mg 1 s (Fig. 6d), the peak at binding energy of 1302.8 eV was attributed to Mg(OH)2 in Cu7Mg3(OH)(T120-R150) [54], which is in agreement with the HRTEM observation (Fig. 5).
In our previous investigation [43], the catalysts derived from Cu(OH)2 showed an excellent performance in acetylene selective hydrogenation. It was found that CuxC served as the catalytic site for hydrogen dissociation whereas acetylene hydrogenation mainly occurred on Cu site. The addition of Mg(OH)2 may affect the fraction of CuxC in the preparation. Therefore, the effect of the composition of Cu and Mg in the precursor on acetylene conversion and ethane selectivity was investigated. Figure 7 compares the catalytic performances of catalysts prepared from various Cu10-nMgn(OH) (n = 0.5, 1, 2, 3, 4) precursors, which were treated with acetylene-containing gas at 120 ℃ for 2 h and then reduced in H2 at 150 °C for 3 h. With the addition of Mg(OH)2, all Cu10-nMgn(OH)(T120-R150) (n = 0.5, 1, 2, 3, 4) showed higher hydrogenation activity than Cu(OH)2(T120-R150) at 100 ℃, probably due to increased amount of CuxC crystallites in Cu10-nMgn(OH)(T120-R150). Cu9Mg1(OH)(T120-R150) exhibited the highest hydrogenation activity, implying that more CuxC crystallites were present. However, a considerably higher amount of unwanted ethane was produced on Cu9Mg1(OH)(T120-R150), leading to a marked ethylene loss. For Cu8Mg2(OH)(T120-R150), acetylene conversion decreased slightly whereas the selectivity to ethane was reduced significantly. Cu7Mg3(OH)(T120-R150) showed the best performance with extremely high hydrogenation activity and an ethylene gain (ethane selectivity < 30%). The outstanding catalytic performance of Cu7Mg3(OH)(T120-R150) might be related with the optimal composition of CuxC and Cu phases. Therefore, Cu7Mg3(OH) was chosen the catalyst precursor in the subsequent investigation.
The influence of acetylene treatment temperature on the catalytic performance of Cu7Mg3(OH)-derived catalysts in acetylene selective hydrogenation is shown in Fig. 8. The Cu7Mg3(OH) precursor was treated with acetylene-containing gas at various temperature in the range of 100–180 ℃ for 2 h and then reduced in H2 at 150 °C for 3 h. Acetylene conversion increased at first and then decreased with the treatment temperature. On one hand, higher acetylene treatment temperature might enhance the decomposition of the in situ generated copper acetylide (CuC2). On the other hand, less amount of intermediate CuC2 was formed at lower treatment temperatures, leading to less formation of CuxC active phase in the subsequent hydrogen reduction.
The influence of acetylene treatment time on the catalytic performance is shown in Fig. 9. The acetylene conversion reached nearly 100% when the precursor was treated at 120 ℃ for 2 or 3 h. However, at longer treatment time (4 h), the conversion decreased, probably because the decomposition of intermediate CuC2 was enhanced at a long thermal treatment time, which is in agreement with our previous results [43].
Under the optimal treatment conditions (120 °C for 2 h), the influence of hydrogen reduction temperature on the catalytic performance in selective hydrogenation of acetylene was investigated (Fig. 10). Cu7Mg3(OH)(T120) was reduced in H2 at various temperatures in the range of 110–190 °C for 3 h. The catalysts prepared by hydrogen reduction in the temperature range of 130 to 170 °C exhibited significantly high activity for acetylene hydrogenation with nearly 100% acetylene conversion at 100 °C, while the catalyst obtained by hydrogen reduction at 190 °C showed a slightly lower acetylene conversion (91%) under the same conditions. Although the rate of CuC2 reduction to yield CuxC was high at higher reduction temperatures, the parallel decomposition of CuC2 was enhanced as well, resulting in less production of CuxC. Interestingly, the catalysts prepared by hydrogen reduction at 130 and 170 ℃ both showed full acetylene conversion, probably due to a balanced reduction and decomposition of CuC2 in the catalyst preparation. Nevertheless, both catalysts showed high selectivity to undesired ethane, and, as a consequence, the optimal reduction temperature was chosen to be 150 °C.
