Abstract
The g-C3N4/Ag/Bi2O2CO3 nanosheets were synthesized using a mixture of precursors containing melamine and nitrates of silver and bismuth through a simple one-pot synthesis strategy. In the presence of ammonium nitrate, g-C3N4/Ag/Bi2O2CO3 composite catalysts with different silver mass ratios were prepared, and the photodegradation performance of the composite samples was evaluated by photodegradation of tetracycline hydrochloride (TC) under simulated solar light irradiation. The synthesized composites were characterized by XPD, XPS, FT-IR, TEM, UV–vis DRS and PL. It was found that the g-C3N4/Ag/Bi2O2CO3 catalyst with Ag mass ratio of 4% exhibited the highest catalytic activity, and the degradation effect was more than 9 times that of pure C3N4 and nearly 5 times that of g-C3N4/Bi2O2CO3.In addition, cyclic experiments showed that g-C3N4/Ag/Bi2O2CO3 composites had certain stability. The photocatalytic performance of the g-C3N4/Ag/Bi2O2CO3 composites was attributable to the heterogeneous junction of Bi2O2CO3 and g-C3N4 and the surface plasmon resonance effect (SPR) of Ag nanoparticles. The energy levels of Bi2O2CO3 and g-C3N4 were properly matched to form a heterojunction, which promoted the separation and transfer of photogenerated electrons and holes, thereby improving the photocatalytic performance. The Ag surface plasmon resonance (SPR) effect not only accelerated the separation and transmission of electron–hole pairs, but also enhanced the absorption of visible light. Finally, based on the above basis, a possible photocatalytic mechanism was proposed.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the development of industry, a large amount of industrial waste was discharged into the water, which was extremely harmful to the environment [1, 2]. To address these hazards, many solutions had been developed, such as membrane filtration [3], chemical oxidation [4], and microbial degradation [5]. However, the use of these methods to treat contamination was unsatisfactory due to secondary pollution and high cost. Therefore, finding a green and efficient method of treating pollutants had become an urgent matter. Recently, the use of semiconductor materials to degrade pollutants using solar energy had attracted great attention [6, 7]. Graphite phase phase carbonitride (g-C3N4) was a low cost, high stability metal-free catalyst. The forbidden band width of g-C3N4 was about 2.7 eV, which was much lower than the forbidden band width of ZnO and TiO2, and can absorb visible light for catalytic degradation reaction [8,9,10]. Based on the above reasons, g-C3N4 had caused great concern. However, g-C3N4 also had serious shortcomings. Most of the conventionally synthesized g-C3N4 was a massive structure, and the bulk g-C3N4 had the disadvantages of small specific surface area, fast internal photo-generated carrier current and low photocatalytic activity [11, 12]. In order to change the shortcomings of bulk g-C3N4, it was proposed to manufacture g-C3N4 with ultra-thin nanosheet structure. In photocatalysis, semiconductor materials with nanosheet structure had rich activity due to relatively large surface area and ultra-thin thickness. Generally, methods for preparing nanosheet structures include thermal oxidation etching and ultrasonic assisted liquid stripping [13, 14]. However, both methods had certain disadvantages. Thermal oxidation etching caused a large amount of interface defects in the semiconductor material, and the yield of ultrasonic assisted liquid stripping was relatively low. Recently, a route for synthesizing g-C3N4 nanosheets using chemical blowing had been reported [15, 16]. In order to increase the activity of g-C3N4, it was necessary to modify g-C3N4. Common modification methods included doping modification, semiconductor recombination, morphology control etc. [17,18,19,20]. In recent years, in the field of photocatalysis, in addition to g-C3N4, Bi-based semiconductor materials had also been