Abstract
This study deals with the use of commercial lipase stabilized onto magnetic particles of iron oxides (Fe3O4/γ-Fe2O3) and applied as a heterogeneous biocatalyst in the synthesis of ethyl esters (biodiesel) in a solvent-free medium on a continuous process. Magnetic particles were synthesized by alkaline co-precipitation, silanized, activated and subsequently used for immobilization of Burkholderia cepacia lipase. The results regarding the lipase immobilization showed an enzyme activity retention of about 53% and an increase in the KM value in about threefold (from 410 to 1262 mmol L−1) and a decrease in the Vmax value in sevenfold (From 12,390 to 1786 U g−1) when compared with the free enzyme. The immobilization process favored the thermal stability and increased the half-time of the enzyme about tenfold at 50 °C. The immobilized derivative was evaluated to aroma production and the activity of esterification was calculated as being 56.7 μmol L−1 g−1 min−1, which corresponds to a productivity value of 0.58 g ester L−1 h−1. The immobilized system was also used to mediate transesterification of kernel oil in a fixed bed reactor operating in a continuous flow with a reaction medium composed of oil and ethanol at a molar ratio of 1:12, 50 °C and space–time of 16 h. The operational stability of the immobilized lipase estimated at 47 days allowed to operate the reactor with high productivity of 38.7 ± 0.7 mg g−1 h−1. Also, the product properties of the ester content (> 96.5%) and kinematic viscosity value (5.32 ± 0.4 mm2 s−1 at 40 °C) meet the requirements of the ANP and ASTM (D6751) for biodiesel fuel.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biodiesel is an alternative biofuel that can be used in an engine suitable for diesel without the need for modifications and has environmental advantages due to the fact that it is made from renewable resources [1]. The raw materials used in the transesterification processes, such as alcohol, oil and the catalyst, when from renewable sources, guarantee a sustainable biofuel that contributes to the establishment of a circular bioeconomy platform [2]. Biodiesel production can be carried out using renewable catalysts, like lipases. Lipases (triacylglycerol acylhydrolases: E.C. 3.1.1.3) are enzymes that belong to the class of serine hydrolases which can be produced by animals, plants, and microorganisms [3]. This enzyme is one the most industrially applied biocatalyst and shows high activity and stability both in aqueous and organic media which allow them to catalyze various chemical reactions like esterification, transesterification, aminolysis, acidolysis, and others [4, 5]. The global lipase market reached USD 425.0 Million in 2018 and is expected to reach USD 590.2 Million in 2023 [6]. In this context, the utilization of lipases as biocatalysts in the valorization of oils and fats has gained considerable interest over the past decades [7, 8]. The enzymatic process is a new technology that shows promise for the synthesis of biodiesel from vegetable oils due to the non-soap formation and pure glycerol as a by-product [9]. The cost of using enzymes, however, is the main limitation for its industrial application in bulk products processing. On the other hand, a continuous transesterification with a high conversion implemented in a fixed bed reactor with a well-immobilized lipase can significantly reduce the costs of this process [9].
Notwithstanding the high potential of lipases, the use of their free form also presents limitations due to the fact they are very sensitive to the environmental reaction system and their recovery and reusability are difficult [10]. Therefore, the use of efficient and feasible techniques of immobilization have been described as the main driver for the utilization of this cluster of enzymes in the production of commodity chemicals, as fuels [11]. Immobilized lipases are also known to have lower inactivation effects when in contact with short-chain alcohols, which, when combined with their higher thermal and mechanic stabilities, make them possible catalysts to be used in different industrial fields, like the biodiesel production [12]. Besides that, exploring the peculiar catalytic mechanism of lipases, called interfacial activation, make it possible to manipulate the enzyme properties (catalytic behavior and stability) through modification of immobilization process conditions: type of immobilization (adsorption, entrapment, covalent attachment and cross-linking), support nature, ionic strength, pH, presence of detergents, chemical agents, and others [10, 13]. Wherefore, a wide library of biocatalysts having different properties can be obtained by altering the immobilization protocol.
An ideal carrier material for enzyme immobilization should be mechanically stable, inert, biologically compatible, resistant against microbial attack and cost-effective [14]. Therefore, several materials (organic and inorganic), have been evaluated as support for enzyme immobilization: polyvinyl alcohol, nanofibers, sepabeads, polyaniline matrix, rice husk, polyurethane, chitosan, niobium oxide, silica, agarose, pullulan, activated carbon, among others [15,16,17,18,19]. Magnetized particles have been stood out as enzyme immobilization matrices due to their particular magnetic properties, which allow easy recovery and a controlled stabilization in packed and fluidized bed reactors through the application of an external magnetic field [20]. Iron oxides, typically magnetite (Fe3O4) and maghemite (γ-Fe2O3), have been described as model immobilization matrices, due to their high surface area, mechanical stability, biocompatibility and low cost synthesis [21]. Surface modifications in such inorganic materials increase its applications and may also give the particles some additional flexibility that facilitates the interaction with the enzymes, leading to increased stability in a covalent bond immobilization process [22]. The utilization of γ-aminopropyltriethoxysilane (γ-APTS) as a functionalizing agent on iron oxide particles has been thoroughly described in the literature [23, 24], which binds to the oxide surface mostly by covalent bonding [23]. Therefore, the use of Magnetic nanoparticles (MNPs) as support for enzyme immobilization can improve the operational stability of enzymes in harsh environments and simplify the downstream separation in enzymatic catalyzed reactions, reinforcing the recyclability.