Table 1 presents acetylene conversion and selectivities to ethane and C4= as a function of reaction temperature in hydrogenation of acetylene in excess ethylene over Cu(OH)2(T120-R150) and Cu7Mg3(OH)(T120-R150). It can be seen that both catalysts gave low selectivities to ethane and C4= when acetylene was not completely converted at low temperatures. However, when acetylene was 100%, large amounts of ethane and C4= were yielded. When the reaction temperature was further increased, both catalysts showed reduced selectivity to C4= at 100% acetylene conversion because high temperature led to enhanced hydrogen dissociation, thus increasing ethane selectivity while decreasing the selectivity to C4=, in agreement with the published results [55,56,57]. Table S2 compares the results in hydrogenation of acetylene in nitrogen (0.5% C2H2/99.5% N2) over Cu(OH)2(T120-R150) and Cu7Mg3(OH)(T120-R150). Similarly, high temperature was unfavorable to the production of C4= at complete conversion of acetylene. Additionally, Cu7Mg3(OH)(T120-R150) yielded less C4= than Cu(OH)2(T120-R150) in the investigated temperature range, probably because the presence of Mg(OH)2 in Cu7Mg3(OH)(T120-R150) might suppress significantly the production of C4= and undesired oligomers [56, 58, 59].
Figure 11 displays the dependence of acetylene conversion and ethane selectivity on H2/C2H2 ratio in acetylene selective hydrogenation catalyzed by Cu7Mg3(OH)(T120-R150) at 100 °C and atmospheric pressure. In the range of 14–30, the acetylene conversion and ethane selectivity increased gradually with H2/C2H2 ratio. At H2/C2H2 ratio of 22, complete acetylene conversion was obtained with an ethane selectivity of 24%. In the range of 24–30, the ethane selectivity markedly increased with H2/C2H2 ratio, causing an ethylene loss. Compared with Cu(OH)2(T120-R150) [43], Cu7Mg3(OH)(T120-R150) exhibited higher hydrogenation activity, with complete acetylene removal in ethylene stream at 100 ℃ and atmospheric pressure.
Copper-based catalysts are known to deactivate quickly due to the formation of green oil, which was accumulated on the catalyst surface [55]. In our investigation, there was no green oil downstream of the quartz tube in the course of a 100-h reaction. The variation of acetylene conversion and ethane selectivity with time on stream in selective hydrogenation of acetylene catalyzed by Cu7Mg3(OH)(T120-R150) are demonstrated in Fig. 12. It is indicated that Cu7Mg3(OH)(T120-R150) was stable in a 100-h run at 100 °C and atmospheric pressure, achieving complete removal of acetylene in ethylene with low selectivity to undesired ethane (24%). The high stability with an ethylene gain might be attributed to the formation of the porous carbonaceous matrix formed by the decomposition of intermediate CuC2 on the external surface of the catalyst, which suppressed the chain growth of linear hydrocarbons because of steric hindrance [41,42,43,44,45]. Additionally, Mg(OH)2 in Cu7Mg3(OH)(T120-R150) helped to suppress the production of C4=, among which 1,3-butadiene is considered to be the precursor of green oil.
4 Conclusions
In summary, a catalyst for selective hydrogenation of acetylene was prepared from copper and magnesium hydroxides through thermal treatment with acetylene-containing gas followed by hydrogen reduction. The catalyst was composed of CuxC, Cu, and Mg(OH)2 enwrapped in a porous carbon matrix. Among them, CuxC was considered to be the highly active site for hydrogen dissociation, Cu mainly acted as the site for selective hydrogenation of acetylene, and the porous carbon layer helped to block the chain growth of linear hydrocarbons. Mg(OH)2 facilitated the formation of highly dispersed CuxC crystallites with enhanced hydrogenation activity, and its basicity was favorable to suppress the formation of C4=. The prepared catalysts exhibited remarkably high performance with complete acetylene conversion, low ethane selectivity (24%), and high stability in removal of acetylene impurity of ethylene stream at 100 °C and atmospheric pressure in a 100-h run. This work provides a new approach to preparing non-noble catalysts for a variety of hydrogenations under mild conditions.