greatly explored and developed [21,22,23]. Bismuth carbonate (Bi2O2CO3) was a typical Aurivillius/Sillén phase compound in Bi-based compounds. Due to its unique photocatalytic activity, low cost and stability, it had been widely concerned [24,25,26,27]. Bi2O2CO3 had a unique layered structure, which was composed of alternating [Bi2O2]2+ and CO32−, which could accelerate the transfer of active substances between layers and improve photocatalytic activity [28]. However, the band gap of Bi2O2CO3 was 2.8–3.5 eV, which could only be corresponding under ultraviolet light. In order to improve the deficiency of g-C3N4 and Bi2O2CO3, and to make good use of the advantages of the two semiconductor materials, it was preferred to modify g-C3N4 with Bi2O2CO3. A heterojunction can be formed between Bi2O2CO3 and g-C3N4, which not only broadens the light absorption range, but also accelerates the transfer of photogenerated carriers [27]. Among these heterojunction materials, Z-scheme heterojunctions exhibited the dual advantages of suppression of recombination of electron-holepairs and reserving high redox ability for both semiconductors [29, 30]. In particular Z-scheme heterojunction photocatalysts with noble metals as bridges showed better photocatalytic performance [31, 32]. Due to the surface plasmon resonance effect (SPR) of the noble metal[33], on the one hand, the light absorption range could be broadened, and on the other hand, it could suppress the superposition electron–hole pair recombination in the semiconductor catalyst, thereby causing the photocatalytic activity of the catalyst to be greatly improved [34, 35]. Among many precious metals, Ag was selected as a load metal due to its low price.
In view of practical application, the preparation method was of great importance. In this work, a simple one-pot synthesis strategy was chosen to synthesize Z-scheme g-C3N4/Ag/Bi2O2CO3 materials. Compared with other preparation methods, the method reduced the cumbersome steps and shortened the synthesis cycle, which provided a possibility for practical application. The performance of the composite photocatalyst was evaluated using tetracycline hydrochloride (TC) degradation. Compared with pure g-C3N4 and g-C3N4/Bi2O2CO3, the photocatalytic activity of the synthesized g-C3N4/Ag/Bi2O2CO3 was significantly improved. Finally, based on the capture agent experiments, the reaction mechanism of the composite was inferred.
2 Experimental Section
All chemicals were reagent grade and used directly withoutany further purification. Chemical agents, Melamine and bismuth nitrate pentahydrate were purchased from Shanghai sinopharm chemical reagent co., LTD., China. Silver nitrate was obtained from tianjin fengchuan chemical reagent technology co., LTD., China. Ammonium nitrate was purchased from Shanghai reagent co., LTD.,China. All the water used in the experiment was deionized water, and the whole experiment process was carried out under the pressure of greenhouse.
A g-C3N4/Ag/Bi2O2CO3 photocatalyst sample was synthesized by a one-step milling roasting method. In a typical process, 3.0 g of melamine, 0.45 g of NH4NO3, 0.032 g of Bi(NO3)3·5H2O, and a portion of silver nitrate are thoroughly milled in a mortar for 30 min at room temperature. The ground mixture was placed in a crucible and the crucible was placed in a muffle furnace. It was heated to 550 °C in a muffle furnace at a heating rate of 5 °C/min, and then held for 2 h. After the muffle furnace was naturally cooled to room temperature, a yellow solid was obtained, which was ground to a fine powder for use. The catalyst used in the study was abbreviated as x wt% CN/Ag/BiOC, where x represented the mass concentration of Ag in the sample. Moreover, a series of composite CN/Ag/BiOC samples with different contents of Bi2O2CO3 were synthesized by the same method. These catalysts were abbreviated as x wt% CN/Ag/BiOC-y, where y represented the amount of Bi2O2CO3 in the catalyst (wt %).