The use of enzymatic catalysis in biodiesel production allows reasonable production values, with substantially more economical attractiveness in continuous reactions when compared to batch systems [25]. Continuous systems in lab scales are usually composed of glass columns with a packed bed in which there is an up- or downflow and the products are recovered on the outlet of the reactor. Packed-bed reactors (PBR) are usually operated at low flow rates, which, despite presenting limitations on productivity values, are known to increase the contact time between the biocatalyst and the substrate, resulting in high conversion rates [26]. In that sense, continuous production of biodiesel employing compacted bed reactor and immobilized lipase is increasingly recurrent [27].
In this context, in the present study, bare iron oxide magnetic particles produced by co-precipitation were functionalized with γ-APTS and evaluated as lipase support to produce biodiesel in a continuous process. The utilization of magnetized biocatalysts in continuous reaction systems is a configuration still rarely reported in the literature. The prepared biocatalyst was evaluated in the transesterification process of the kernel oil obtained from palm almond (Elaeis guineensis), differing of palm oil which is extracted from the pulp (mesocarp) of the palm fruit. Kernel oil was chosen as the fatty component for the reaction due to its composition, regarded as a lauric oil, and for having been represented as a potential feedstock in previous studies using enzyme-assisted production of biodiesel [28].
2 Material and Methods
2.1 Materials
Commercial lipase from Burkholderia cepacia, Iron (III) chloride (FeCl3, > 97%), Iron (II) chloride tetrahydrate (FeCl2⋅4H2O, 99%), and γ-aminopropyltriethoxysilane (γ-APTS) were acquired from Sigma-Aldrich® (Missouri). Polyethylene glycol (PEG, MW 1,500) was acquired from Synth (São Paulo, Brazil). Glutaraldehyde, Arabic gum, heptane and anhydrous ethanol (99.7%) were supplied by Cromoline (São Paulo, Brazil). Olive oil with low acidity (Carbonell®) was purchased in the local market. Other reagents and solvents were of standard laboratory grade. Kernel oil was supplied by Agropalma-Para Brasil.
The composition of the oil is found in Table 1. The fatty acid composition was determined as Fatty acid methyl ester (FAME) by gas chromatography according to adapted American Oil Chemists’ Society (AOCS) official method Ce 1–62 [29]. The analysis was performed in Varian CP 3800 chromatograph equipped with an auto-injector (CP 8410) and a flame ionization detector (FID). A TR FAME capillary column was used with the following features: 30 m length, 0.25 mm internal diameter, and 0.25 μm film thickness. Nitrogen gas was used as the mobile phase at a flow rate of 1.0 mLmin−1 and sample injection of 1 μL. The oven temperature was kept at 50 °C for 1 min, ramped to 150 °C at 15 °C min−1, raised to 180 °C at 2 °C min−1 and then to 250 °C at 15 °C min−1 and held for 5 min. The temperature of the detector and injector were set at 250 and 200 °C, respectively. Fatty acid peaks were identified according to a standard mixture (GLC standard461, Nu-Chek Prep, Elysian, MN, USA) and retention times.
2.2 Synthesis of Magnetic Particles of Iron Oxide and Support Preparation
The magnetic particles were prepared by co-precipitation of Fe+2 and Fe+3 ions in alkaline medium. In 300 mL of ultrapure water, 30 mL of 0.6 mol L−1 FeCl3 and 30 mL of 1.1 mol L−1 FeCl2⋅4H2O, were gently added under vigorous agitation. The temperature of the medium was raised to 80 °C and the pH was adjusted to 11 with NaOH 4 mol L−1, and the system was maintained under mechanical agitation for 30 min [30]. The obtained mixture was allowed to stand over a magnetic field (magnet) for the separation of the magnetic particles. The precipitate was recovered and washed with ultrapure water and a solution of water and ethyl acetate (1:1) until attaining pH 7, followed by drying at 100 °C for 24 h [31].
To be used as immobilization support, the magnetic particles were activated by a procedure in two steps: silanization of support with γ-aminopropyltriethoxysilane (γ-APTS) and activation with glutaraldehyde as described by Soares et al. [32]. Briefly, in a 125-mL round-bottom flask 3 mL of γ-APTS (0.5% v v−1 in heptane) was added for each gram of support and the reaction was performed in a rotary evaporator at 75 °C for 3 h. The precipitate was recovered by vacuum filtration, washed with heptane for removal of the silane and posterior oven-dried at 108 °C for 18 h. The silanized particles were posteriorly immersed in a solution of 2.5% glutaraldehyde in hydrogen phosphate buffer (0.1 mol L−1, pH 7) and maintained under agitation for 1 h at room temperature. After this procedure, the precipitate was recovered by vacuum filtration, washed with distilled water for removal of glutaraldehyde excess. The silanized and activated particles were oven-dried at 60 °C for 18 h.