References
Huang F, Deng Y, Chen Y, Cai X, Peng M, Jia Z, Ren P, Xiao D, Wen X, Wang N, Liu H, Ma D (2018) Atomically Dispersed Pd on Nanodiamond/Graphene Hybrid for Selective Hydrogenation of Acetylene. J Am Chem Soc 140:13142–13146
Chai M, Liu X, Li L, Pei G, Ren Y, Su Y, Cheng H, Wang A, Zhang T (2017) SiO2-Supported Au-Ni Bimetallic Catalyst for the Selective Hydrogenation of Acetylene. Chin J Catal 38:1338–1346
Kim SK, Kim C, Lee JH, Kim J, Lee H, Moon SH (2013) Performance of Shape-Controlled Pd Nanoparticles in the Selective Hydrogenation of Acetylene. J Catal 306:146–154
Liu H, Chai M, Pei G, Liu X, Li L, Kang L, Wang A, Zhang T (2020) Effect of IB-Metal on Ni/SiO2 Catalyst for Selective Hydrogenation of Acetylene. Chin J Catal 41:1099–1108
Rijo B, Lemos F, Fonseca I, Vilelas A (2020) Development of a Model for an Industrial Acetylene Hydrogenation Reactor Using Plant Data - Part I. Chem Eng J 379:122390
Pradier CM, Mazina M, Berthier Y, Oudar J (1994) Hydrogenation of acetylene on palladium. J Mol Catal 89:211–220
Sárkány A, Beck A, Horváth A, Révay Z, Guczi L (2003) Acetylene Hydrogenation on Sol-derived Pd/SiO2. Appl Catal A: Gen 253:283–292
Yang Z, Li Y, Cao Y, Zhao X, Chen W, Zhang J, Qian G, Peng C, Gong X, Duan X (2022) Al2O3 Microrods Supported Pd Catalysts for Semi-Hydrogenation of Acetylene: Acidic Properties Tuned Reaction Kinetics Behaviors. Chem Eng J 445:136681
Cao Y, Ge X, Li Y, Si R, Sui Z, Zhou J, Duan X, Zhou X (2021) Structural and Kinetics Understanding of Support Effects in Pd-Catalyzed Semi-Hydrogenation of Acetylene. Engineering 7:103–110
Liu Y, Fu F, McCue A, Jones W, Rao D, Feng J, He Y, Li D (2020) Adsorbate-Induced Structural Evolution of Pd Catalyst for Selective Hydrogenation of Acetylene. ACS Catal 10:15048–15059
McCue AJ, Anderson JA (2015) CO Induced Surface Segregation as a Means of Improving Surface Composition and Enhancing Performance of CuPd Bimetallic Catalysts. J Catal 329:538–546
Borodziński A, Bond GC (2008) Selective Hydrogenation of Ethyne in Ethene-Rich Streams on Palladium Catalysts, Part 2: Steady-State Kinetics and Effects of Palladium Particle Size, Carbon Monoxide, and Promoters. Catal Rev-Sci Eng 50:379–469
Nikolaev SA, Zanaveskin LN, Smirnov VV, Averyanov VA, Zanaveskin KL (2009) Catalytic Hydrogenation of Alkyne and Alkadiene Impurities in Alkenes. Practical and Theoretical Aspects. Russ Chem Rev 78:231–247
Bukhtiyarov AV, Panafidin MA, Prosvirin IP, Mashkovsky IS, Markov PV, Rassolov AV, Smirnova NS, Baeva GN, Rameshan C, Rameshan R, Zubavichus YV, Bukhtiyarov VI, Stakheev AY (2022) Boosting the Activity of PdAg2/Al2O3 Supported Catalysts towards the Selective Acetylene Hydrogenation by Means of CO-Induced Segregation: A Combined NAP XPS and Mass-Spectrometry Study. Appl Surf Sci 604:154497
Afonasenko TN, Temerev VL, Shlyapin DA, Tsyrul’Nikov PG (2019) Liquid-Phase Hydrogenation of Acetylene to Ethylene in a Flow on Pd/Al2O3 and Pd-Ga/Al2O3 Catalysts in the Presence of CO. Russ J Appl Chem 92:128–134
Wang J, Xu H, Che C, Zhu J, Cheng D (2023) Rational Design of PdAg Catalysts for Acetylene Selective Hydrogenation via Structural Descriptor-Based Screening Strategy. ACS Catal 13:433–444