X-ray powder diffraction (XRD) patterns of the samples were examined on the Bruker D8 X-ray diffraction instrument with CuKa gamma ray (λ = 1.5418A), and the CuKa ray scanning range is 5° to 90°. High resolution transmission electron microscopic (HRTEM) mages were acquired with FEI-Tecnai-G20 TEM microscope. The chemical composition and chemical state of CN/Ag/BiOC complex were analyzed by X ray photoelectron spectroscopy (XPS) on Escalab 250Xi X-ray photoelectron spectrometer with Al Ka excitation source. The FT-IR experiment was carried out on a NiCOLET 380 type FT-IR spectrometer (hermo Electron Corporation) using KBr as the background, and the scanning range was selected to be 4000–400 cm−1. Diffuse reflectance spectra were recorded using a TU-1950 dual-beam UV spectrophotometer with barium sulfate as the background and a wavelength range of 200 nm to 800 nm. Photoluminescence (PL) spectra were performed on a FL970 fluorescence spectrophotometer with an excitation wavelength of365 nm. The photocurrent response under simulated solar light irradiation was recorded with a CHI-660D electrochemical workstation in a sandwich-type configuration, a Pt slice as the counter electrode, a saturated calomel electrode (SCE) as the reference, and 0.1 M Na2SO4 solution as electrolyte. A 300W xenon arc lamp equipped with a simulated solar light filter (HSX-F300, Beijing NBeT Technology Co., Ltd) calibrated to 100 mW/cm2, which was measured with a radiometer (CEL-NP2000, Beijing Au-light Co., Ltd), employed as the light source. Electrochemical impedance spectroscopy (EIS) Nyquist plots were obtained at 0.6 V with small AC amplitude of 5 mV in the frequency range of 0.1–105 Hz. All experiments were carried out under ambient conditions.
In the experimental setup, a 500 W xenon lamp was employed as a light source. The photocatalytic activity of the CN/Ag/BiOC sample was determined by degrading the simulated pollutant tetracycline (TC). The photodegradation reaction was carried out in a photochemical reactor. Before photodegradation, about 50 mg of catalyst was added to 50 ml of TC solution (1 × 10−4 mol·L−1), and the solution was magnetically stirred in the dark for 3 h to reach the adsorption–desorption equilibrium between the catalyst and tetracycline. Then the solution was exposed to simulated solar light irradiation under magnetic stirring. During the degradation process, About 3 mL aliquot was periodically taken out from the reactor and centrifuged immediately every 10 min. The absorbance of the solution was then measured at 357 nm using a UV–Vis spectrophotometer (UV-2550, Shimadzu) to analyze the degradation of the TC solution. The formula for calculating the photocatalytic degradation efficiency was as follows:
Where t represented time, C represented the concentration of TC, C0 was the initial concentration of TC, and Ct was the concentration of TC at time t.
To test the stability of the catalyst sample, a sample reuse experiment was performed. The catalyst was washed with water and briefly sonicated before each interval of the experiment.
3 Results and Discussion
A simple synthesis process for the catalyst sample was shown in Fig. 1. Bismuth nitrate, silver nitrate and ammonium nitrate were encapsulated or adhered by melamine during mixing with melamine. During the calcination process, the melamine molecules were heat-shirked into a 2D carbon nitride layer, and the 2D carbon nitride layer was deposited to form a 3D g-C3N4. During the heating process, ammonium nitrate, bismuth nitrate and silver nitrate would decompose, releasing NH3, NO, NO2 and other gases. These gases acted as a template to accelerate the formation of the composite and made the material have a large number of pore structures. At the same time, metallic silver nanoparticles and solid Bi2O2CO3 products appeared on the g-C3N4 nanosheets.
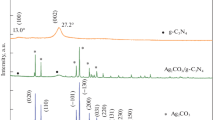
The crystal structures of the synthesized samples were analyzed by XRD diffraction pattern, as shown in Fig. 2. No excess peak was found in the XRD pattern, indicating that the synthesized sample did not contain any impurities. In the XRD pattern of g-C3N4, there were two significant characteristic peaks at 13.4° and 27.4° [36]. The characteristic peak at 13.4° corresponded to the (100) diffractive surface of g-C3N4, which corresponded to the interplanar structural packing. The diffraction peak at 27.4° corresponded to the (002) crystal plane of g-C3N4, reflecting the interlayer deposition characteristics of the conjugated aromatic system. For the CN/BiOC composites, no diffraction peak corresponding to Bi2O2CO3 was found, which may be caused by the relatively small amount of bismuth species in the synthesis. However, compared with the XRD pattern of pure g-C3N4 material, the diffraction intensity of the CN/BiOC material at 27.4° was greatly increased, which was sharper, indicating that the addition of Bismuth nitrate in the synthesis increased the crystallinity of the material. The XRD of CN/Ag/BOC composites with different contents of Bi2O2CO3 were prepared in order to verify the presence of Bi2O2CO3 in the composite. As shown in Fig. 2, it was obvious that the diffraction peaks of tetragonal phase Bi2O2CO3 were found as the content of Bi2O2CO3 is 10wt % [23]. No diffraction peak of Ag was found in the XRD patterns of CN/Ag/BiOC and Ag/CN materials, which may be caused by the relatively small amount of Ag species in the synthesis. The presence of Ag in the CN/Ag/BiOC samples was confirmed by the following TEM and XPS images.