2.3 Lipase Immobilization
The enzyme was immobilized onto the prepared magnetized particles by covalent bonding following the methodology described by Carvalho et al. [33]. Briefly, each 1 g (dry matter basis) of support received 300 mg of crude lipase and 100 μL of an aqueous solution containing 5 mg mL−1 of polyethylene glycol (1500 molecular weight). The enzyme-support suspensions were kept under constant stirring for 2 h at 30 °C and then left to rest for 18 h at 4 °C [31]. Immobilized derivatives were recovered by vacuum filtration and washed with hexane to remove excess moisture (less than 15%). The enzymatic activity was determined by the hydrolysis of olive oil and the immobilization yield (η%) was determined taking into consideration the relationship between the overall activity of the immobilized enzyme and the activity of the initial enzyme solution.
2.4 Transesterification of Kernel Oil with Ethanol
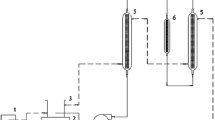
Continuous synthesis of biodiesel was carried out in a packed-bed reactor with a reflux con-denser system connected to the feeding vessel to avoid the loss of ethanol. The substrate was prepared at a 1:12 molar ratio ethanol: oil. The temperature was maintained at 50 °C using a water jacket connected to a circulating water bath. In the reaction, two flow rates were evaluated, corresponding to space times of 10 and 16 h, this resulted in a working volume of 285 cm3. For each run, lipase immobilized on magnetic support (about 13 g and 1800 U g−1) was used. The system was stabilized by substrate circulation for a period of two space-times. Substrate was then pumped continuously (Peristaltic Pump SJ-1211, Atto Bioscience and Biotechnology, Tokyo, Japan) from a reservoir through marprene tubing (Watson Marlow 913.AJ05.016) into the bottom of the bioreactor at the selected flow rate.
Periodic samples were withdrawn, purified, and used to quantify Fatty acid ethyl esters (FAEE) by gas chromatography, viscosity and density values. Figure 1 depicts the representation of the apparatus used in this reaction. Figure S1 (Supplementary Material) shows the actual reaction system used in the work.
2.5 Analytical Methods
The morphological characteristics of the magnetic support of iron oxide were assessed by scanning electron microscopy - SEM (BELSORP-mini II, MicrotracBEL Corporation). The specific surface area was obtained by the BET method by using the technique of nitrogen physical adsorption at 77.3 K. The samples were previously heated at 100 °C for 3 h under vacuum. Structural identification of the magnetized support was made on an X-ray diffractometer (XRD 6000, Shimadzu) by using CuKα radiation (λ = 1.5418 Å, 40 kV, and 30 mA). The diffraction patterns were obtained in an angular range of 10° ≤ 2θ ≤ 70º. Samples were also submitted to FTIR analysis (Spectrum GX, Perkin Elmer) in a range of 4000–400 cm−1 wavelength, samples were prepared using KBr pellets. The magnetization profile of the iron oxides used as support was obtained through the use of a Physical Properties Measurement System (VSM-PPMS EverCool II) (Quantum Design) using field-cooling regime and zero-field cooling, with the aid of a four-probe method used to characterize the electrical resistance as a function of temperature on a range from 3 to 300 K following the description by Corrêa et al. [34].
The hydrolytic activities of free and immobilized lipase derivatives were determined by the modified olive oil emulsion method according to Soares et al. [32]. One unit of enzyme activity (U) was defined as the amount of enzyme required to release one micromole of free fatty acid per minute under the assay conditions (37 °C, pH 7.0, 150 rpm). Free and immobilized lipases were also tested for their thermal stability and kinetic properties. Thermal stability was determined by incubation in phosphate buffer (100 mmol L−1, pH 8.0) at 50 °C for 180 min. Periodic samples were withdrawn through 30 min intervals to assay the residual hydrolytic activity. Thermal deactivation constant (kd) and half-life time (t1/2) were calculated according to the methodology described by Pereira et al. [35] based on the Arrhenius equation. The kinetic properties were estimated using olive oil hydrolysis assay in a range of olive oil concentration from 10 to 50% in the emulsion system (Arabic gum aqueous solution, 7 wt%). Kinect parameters Vmax (maximum reaction rate) and KM (Michaelis–Menten constant) were calculated using fitting equations available on OriginPro® (version 8.0).
The crude produced ethyl ester samples were purified by water washing to remove the free glycerol generated. Residual alcohol was then recovered by a rotary evaporator [36]. Alkyl ester contents were determined by gas chromatography using a Varian CG 3800 (Varian Inc., Palo Alto, California, USA) equipped with a flame ionization detector and a 5% DEGS CHR-WHP 80/100 mesh 6 ft 2.0 mm ID/800 OD column (Restek Frankel Analytic Instruments Ltd., São Paulo, Brazil) according to the method described by Urioste et al. [37]. Absolute viscosity was determined by a DV-II viscometer (Brookfield Viscometers Ltd, England) using spindle no. 42 at 40 ± 0.1 °C. Density was determined on a DMA 35 N EX digital densitometer (Anton Paar, Graz, Austria) at 20 °C [38]. Experiments and analysis were made in triplicate.
3 Results and Discussion
3.1 Characterization: Support and Immobilized Enzyme
According to the BET analysis the specific surface area of functionalized-iron oxide support corresponded to 49.0 ± 2.5 m2 g−1, and the pore volume, 0.07 cm3 g−1. The produced particles presented slightly smaller specific surface area than those obtained by Ali et al. [39] (75.3 m2 g−1, 15.4 nm). The scanning electron microscopy (Fig. 2) of the magnetic support shows an amorphous morphology with a tendency to cubical-shaped forms. Moreover, the particles have small precipitates encrusted in the surface, aspect similar to those found by Icart et al. [40].