Ma R, He Y, Feng J, Hu Z, Van Tendeloo G, Li D (2019) A Facile Synthesis of Ag@PdAg Core-Shell Architecture for Efficient Purification of Ethene Feedstock. J Catal 369:440–449
Chen L, Li X, Ma S, Hu Y, Shang C, Liu Z (2022) Highly Selective Low-Temperature Acetylene Semihydrogenation Guided by Multiscale Machine Learning. ACS Catal 12:14872–14881
Li X, Chen L, Shang C, Liu Z (2021) In Situ Surface Structures of PdAg Catalyst and Their Influence on Acetylene Semihydrogenation Revealed by Machine Learning and Experiment. J Am Chem Soc 143:6281–6292
Wang Y, Qi Y, Fan M, Wang B, Ling L, Zhang R (2022) C2H2 Semi-Hydrogenation on the PdxMy Cluster/Graphdiyne Catalysts: Effects of Cluster Composition and Size on the Activity and Selectivity. Green Energy Environ 7:500–511
Huang F, Peng M, Chen Y, Cai X, Qin X, Wang N, Xiao D, Jin L, Wang G, Wen X, Liu H, Ma D (2022) Low-Temperature Acetylene Semi-Hydrogenation over the Pd1-Cu1 Dual-Atom Catalyst. J Am Chem Soc 144:18485–18493
Yang T, Feng Y, Ma R, Li Q, Yan H, Liu Y, He Y, Miller JT, Li D (2021) Improvement of Selectivity in Acetylene Hydrogenation with Comparable Activity over Ordered PdCu Catalysts Induced by Post-treatment. ACS Appl Mater Inter 13:706–716
Yuan Z, Liu L, Ru W, Zhou D, Kuang Y, Feng J, Liu B, Sun X (2022) 3D Printed Hierarchical Spinet Monolithic Catalysts for Highly Efficient Semi-Hydrogenation of Acetylene. Nano Res 15:6010–6018
Qin C, Guo Q, Guo J, Chen P (2021) Atomically Dispersed Pd Atoms on a Simple MgO Support with an Ultralow Loading for Selective Hydrogenation of Acetylene to Ethylene. Chem-Asian J 16:1225–1228
Guo Y, Qi H, Su Y, Jiang Q, Cui Y, Li L, Qiao B (2021) High Performance of Single-Atom Catalyst Pd1/MgO for Semi-Hydrogenation of Acetylene to Ethylene in Excess Ethylene. ChemNanoMat 7:526–529
Lomonosov V, Wayman TMR, Hopper ER, Ivanov YP, Divitini G, Ringe E (2023) Plasmonic Magnesium Nanoparticles Decorated with Palladium Catalyze Thermal and Light-Driven Hydrogenation of Acetylene. Nanoscale 15:7420–7429
Dasgupta A, He H, Gong R, Shang S, Zimmerer EK, Meyer RJ, Liu Z, Janik MJ, Rioux RM (2022) Atomic Control of Active-Site Ensembles in Ordered Alloys to Enhance Hydrogenation Selectivity. Nat Chem 14:523–529
Wang S, Zhu J, Si J, Zhao G, Liu Y, Lu Y (2020) High-Performance Pd/Brass-fiber Catalyst for Selective Hydrogenation of Acetylene : Effect of Calcination-Assisted Endogenous Growth of ZnO-CuOx on Brass-Fiber. J Catal 382:295–304
Niu Y, Wang Y, Chen J, Li S, Huang X, Willinger M, Zhang W, Liu Y, Zhang B (2022) Patterning the Consecutive Pd3 to Pd1 on Pd2Ga Surface via Temperature-Promoted Reactive Metal-Support Interaction. Sci Adv 8: eabq5751
He Y, Liang L, Liu Y, Feng J, Ma C, Li D (2014) Partial hydrogenation of acetylene using highly stable dispersed bimetallic Pd-Ga/MgO-Al2O3 catalyst. J Catal 309:166–173
Menezes WG, Altmann L, Zielasek V, Thiel K, Bäumer M (2013) Bimetallic Co-Pd Catalysts: Study of Preparation Methods and Their Influence on the Selective Hydrogenation of Acetylene. J Catal 300:125–135
Yurpalova DV, Afonasenko TN, Prosvirin IP, Bukhtiyarov AV, Panafidin MA, Vinokurov ZS, Trenikhin MV, Gerasimov EY, Gulyaeva TI, Kovtunova LM, Shlyapin DA (2023) Selective Hydrogenation of Acetylene over Pd-Co/C Catalysts: The Modifying Effect of Cobalt. Catalysts 13:739