The morphology and microstructure of the samples were investigated by the high-resolution transmission electron microscopy (HRTEM). As shown in Fig. 3, it could be observed in Fig. 3a that the synthesized CN/Ag/BiOC sample was a two-dimensional irregular layered nanosheet material and the bismuth species were well dispersed on the g-C3N4nanosheets. The attached small nanocrystals had a lattice fringe spacing of 0.372 nm, which could be ascribed to the (011) planes of tetragonal phase Bi2O2CO3 [37] (Fig. 3b). In addition, the lattice fringe of d = 0.210 nm was measured to correspond to the (200) crystal plane of Ag [38], indicating metal Ag Nanoparticles were present in CN/Ag/BiOC samples.
The FT-IR spectroscopy was used to reveal the interfacial interaction between g-C3N4 and Bi2O2CO3 in composite samples. The FT-IR spectra of g-C3N4 and different catalyst samples were shown in Fig. 4. It could be seen from the figure that g-C3N4 had a strong absorption band in the range of 900–1800 cm−1, which could be ascribed to the C=N and aromatic C–N stretching vibration modes [39]. The band at 810 cm−1 was associated with the breathing modes of s-triazine ring system. The broad band at 3294 cm−1 could be assigned to the stretching mode of NH2 or N–H groups. In the case of Bi2O2CO3, the absorption peaks at about 551 and 848 cm−1 assigned to the stretching vibrations of the Bi-O and the bending mode of the CO32− group, respectively, while the peaks at about 1400 cm−1 were associated with the stretching vibrations of C–O and C=O [40] respectively. The FTIR results of g-C3N4/Ag/Bi2O2CO3 nanocomposites with different weight ratios were very similar to those of pure g-C3N4, which may be due to the weak vibration mode of Bi2O2CO3 and the strong IR response of g-C3N4.
The surface elemental composition and chemical state of the different elements in 4wt% CN/Ag/BiOC sample were investigated by XPS measurement. According to the Fig. 5a, the Bi 4f spectrum showed two distinct peaks at 158.9 and 164.3 eV, attributed to Bi 4f7/2 and Bi 4f5/2, yielding up shift of 0.7 and 0.9 eV in comparison with 158.2 and 163.4 eV for pure Bi2O2CO3, respectively [41]. The upshift of binding energy may be attributed to the interaction between Bi2O2CO3 and g-C3N4 [42]. As shown in Fig. 5b, the N 1 s XPS spectrum was devided into three component peaks at 398.3, 398.5, 400.2 eV, indexed as the C-N sp3 bonds, C = N sp2 bonds, and the interaction of g-C3N4 sheets with Bi2O2CO3, respectively [43]. Figure 5c exhibited the Ag 3d XPS spectrum of 4wt %CN/Ag/BiOC and the binding energies of the Ag 3d3/2 and Ag 3d5/2 peaks as 373.8 and 367.8 eV were found respectively. According to this result, the Ag existed predominantly in the metallic form [44]. From Fig. 5d, it c0uld also be seen that the C 1 s peak was mainly devided into three peaks located at about 284.6, 287.5 and 288.3 eV, respectively. The peak located at 284.6 eV could be indexed as the signal of standard reference carbon [45].The peak located at 287.5 and 288.3 eV could be ascribed to C = N sp2 bonds and the overlapping of sp2 C atoms in carbonate in Bi2O2CO3 and g-C3N4 [46]. In Fig. 5e, the XPS spectrum of O1s displayed two distinct peaks at 531.5 and 532.5 eV, corresponding to O2−ions in the Bi-O bonds and the chemisorbed oxygen due to hydroxyl radicals [47]. According to these results, it revealed the coexistence of Bi2O2CO3, g-C3N4 and Ag in this CN/Ag/BiOC system, which was in good agreement with the above analysis.