The production of iron oxide particles from aqueous Fe2+/Fe3+ salts solution by alkaline co-precipitation is a simple and easily reproducible method that results in a large amount of nano-sized magnetic particles. The solid is formed in two steps with short burst nucleation as a result of critical supersaturation of species followed by the slow growth of the nuclei by diffusion of the solutes to the crystal surface. However, the size and shape of particles will be responses to several factors as iron salt used, precipitating agent, Fe2+/Fe3+ ratio, pH, temperature, ionic strength of the medium, stirring rate, among other factors [39, 41, 42]. According to Ali et al. [39], the precipitating agents have an important effect on the size and shape of magnetite because their strength plays a vital role in the control of the growth rate of the facets of the crystal in different directions. The authors observed that the use of NH4OH as a precipitating agent result predominantly in spherical-shaped magnetite particles while with NaOH the particles were cubic. In the first case, the spherical-shape occurred due to its least surface free-energy. When NaOH is used, cubic-shaped is dominant due to faster crystal growth. Another fact that must be taken into account is the high surface energy of the Fe3O4 (γ-Fe2O3) particles, a tendency to form aggregates, and this phenomenon occurs most pronounced with uncoated particles [39]. This, in some cases, could result in a loss of original support shape.
XRD pattern of the iron oxide support functionalized with γ-APTS is displayed in Fig. 3. Despite the noise of the spectral line, it is possible to identify peaks at 30.0°, 35.5°, 43.1°, 53.4°, 57.0°, 63.0°, and 74.5°. These peaks are consistent with the inverse cubic spinel structure of pure magnetite (Fe3O4) [43] and also pure maghemite (γ-Fe2O3) [44, 45]. Therefore, these results indicate that the magnetic particles produced in the present study are composed of both Fe3O4 and γ-Fe2O3. The results also show that the functionalization of iron particles with γ-APTES did not affect its crystalline structure allowing the magnetic behavior to be preserved, similar to reports in the literature [23, 24, 43]. The magnetization profile of the supporting matrix depicted in Fig. 4 demonstrated low reminiscent coercivity and magnetization values, which is an indication of the superparamagnetic behavior, presenting characteristics similar to the indications reported by Henriques et al. [46], Teja et al. [47] and Bento et al. [30].
Figure 5 shows the FTIR spectra for the functionalized-magnetic support. The band at 3400 cm−1 is assigned to hydroxyl-groups from water attached to the iron (1). Another characteristic band, at 1630 cm−1, can be attributed to the angular deformation of δH–O–H (2). The peak at 1000–1100 cm−1 is due to the stretching vibrations of Si–O from γ-APTS, and the bands between 700–500 cm−1 correspond to the Fe–O stretching vibrations of Fe3O4 and γ-Fe2O3 (4) [23, 24, 43, 48].
The results regarding the immobilization procedure of lipase onto iron-oxide support indicate a successful approach, with about 53% of activity recovered in the magnetized support. The immobilized enzyme–substrate affinity showed an increase in the KM value in about threefold (from 410 to 1262 mmol L−1) and a decrease in the Vmax value in sevenfold (From 12,390 to 1786 U g−1) when compared with the free enzyme (Table 2), probably due to conformational changes as a result of the immobilization process or steric hindrance caused by the support, which may cause some difficulties in the mass transfer in the catalytic system hindering the diffusion of the substrate to the enzyme active site, reducing the substrate affinity. The same kinetic behavior was observed when Candida rugosa lipase (CRL) was immobilized onto magnetic framework composite [49]. The Vmax value of free CRL was 0.357 μmol/min and the immobilized form was 0.307 μmol/min while the KM value of immobilized lipase was higher than the free counterpart.
In order to improve the characteristics of the magnetic particles for lipase immobilization, their surfaces were modified with γ-aminopropyltriethoxysilane (silanization treatment), an organosilane which is a commonly used coupling agent because of its amino group [50]. This bifunctional molecule can bind covalently to the free –OH groups at the surface of support producing the amino-terminated iron oxide nanoparticles (modified surface –NH2). After the treatment with a second bifunctional agent, glutaraldehyde, can occur the formation of a Schiff base after reaction with aminoalkyl groups, which can covalently immobilize the lipase molecules [32]. It is challenging to give the exact mechanism of the enzyme immobilization process using glutaraldehyde-activated supports since it is possible to find three distinct kinds of interactions under certain experimental conditions: anionic exchange, hydrophobic and covalent bonding [51]. The activation degree of the support has an important effect on immobilization mechanisms. Herein, were used very lowly activated amino supports (solution of 2.5% glutaraldehyde), aqueous-buffered medium with moderate ionic strength, so it is possible to allow the covalent reaction by the most reactive amino group on the lipase with the support [13, 51]. However, other possibilities of lipase-support interactions, like as via interfacial activation, cannot be discarded due to the moderate hydrophobicity of the glutaraldehyde and the absence of detergents.
Improvements in enzyme thermostability are very attractive to make the enzymatic process economically viable. Table 2 also shows the values of the thermal deactivation constant and half-time at 50 °C for the lipase immobilized onto iron-oxide support. It can be seen that immobilization favored the thermal stability and increased the half-time of the enzyme by about tenfold when compared with the free enzyme which can improve the enzyme operational stability in a wider range of temperature. Improvements of thermal stability parameters also were observed when lipase from Candida rugosa was immobilized onto magnetized poly(STY-co-DVB) reaching a thermal stabilization factor of 50 [30]. Additional data can be found as supplementary figures S2 regarding the thermal activity of free and immobilized lipase.