Cao Y, Sui Z, Zhu Y, Zhou X, Chen D (2017) Selective Hydrogenation of Acetylene over Pd-In/Al2O3 Catalyst: Promotional Effect of Indium and Composition-Dependent Performance. ACS Catal 7:7835–7846
Feng Q, Zhao S, Wang Y, Dong J, Chen W, He D, Wang D, Yang J, Zhu Y, Zhu H, Gu L, Li Z, Liu Y, Yu R, Li J, Li Y (2017) Isolated Single-Atom Pd Sites in Intermetallic Nanostructures: High Catalytic Selectivity for Semihydrogenation of Alkynes. J Am Chem Soc 139:7294–7301
Wang L, Li F, Chen Y, Chen J (2019) Selective Hydrogenation of Acetylene on SiO2-Supported Ni-Ga Alloy and Intermetallic Compound. J Energy Chem 29:40–49
Cao Y, Zhang H, Ji S, Sui Z, Jiang Z, Wang D, Zaera F, Zhou X, Duan X, Li Y (2020) Adsorption Site Regulation to Guide Atomic Design of Ni-Ga Catalysts for Acetylene Semi-Hydrogenation. Angew Chem Int Ed 59:11647–11652
Fu F, Liu Y, Li Y, Fu B, Zheng L, Feng J, Li D (2021) Interfacial Bifunctional Effect Promoted Non-Noble Cu/FeyMgOx Catalysts for Selective Hydrogenation of Acetylene. ACS Catal 11:11117–11128
Shi X, Lin Y, Huang L, Sun Z, Yang Y, Zhou X, Vovk E, Liu X, Huang X, Sun M, Wei S, Lu J (2020) Copper Catalysts in Semihydrogenation of Acetylene : From Single Atoms to Nanoparticles. ACS Catal 10:3495–3504
Huang F, Peng M, Liu H, Ma D (2023) Atomically Dispersed Metals on Nanodiamond-Derived Hybrid Materials for Heterogeneous Catalysis. ACC Mater Res 4:223–236
Huang F, Deng Y, Chen Y, Cai X, Peng M, Jia Z, Xie J, Xiao D, Wen X, Wang N, Jiang Z, Liu H, Ma D (2019) Anchoring Cu1 Species over Nanodiamond-Graphene for Semi-Hydrogenation of Acetylene. Nat Commun 10:4431
Lu C, Wang Y, Zhang R, Wang B, Wang A (2020) Preparation of an Unsupported Copper-Based Catalyst for Selective Hydrogenation of Acetylene from Cu2O Nanocubes. ACS Appl Mater Inter 12:46027–46036
Lu C, Zeng A, Wang Y, Wang A (2021) High-Performance Catalysts Derived from Cupric Subcarbonate for Selective Hydrogenation of Acetylene in an Ethylene Stream. Eur J Inorg Chem 2021:997–1004
Lu C, Zeng A, Wang Y, Wang A (2021) Copper-Based Catalysts for Selective Hydrogenation of Acetylene Derived from Cu(OH)2. ACS Omega 6:3363–3371
Zeng A, Lu C, Xu B, Wang A, Liu Y, Sun Z, Wang Y (2023) A Highly Active Catalyst Derived from CuO Particles for Selective Hydrogenation of Acetylene in Large Excess Ethylene. Phys Chem Chem Phys 25:14598–14605
Lu C, Zeng A, Wang Y, Wang A (2022) Enhanced Hydrogenation Activity over a Zn-Modified Cu-Based Catalyst in Acetylene Hydrogenation. Ind Eng Chem Res 61:18696–18702
Yao Y, Yu Z, Lu C, Sun F, Wang Y, Sun Z, Liu Y, Wang A (2022) Highly Efficient Cu-Based Catalysts for Selective Hydrogenation of Furfural: A Key Role of Copper Carbide. Renew Energy 197:69–78
Liu S, Yu Z, Lu C, Wang Y, Sun F, Sun Z, Liu Y, Shi C, Wang A (2023) Copper Carbide Composite Catalyst for Hydrogenolysis of Glycerol to 1,2-Propanediol. Fuel 334:126763
Zhao Z, Zhao J, Chang X, Zha S, Zeng L, Gong J (2019) Competition of C-C Bond Formation and C-H Bond Formation for Acetylene Hydrogenation on Transition Metals: A Density Functional Theory Study. AIChE J 65:1059–1066