The UV–vis diffuse reflectance (UV–vis DRS) spectra of as-prepared samples were shown in Fig. 6. It could be seen that the absorption edge of the pure Bi2O2CO3and g-C3N4occur at about 403 nm and 459 nm, respectively, which suggested that the Bi2O2CO3 could only absorb UV light, while the g-C3N4 had strong absorption in visible light region. The absorption features of CN/BiOC and Ag/CN were similar to the g-C3N4, while the absorption intensities of both CN/BiOC and Ag/CN were stronger than the g-C3N4. The optical absorption of CN/Ag/BiOC shifted significantly to longer wavelengths and displayed higher absorption intensity over the range from 200 to 600 nm, in contrast with all other samples. This result was consistent with the literature [40]. According to the literature, the g-C3N4 could serve as an effective visible-light sensibilizer for Bi2O2CO3. So this result may be mainly ascribed to synergic effect between the SPR of Ag metal and the addition of Bi2O2CO3.
The photocatalytic activities of the as-preparedCN/Ag/BiOC composites were evaluated by photocatalytic removal of TC under the simulated solar light irradiation. As shown in Fig. 7a, in the absence of the photocatalyst, TC self-photodegradation was almost unobserved within 240 min, revealing that TC was quite stable toward incident light. The g-C3N4 exhibited the poorest catalytic activity among these catalysts and about 15% of TC was decomposed after irradiation for 240 min. Furthermore, it could be seen that CN/BiOC and CN/Ag/BiOC heterostructures all exhibited higher photocatalytic activity in the degradation of TC relative to the g-C3N4 sample. Specifically, the photocatalytic activities of CN/Ag/BiOC nanocomposites gradually increase with the weight of Ag from 1wt% to 4wt%. The decomposition rate would decrease again if more Ag was added into the CN/BiOC. Hence, the as-prepared 4wt%CN/Ag/BiOC nanocomposite was the optimal, and the maximum degradation effificiency of 98.1% was attained after the irradiation for 240 min.
Following a Langmuir–Hinshelwood kinetic model, the photocatalytic degradation of TC at low concentrations followed the pesudo-fifirst-order kinetics. Figure 7b exhibited the photocatalytic degradation kinetics of TC and the 4wt% CN/Ag/BiOC nanocomposite possessed the optimum activity among all the samples with a rate constant of 29.5 × 10−3 min−1, which was nearly 9 times that of pure g-C3N4 (k = 0.032 min−1) and nearly 5 times that of g-C3N4/Bi2O2CO3(k = 0.055 min−1).
The stability of the composite photocatalyst was evaluated by performing cycle experiments on the degradation of TC under simulated solar light irradiation, using 4wt% CN/Ag/BiOC. As shown in Fig. 8a, the photocatalytic efficiency showed very little variation after five successive cycles, which indicated that the CN/Ag/BiOC possessed excellent long term stability. These results suggested that the CN/Ag/BiOC were promising solar light photocatalyst for practical applications. Figure 8 (b) showed the XRD patterns of 4wt%CN/Ag/BiOC composite before and after five recycles for the photodegradation of TC under simulated solar light irradiation. It was obvious that the used 4wt%CN/Ag/BiOC composite showed similar XRD pattern compared to the fresh 4wt%CN/Ag/BiOC composite, which could prove the chemical stability of CN/Ag/BiOC photocatalyst in the photodegradation of TC solution.