3.2 Evaluation of Industrial Application
3.2.1 Esterification Reaction: Butyl Butyrate Synthesis
In order to evaluate the biocatalyst efficiency, the first tested industrial application of the prepared magnetic biocatalyst was the aroma production by the batch esterification of n-butanol and butyric acid following the parameters described by Bento et al. [30]. The results showed that butyl butyrate was successfully synthesized by the produced immobilized derivative as indicated in the Fig. 6, which shows the enzymatic conversion of butanol and butyric acid to butyl butyrate over time.
Esterification activity was calculated as being 56.7 μmol L−1 g−1 min−1, which corresponds to a productivity value of 0.58 g ester L−1 h−1 under the applied conditions. A similar result was obtained when Candida Rugosa lipase was immobilized on magnetized poly(STY-co-DVB) which showed a productivity value of 0.80 g ester L−1 h−1 [30]. In another study, Candida Rugosa lipase was immobilized on chemically modified cellulignin and evaluated in the same esterification reaction showing a productivity 1.3 g L−1 butyl butyrate h−1 [52], indicating the herein analyzed catalyst presents potential to be applied in industrial aroma synthesis.
3.2.2 Synthesis of Ethyl Esters from Kernel Oil Under Continuous Process
Coconut oil has a similar profile to kernel oil, both having lauric acid as a predominant component in their compositions. Thus, an ethanolysis of coconut oil was carried out in a batch system to test the biocatalyst proposed in this work, before applying it in a continuous reaction with Kernel oil. The conversion profile of the reaction is demonstrated in the supplementary material, figure S3, which provided ethyl esters at a content of 99.53% and viscosity of 3.78 mm2 s−1 at 40 °C, which fits the Brazilian and international standards for biodiesel. The results are comparable with the data demonstrated by Mijone et al. [31], who also addressed the use of B. cepacia lipase immobilized on a magnetic hybrid matrix using coconut oil and ethanol.
These results indicated the prepared magnetic biocatalyst can act in the catalysis of lauric acid predominant oils, achieving high yields, which make it appropriate for continuous reactions tests using similar raw materials.
The continuous system applying the prepared biocatalyst in a packed bed reactor operated with higher conversions when a higher space–time was considered (16 h). The effect of different space-times (10 h and 16 h) is described in the supplementary material as Table S1 and Figure S4. Figure 7 shows the profiles of the esters formation during the transesterification reaction, being evidenced by the esters ethyl laurate (C12) and ethyl oleate (C18:1) which are the fatty acids of major proportion in the raw material. The kernel oil has lauric acid as a prevalent component in its compositions. This feature can enable the transesterification reaction, due to lauric esters consist of short chains which interact efficiently with ethanol and lipase [33]. The stability of the system was attained after four space times and the ester content was maintained at high levels (around 96%) for more than 15 days. Under these conditions, the half-time (t1/2) of biocatalyst was estimated in 47 days, with a corresponding kd of 6.1 × 10–4 h−1. The supplementary figure S5 demonstrates the robustness and reproducibility of the PBR used in this work through the triplicate run.
Profile of ethyl esters formation in the continuous transesterification of kernel oil and ethanol using Burkholderia cepacian lipase immobilized in iron oxides (C8:0 = White circle; C10:0 = Black circle; C12:0 = Black up pointing triangle; C14:0 = Black down pointing triangle; C16:0 = White square; C18:0 = Black left pointing small triangle; C18:1 = Black right pointing small triangle; C18:2 = Black square; Total = Black circle)
Great attention has been given to the iron-oxides particles to be used as enzyme-carrier. Besides the already mentioned advantages and attractive characteristics, many studies have been carried out applying iron-oxides particles coated or combined with organic matrices, for example, Fe3O4-chitosan microspheres [53], Fe3O4/γ-Fe2O3 coated with poly(STY-co-DVB) [30], Fe3O4 core coated with silica shell [54] and Fe3O4 coated with polydopamine film [55]. However, their use in continuous biodiesel synthesis is still remotely reported.
In this context, the results of the transesterification reaction obtained in the present study were favorably compared with the data of Ramos et al. [56], which employed the same reactor configuration and similar reactional conditions but with lipase immobilized on a hybrid support SiO2-PVA (Table 3). It can be observed that the functionalized Fe3O4/γ-Fe2O3 particles provided a more stable biocatalyst with half-time almost threefold higher than the silica-based biocatalyst (47 and 17 days, respectively). Further, the values of FAEE content and yield achieved (~ 62 wt% and 96%, respectively) were also higher than the values obtained with the hybrid biocatalyst (~ 54 wt% and 87%, respectively). The iron-oxide particles are smaller than the SiO2-PVA matrix and have low porosity but due to the functionalization, the enzyme attachment onto the surface is probably stronger due to its covalent bonding nature. SiO2-PVA-based biocatalysts are highly porous; however, the structure allows the enzyme to bind by both covalent bonding and physical adsorption, which would result in a higher immobilization yield when compared to the magnetic support. However, limitations such as loss of activity related to the leaching of adsorption-bound enzymes and the high affinity for glycerol can compromise the continuous process. As it is formed, glycerol is adsorbed and accumulates on the support, decreasing the enzyme activity by substrate diffusion limitation [36, 56, 57].