Bao H, Zhang W, Hua Q, Jiang Z, Yang J, Huang W (2011) Crystal-Plane-Controlled Surface Restructuring and Catalytic Performance of Oxide Nanocrystals. Angew Chem Int Ed 50:12294–12298
Fox EB, Velu S, Engelhard MH, Chin Y, Miller JT, Kropf J, Song C (2008) Characterization of CeO2-Supported Cu-Pd Bimetallic Catalyst for the Oxygen-Assisted Water-Gas Shift Reaction. J Catal 260:358–370
Wang J, Li C, Zhu Y, Boscoboinik JA, Zhou G (2022) In Situ Monitoring of H2-Induced Nonstoichiometry in Cu2O. J Phys Chem Lett 13:5597–5604
Kim S, Son Y, Choi K, Kim S, Son Y, Park J, Lee JH, Jang J (2018) Highly Active Bifunctional Electrocatalysts for Oxygen Evolution and Reduction in Zn-Air Batteries. Chemsuschem 11:4203–4208
Martínez MT, Callejas MA, Benito AM, Cochet M, Seeger T, Ansón A, Schreiber J, Gordon C, Marhic C, Chauvet O, Fierro JLG, Maser WK (2003) Sensitivity of Single Wall Carbon Nanotubes to Oxidative Processing: Structural Modification, Intercalation and Functionalisation. Carbon 41:2247–2256
Zhang F, Zhang C, Zeng R, Song L, Guo L, Huang X (2016) Corrosion Resistance of the Superhydrophobic Mg(OH)2/Mg-Al Layered Double Hydroxide Coatings on Magnesium Alloys. Metals 6:85
Bridier B, Pérez-Ramírez J (2010) Cooperative Effects in Ternary Cu-Ni-Fe Catalysts Lead to Enhanced Alkene Selectivity in Alkyne Hydrogenation. J Am Chem Soc 132:4321–4327
Bridier B, López N, Pérez-Ramírez J (2010) Partial Hydrogenation of Propyne over Copper-Based Catalysts and Comparison with Nickel-Based Analogues. J Catal 269:80–92
Koeppel RA, Wehrli JT, Wainwright MS, Trimma DL, Cant NW (1994) Selective Hydrogenation of C-4-alkynes over a Copper on Silica Catalyst. Appl Catal 120:163–177
Che C, Wang B, Shan C, Chen H, Liu W, Tang Y (2017) An Effective Strategy to Prepare Pd-Ag/MgCO3@alpha-Al2O3 Catalyst for Selective Hydrogenation of Acetylene. Catal Lett 147:483–490
Wehrli JT, Thomas DJ, Wainwright MS, Trimma DL, Cant NW (1991) Selective Hydrogenation of Propyne over Supported Copper Catalysts: Influence of Support. Appl Catal 70:253–262
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22172015, 21972014, 22172012, and 22202024).
Funding
National Natural Science Foundation of China,21972014,22172012,22172015,22202024
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interest
The authors declare no competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10562_2024_4686_MOESM1_ESM.docx
Supplementary file1 (DOCX 1070 KB) Fig. S1, HRTEM image of Cu 7 Mg 3 (OH); Fig. S2, TG-DSC profiles of Cu(OH) 2 , Mg(OH) 2 and Cu 7 Mg 3 (OH); Fig. S3, EDS elementary mapping images of Cu 7 Mg 3 (OH)(T120-R150); Fig. S4, N 2 adsorption–desorption isotherms of Cu 7 Mg 3 (OH), Cu 7 Mg 3 (OH)(T120) and Cu 7 Mg 3 (OH)(T120-R150); Fig. S5, H 2 -TPR curve of Cu 7 Mg 3 (OH)(T120); Table S1, Specific surface areas of Cu 7 Mg 3 (OH), Cu 7 Mg 3 (OH)(T120) and Cu 7 Mg 3 (OH)(T120-R150); Table S2, Product selectivity in hydrogenation of acetylene in N 2 catalyzed by Cu(OH) 2 (T120-R150) and Cu 7 Mg 3 (OH)(T120-R150).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Zeng, A., Xu, B. et al. Promotion of Mg(OH)2 in Cu-Based Catalysts for Selective Hydrogenation of Acetylene. Catal Lett 154, 5171–5183 (2024). https://doi.org/10.1007/s10562-024-04686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-024-04686-y