Photoluminescence spectra (PL spectra) descend from the recombination of the free charge carriers. So the separation efficiency of the photogenerated electron–hole pairs of as-prepared composites were characterized by PL spectra. In generally, the higher the PL emission intensity indicated the higher the recombination of the photogenerated charge carriers and the lower the photocatalytic activity [49]. The PL spectra of g-C3N4, Ag/CN, CN/BiOC and 4wt%CN/Ag/BiOC at the excitation wavelength of 368 nm are shown in Fig. 9a. It was obvious that the g-C3N4 exhibited the highest intensity among all the samples. When the g-C3N4 combined with Ag and Bi2O2CO3, the intensity of the PL emission decreased. The 4wt%CN/Ag/BiOC exhibited the lowest intensity which was consistent with its best degradation efficiency. Therefore, incorporation of Ag and Bi2O2CO3 nanoparticles on the surface of g-C3N4 was highly favorable for efficient separation of photoinduced charge carriers, and thereby improves the photocatalytic activity of g-C3N4. Photoelectrochemical (PEC) experiment was accomplished to perform light-induced electron migration property. Transient photocurrent of g-C3N4, Ag/CN, CN/BiOC and 4wt% CN/Ag/BiOC were shown in Fig. 9b. As revealed in Fig. 8b, the 4wt% CN/Ag/BiOC possessed the highest photocurrent intensity, in which the enhanced photocurrent exemplified the higher separation of charge carriers. Meanwhile, these samples were tested by electrochemical impedance spectra (EIS) to investigate charge transfer rates. The EIS plots (Fig. 9c) illustrated that 4wt% CN/Ag/BiOC sample presented a relatively smaller arc radius of EIS Nyquist plot compared with the other samples, suggesting the relatively faster charge transfer in the 4wt% CN/Ag/BiOC sample. In other words, the 4wt% CN/Ag/BiOC exhibited lower resistance than the other samples and could facilitate the separation and immigration of photogenerated carriers under the light irradiation.
Figure 10a was a histogram of the effect of different scavengers on the photocatalytic process. The degradation rates of TC were drastically reduced after adding TEMPOL (⋅O2− scavenger). When the formic acid (h+ scavenger) was added to the solution, the degradation rates of TC were slightly reduced. But the degradation rates of TC was enhanced in the presence of isopropanol (⋅OH scavenger). This may have been attributed to the isopropanol greatly promoting the separation efficiency of electrons. Then, more electrons combined with O2 to form ⋅O2−, leading to increased degradation activity [48]. Compared with those without any scavenger, the degradation rate decreased from 88 to 78%, 59%, respectively, which indicated that ⋅O2− is mainly responsible for this photocatalytic reaction. According to the above results and some previous literature [27], a possible mechanism for the photocatalytic degradation of TC over CN/Ag/BiOC nanocomposites was proposed (Fig. 10b). As shown in Fig. 10b, the band-edge positions of the conduction band (CB) and the valence band (VB) of g-C3N4 were approximate − 1.13 eV and 1.57 eV, respectively, which could be known according to the results in the previous reports [27]. Correspondingly, the band-edge positions of CB and VB of Bi2O2CO3 were around 0.50 eV and 2.58 eV, respectively [50]. When the heterojunction CN/Ag/BiOC were exposed to the simulated solar light, the electrons in the VB of g-C3N4 and Bi2O2CO3 were excited to the CB. The CN/Ag/BiOC was Z-scheme heterojunction and Ag NPs were paired between Bi2O2CO3 and g-C3N4. The CB potential of Bi2O2CO3 was more negative than that of Ag due to its high Schottky barriers at the interface of the semiconductor–metal, thus the photoinduced electrons of Bi2O2CO3 would shift to metallic Ag and combined with the holes of g-C3N4. The electrons of Bi2O2CO3 and holes of g-C3N4 would annihilate in Ag NPs. This electron increased the separation rate of photogenerated electron–hole pairs and prolongs the lifetime of free electrons and holes. Meanwhile, the surface plasmon resonance (SPR) effect of Ag nanoparticles enhanced the adsorption of light and rapid separation and transportation of photogenerated electrons-holes. Moreover, the photogenerated electrons on the CB of g-C3N4 captured O2 to produce •O2– and then the •O2– oxidized the TC organic molecule. The holes on the VB of Bi2O2CO3 could degrade TC organic molecule through direct oxidation. Therefore, CN/Ag/BiOC composites possess ed higher photocatalytic activity than the Bi2O2CO3 and g-C3N4.