One way to verify the framing of alkyl esters produced within the required specifications for biodiesel fuel is to monitor the viscosity of the transesterified oil [58], since lowering the high viscosity of the oil is an essential characteristic of the process to allow its use as fuel in diesel engines. In the present study, the average viscosity of the product at the end of the reaction was 5.32 ± 0.42 mm2 s−1. This value fits the specifications of the Brazilian standard based on ANP Resolution No. 45/2014 and by the American National Standard ASTM (D6751).
4 Conclusions
A facile prepared magnetic nanoparticle was successfully synthesized in this study, giving rise to nanosupports for immobilization of enzymes. B. cepacia lipase immobilized on functionalized Fe3O4/γ-Fe2O3 particles showed good performance as a catalyst in the ethanolysis of kernel oil in a continuous process. The biocatalyst half-life of 47 days allowed a stable operational system achieving high conversion values over a period of 17 days. The continuous ethanolysis of kernel oil achieved ethyl esters productivity of 38.7 mg g−1 h−1 and the final product showed viscosity of 5.32 mm2 s−1 at 40 °C, which meets the specifications established by ANP Resolution 45/2014 and by ASTM (D6751) for biodiesel. Thus, it was concluded that this magnetic support is promising to be applied for immobilization of other enzymes, producing biocatalysts with elevated features. In addition, the possible application of this system for different industrial sectors in a continuous process may have process advantages such as higher productivity and enzyme reuse.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Yusoff MNAM, Zulkifli NWM, Sukiman NL et al (2021) Sustainability of palm biodiesel in transportation: a review on biofuel standard, policy and international collaboration between Malaysia and Colombia. Bioenergy Res 14:43–60. https://doi.org/10.1007/s12155-020-10165-0
Alagumalai A, Mahian O, Hollmann F, Zhang W (2021) Environmentally benign solid catalysts for sustainable biodiesel production: a critical review. Sci Total Environ 768:144856. https://doi.org/10.1016/J.SCITOTENV.2020.144856
Tacin MV, Costa-Silva TA, de Paula AV et al (2020) Microbial lipase: a new approach for a heterogeneous biocatalyst. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2020.1855442
Mehta A, Guleria S, Sharma R, Gupta R (2021) The lipases and their applications with emphasis on food industry. Elsevier Inc.
Dhawane SH, Chowdhury S, Halder G (2019) Lipase immobilised carbonaceous catalyst assisted enzymatic transesterification of Mesua ferrea oil. Energy Convers Manage 184:671–680. https://doi.org/10.1016/J.ENCONMAN.2019.01.038
Chandra P, Enespa SR, Arora PK (2020) Microbial lipases and their industrial applications: a comprehensive review. BioMed Central. https://doi.org/10.1186/s12934-020-01428-8
Costa-Silva TA, Carvalho AKF, Souza CRF et al (2017) Enzymatic transesterification of coconut oil using chitosan-immobilized lipase produced by fluidized-bed system. Energy Fuels 31:12209–12216. https://doi.org/10.1021/acs.energyfuels.7b02033
Carvalho AKF, Bento HBS, Lima R, De Castro HF (2020) Use and reusability of the Na/Nb2O5 catalyst in the ethanolysis of different feedstocks for biofuel production: confirmation of heterogeneity of the catalyst. Energy Fuels 34:7105–7111. https://doi.org/10.1021/acs.energyfuels.0c00246
Lv L, Dai L, Du W, Liu D (2021) Progress in enzymatic biodiesel production and commercialization. Processes 9:1–10. https://doi.org/10.3390/pr9020355
Rafiee F, Rezaee M (2021) Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int J Biol Macromol 179:170–195. https://doi.org/10.1016/j.ijbiomac.2021.02.198
Jegannathan KR, Eng-Seng C, Ravindra P (2011) Economic assessment of biodiesel production: comparison of alkali and biocatalyst processes. Renew Sustain Energy Rev 15:745–751
Maceiras R, Vega M, Costa C et al (2011) Enzyme deactivation during biodiesel production. Chem Eng J 166:358–361. https://doi.org/10.1016/j.cej.2010.11.007
Barbosa O, Torres R, Ortiz C et al (2013) Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromol 14:2433–2462. https://doi.org/10.1021/bm400762h
Zahirinejad S, Hemmati R, Homaei A et al (2021) Nano-organic supports for enzyme immobilization: scopes and Perspectives. Colloids Surfaces B Biointerfaces 204:111774. https://doi.org/10.1016/j.colsurfb.2021.111774
Costa Silva TA, Souza CRF, Said S, Oliveira WP (2015) Drying of enzyme immobilized on eco-friendly supports. Afr J Biotechnol 14:3019–3026. https://doi.org/10.5897/AJB2015.14830
Carneiro LABC, Costa-Silva TA, Souza CRF et al (2014) Immobilization of lipases produced by the endophytic fungus Cercospora kikuchii on chitosan microparticles. Braz Arch Biol Technol 57:578–586. https://doi.org/10.1590/S1516-8913201402174