4 Conclusion
In summary, we had constructed a new type of CN/Ag/BiOC composite photocatalyst through a simple one-country synthesis strategy. It was found that the photocatalytic activity of CN/BiOC under simulated solar light was significantly enhanced after the introduction of Ag nanoparticles. When the weight content of Ag was 4%, the photocatalytic activity of CN/Ag/BiOC was optimum. In addition, the mechanism of photocatalytic degradation, UV–vis DRS, PL spectroscopy were studied. These results indicated that the matching conduction band levels of g-C3N4 and Bi2O2CO3 and the surface plasmon resonance (SPR) effect of Ag nanoparticles led to enhanced light adsorption capacity of the catalyst, and the separation of photogenerated electron–hole pairs was accelerated. This work provided a new strategy for the degradation of tetracycline (TC) by ternary plasma photocatalysts.
References
Qian X, Xu L, Zhu Y, Yu H, Niu J (2021) Chem Eng J 420:127615–127620
Zhou C, Lai C, Huang D, Zeng G, Zhang C, Cheng M, Hu H, Wan J, Xiong W, Wen M, Wen X, Qin L (2018) Appl Catal B Environ 220:202–210
Müller A, Xu Z, Greiner A (2022) Mater Eng 307:2200238–2200245
Alazaiza M, Albahnasawi A, Copty N, Ali G, Bashir M, Abu A, Abushammala M, Nassani D, Maskari T (2022) Global NEST J 24(1):74–86
Pang S, Lin Z, Li J, Zhang Y, Mishra S, Bhatt P, Chen S (2022) Front Microbiol 13:713375–713381
Jin B, Cho Y, Zhang Y, Li P, Zhan K, Lee KS, Park JH (2019) Nano Energy 66:104110–104118
Chandrasekaran S, Chung J, Kim E, Hur S (2016) Chem Eng J 290:465–476
Fu J, Bie C, Cheng B, Jiang C, Yu J (2018) ACS Sustain Chem Eng 6:2767–2779
Liu C, Zhang Y, Dong F, Reshak A, Ye L, Pinna N, Zeng C, Zhang T, Huang H (2017) Appl Catal B Environ 203:465–474
Zhu B, Zhang L, Cheng B, Yu J (2018) Appl Catal B Environ 224:983–999
Mamba G, Mishra A (2016) Appl Catal B Environ 198:347–377
Wang L, Wang C, Hu X, Xue H, Pang H (2016) Chem–An Asian J 11: 3305–3328
Zhang H, Guo L, Zhao L, Wan B, Yang Y (2015) J Phys Chem Lett 6:958–963
Lin Q, Li L, Liang S, Liu M, Bi J, Wu L (2015) Appl Catal B Environ 163:135–142
Yan J, Zhou C, Li P, Chen B, Zhang S, Dong X, Xi F, Liu J (2016) Colloid Surface A 5:257–264
Luo L, Zhang A, Janik M, Li K, Song C, Guo X (2017) Mater Lett 188:130–133
Wang X, Chen X, Thomas A, Fu X, Antonietti M (2009) Adv Mater 21:1609–1612
Meng J, Lan Z, Chen T, Lin Q, Liu H, Wei X, Lu Y, Li J, Zhang Z (2018) J Phys Chem C 122:24725–24731
Maeda K, Wang X, Nishihara Y, Lu D (2009) J Phys Chem C 113:4940–4947
Sun J, Zhang J, Zhang M, Antonietti M, Fu X, Wang X (2012) Nat Commun 3:1139–1144
Zheng J, Jiao Z (2017) J Coll Inter Sci 488:234–239