Aggarwal S, Chakravarty A, Ikram S (2021) A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization and thereof strategies. Int J Biol Macromol 167:962–986. https://doi.org/10.1016/j.ijbiomac.2020.11.052
Costa-Silva TA, Carvalho AKF, Souza CRF et al (2021) Enhancement lipase activity via immobilization onto chitosan beads used as seed particles during fluidized bed drying: application in butyl butyrate production. Appl Catal A Gen 622:118217. https://doi.org/10.1016/j.apcata.2021.118217
Dhawane SH, Kumar T, Halder G (2019) Insight into biodiesel synthesis using biocatalyst designed through lipase immobilization onto waste derived microporous carbonaceous support. Process Safety Environ Protect 124:231–239. https://doi.org/10.1016/j.psep.2019.02.021
Costa-Silva TA, Marques PS, Souza CRF et al (2015) Enzyme encapsulation in magnetic chitosan-Fe3O4 microparticles. J Microencapsul 32:16–21. https://doi.org/10.3109/02652048.2014.940013
Wang X, Dou P, Zhao P et al (2009) Immobilization of lipases onto magnetic Fe3O4 nanoparticles for application in biodiesel production. Chemsuschem 2:947–950. https://doi.org/10.1002/cssc.200900174
Bilal M, Zhao Y, Rasheed T, Iqbal HMN (2018) Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review. Int J Biol Macromol 120:2530–2544
Yamaura M, Camilo RL, Sampaio LC et al (2004) Preparation and characterization of (3-aminopropyl) triethoxysilane-coated magnetite nanoparticles. J Magn Magn Mater 279:210–217. https://doi.org/10.1016/j.jmmm.2004.01.094
Hajar M, Vahabzadeh F (2016) Biolubricant production from castor oil in a magnetically stabilized fluidized bed reactor using lipase immobilized on Fe3O4 nanoparticles. Ind Crops Prod 94:544–556. https://doi.org/10.1016/j.indcrop.2016.09.030
Halim SFA, Kamaruddin AH, Fernando WJN (2009) Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: optimization using Response surface methodology (RSM) and mass transfer studies. Bioresour Technol 100:710–716. https://doi.org/10.1016/j.biortech.2008.07.031
Zhang C, Xing XH (2011) Enzyme bioreactors, 2nd Edn, Elsevier B.V.
Cortez DV, Reis C, Perez VH, De Castro HF (2018) The realm of lipases in biodiesel production. In: sustainable biotechnology- enzymatic resources of renewable energy. Springer International Publishing, pp. 247–288
Cardoso A, Laviola BG, Santos GS et al (2017) Opportunities and challenges for sustainable production of A. aculeata through agroforestry systems. Ind Crops Prod 107:573–580. https://doi.org/10.1016/j.indcrop.2017.04.023
AOCS (2004) American oil chemists’ society official methods and recommended practices of the AOCS, 5th edn. AOCS Press, Champaign
Bento HBS, de Castro HF, de Oliveira PC, Freitas L (2017) Magnetized poly (STY-co-DVB) as a matrix for immobilizing microbial lipase to be used in biotransformation. J Magn Magn Mater 426:95–101. https://doi.org/10.1016/j.jmmm.2016.11.061
Mijone PD, Bôas RNV, Bento HBS et al (2020) Coating and incorporation of iron oxides into a magnetic-polymer composite to be used as lipase support for ester syntheses. Renew Energy 149:1167–1173. https://doi.org/10.1016/j.renene.2019.10.100
Soares CMF, De Castro HF, De Moraes FF, Zanin GM (1999) Characterization and utilization of candida rugosa lipase immobilized on controlled pore silica. Appl Biochem Biotechnol 79:745–758. https://doi.org/10.1385/abab:79:1-3:745
Carvalho AKF, Da Rós PCM, Teixeira LF et al (2013) Assessing the potential of non-edible oils and residual fat to be used as a feedstock source in the enzymatic ethanolysis reaction. Ind Crops Prod 50:485–493. https://doi.org/10.1016/j.indcrop.2013.07.040
Correa LE, Nova JCC, de Faria LR et al (2019) Evidence of multiband behavior in a new superconductor Ta0.8Zr0.2B with FeB-prototype structure. J Alloys Compd 803:597–600. https://doi.org/10.1016/j.jallcom.2019.06.186
Pereira EB, De Castro HF, De Moraes FF, Zanin GM (2001) Kinetic studies of lipase from Candida rugosa: a comparative study between free and immobilized enzyme onto porous chitosan beads. Applied biochemistry and biotechnology - part A enzyme engineering and biotechnology. Springer, New York, pp 739–752
Costa e Silva W, Freitas L, Oliveira PC, de Castro HF (2016) Continuous enzymatic biodiesel production from coconut oil in two-stage packed-bed reactor incorporating an extracting column to remove glycerol formed as by-product. Bioprocess Biosyst Eng 39:1611–1617. https://doi.org/10.1007/s00449-016-1636-3
Urioste D, Castro MBA, Biaggio FC, De Castro HF (2008) Síntese de padrões cromatográficos e estabelecimento de método para dosagem da composição de ésteres de ácidos graxos presentes no biodiesel a partir do óleo de babaçu. Quim Nova 31:407–412. https://doi.org/10.1590/S0100-40422008000200038
Martin LS, Ceron A, Oliveira PC et al (2018) Different organic components on silica hybrid matrices modulate the lipase inhibition by the glycerol formed in continuous transesterification reactions. J Ind Eng Chem 62:462–470. https://doi.org/10.1016/j.jiec.2018.01.029