Zheng J, Jiao Z (2017) J Coll Inter Sci 504:620–625
Duan Y, Yao H, Li J, Shang X, Jia D, Li C (2019) Water Sci Technol 146:1494–1502
Yu L, Zhang X, Li G, Cao Y, Shao Y, Li D (2016) Appl Catal B 187:301–309
Wang Q, Yun G, Bai Y, An N, Lian J, Huang H, Su B (2014) Appl Surf Sci 313:537–544
Hu D, Zhang K, Yang Q, Wang M, Xi Y, Hu C (2014) Appl Surf Sci 316:93–101
Zhang Z, Lin S, Cui W, Li X, Li H (2019) Mater Lett 234:264–268
Hu R, Xiao X, Tu S, Zuo X, Nan J (2015) Appl Catal B 163:510–519
He Y, Zhang L, Teng B, Fan M (2015) Environ Sci Technol 49(1):649–656
Chen Y, Zhao C, Ma S, Xing P, Hu X, Wu Y, He Y (2019) Inorg Chem Front 6:3083–3092
Chen P, Chen L, Ge S, Zhang W, Wu M, Xing P, Rotamond TB, Lin H, Wu Y, He Y (2020) Int J Hydrogen Energy 45(28):14354–14367
Chen L, Wang J, Li X, Zhao C, Hu X, Wu Y, He Y (2022) Inorg Chem Front 9:2714–2724
Di J, Xia J, Ji M, Wang B, Yin S, Huang Y, Chen Z, Li H (2016) Appl Catal B-Environ 188:376–387
Qua S, Xiong Y (2018) J Photoch Photobio A 365:23–31
Gao X, Shang Y, Liu L, Nie W (2019) Opt Mater 88:229–237
Dong F, Zhao Z, Xiong T, Ni Z, Zhang W, Sun Y, Ho W (2013) ACS Appl Mater Interfaces 5:11392–11401
Ma Y, Bian Y, Tan P, Shang Y, Liu Y, Wu L, Zhu A, Li W, Xiong X, Pan J (2017) J Colloid Interf Sci 497:144–154
Meia F, Daia K, Zhang J, Li W, Liang C (2019) Appl Surf Sci 488:151–160
Li Y, Zhan J, Huang L, Xu H, Li H, Zhang R, Wu S (2014) RSC Adv 4:11831–11839
Tian N, Huang H, Guo Y, He Y, Zhang Y (2014) Appl Surf Sci 322:249–254
Zhang Q, Wang H, Hu S, Lu G, Bai J, Kang X, Liu D, Gui J (2015) RSC Adv 5:42736–42743
Yang M, Hu S, Li F, Fan Z, Wang F, Liu D, Gui J (2014) Ceram Int 40:11963–11969
Jiang W, Luo W, Zong R, Yao W, Li Z, Zhu Y (2016) Small 12:4370–4378
Wang X, Fan H, Ren P, Yu H, Li J (2012) Mater Res Bull 47:1734–1741
Wang S, Li D, Sun C, Yang S, Guan Y, He H (2014) Appl Catal B Environ 144:885–891
Martha S, Nashim A, Parida K (2013) J Mater Chem A 1:7816–7823
Xu J, Harmer J, Li G, Chapman T, Collier P, Longworth S (2010) Tsang S 46:1887–1889
Jiang J, Gao J, Li T, Chen Y, Wu Q (2019) XieT, Lin Y, Dong S. J Colloid Interf Sci 554:531–543
Hong Y, Li C, Fang Z, Luo B, Shi W (2017) Carbon 121:463–471
Yang C, Xue Z, Qin J, Sawangphruk M, Rajendran S, Zhang X, Liu R (2019) J Phys Chem C 123:4795–4804
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation (ZR2020MB036)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, J., Liu, G., Feng, X. et al. Enhanced Photocatalytic Performance of Z-Scheme Design of Bi2O2CO3/Ag/g-C3N4 Photocatalyst. Catal Lett 154, 2265–2274 (2024). https://doi.org/10.1007/s10562-023-04385-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04385-0