Ali S, Khan SA, Yamani ZH et al (2019) Shape- and size-controlled superparamagnetic iron oxide nanoparticles using various reducing agents and their relaxometric properties by Xigo acorn area. Appl Nanosci 9:479–489. https://doi.org/10.1007/s13204-018-0907-5
Icart LP, Dos Santos ERF, Pereira ED et al (2016) PLA-b-PEG/magnetite hyperthermic agent prepared by Ugi four component condensation. Express Polym Lett 10:188–203. https://doi.org/10.3144/expresspolymlett.2016.18
Laurent S, Forge D, Port M et al (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem Rev 108:2064–2110. https://doi.org/10.1021/cr068445e
Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chemie Int Ed 46:1222–1244
Miao C, Yang L, Wang Z et al (2018) Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 224:774–782. https://doi.org/10.1016/j.fuel.2018.02.149
Ramos Guivar JA, Sadrollahi E, Menzel D et al (2017) Magnetic, structural and surface properties of functionalized maghemite nanoparticles for copper and lead adsorption. RSC Adv 7:28763–28779. https://doi.org/10.1039/c7ra02750h
Nazari M, Ghasemi N, Maddah H, Motlagh MM (2014) Synthesis and characterization of maghemite nanopowders by chemical precipitation method. J Nanostruct Chem 4:1–5. https://doi.org/10.1007/s40097-014-0099-9
Henriques RO, Bork JA, Fernandez-Lorente G et al (2018) Co-immobilization of lipases and Β-D-galactosidase onto magnetic nanoparticle supports: biochemical characterization. Mol Catal 453:12–21. https://doi.org/10.1016/j.mcat.2018.04.022
Teja AS, Koh PY (2009) Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Charact Mater 55:22–45
Galindo-Gonzalez C, Gantz S, Ourry L et al (2014) Elaboration and rheological investigation of magnetic sensitive nanocomposite biopolymer networks. Macromolecules 47:3136–3144. https://doi.org/10.1021/ma402655g
Ji Y, Wu Z, Zhang P et al (2021) Enzyme-functionalized magnetic framework composite fabricated by one-pot encapsulation of lipase and Fe3O4 nanoparticle into metal–organic framework. Biochem Eng J. https://doi.org/10.1016/j.bej.2021.107962
Cui Y, Li Y, Yang Y et al (2010) Facile synthesis of amino-silane modified superparamagnetic Fe3O4 nanoparticles and application for lipase immobilization. J Biotechnol 150:171–174. https://doi.org/10.1016/j.jbiotec.2010.07.013
Okura NS, Sabi GJ, Crivellenti MC et al (2020) Improved immobilization of lipase from Thermomyces lanuginosus on a new chitosan-based heterofunctional support: mixed ion exchange plus hydrophobic interactions. Int J Biol Macromol 163:550–561. https://doi.org/10.1016/j.ijbiomac.2020.07.021
Perez VH, Silva GS, Gomes FM, De Castro HF (2007) Influence of the functional activating agent on the biochemical and kinetic properties of Candida rugosa lipase immobilized on chemically modified cellulignin. Biochem Eng J 34:13–19. https://doi.org/10.1016/j.bej.2006.11.012
Xie W, Wang J (2012) Immobilized lipase on magnetic chitosan microspheres for transesterification of soybean oil. Biomass Bioenerg 36:373–380. https://doi.org/10.1016/j.biombioe.2011.11.006
Tran DT, Chen CL, Chang JS (2012) Immobilization of Burkholderia sp. lipase on a ferric silica nanocomposite for biodiesel production. J Biotechnol 158:112–119. https://doi.org/10.1016/j.jbiotec.2012.01.018
Andrade MFC, Parussulo ALA, Netto CGCM et al (2016) Lipase immobilized on polydopamine-coated magnetite nanoparticles for biodiesel production from soybean oil. Biofuel Res J 3:403–409. https://doi.org/10.18331/BRJ2016.3.2.5
Ramos L, Martin LS, Santos JC, De Castro HF (2017) Combined use of a two-stage packed bed reactor with a glycerol extraction column for enzymatic biodiesel synthesis from macaw palm oil. Ind Eng Chem Res 56:1–7. https://doi.org/10.1021/acs.iecr.6b03811
Dors G, Freitas L, Mendes AA et al (2012) Transesterification of palm oil catalyzed by pseudomonas fluorescens lipase in a packed-bed reactor. Energy Fuels 26:5977–5982. https://doi.org/10.1021/ef300931y
Knothe G, Steidley KR (2005) Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 84:1059–1065. https://doi.org/10.1016/j.fuel.2005.01.016
Acknowledgements
The authors are grateful for the valuable guidance and mentorship of Dr. De Castro (in memoriam). The authors are also thankful for the support provided by Dr. A. J. S. Machado and Mr. L. E. Corrêa for the PPMS analyses.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP) (Process number 2020/15513-7) and CAPES (Finance code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent for Publication
All authors declare consent for publication of this article, being represented by the author for correspondence.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bento, H.B.S., Reis, C.E.R., Pinto, P.A. et al. Continuous Synthesis of Biodiesel from Outstanding Kernel Oil in a Packed Bed Reactor Using Burkholderia cepacia Lipase Immobilized on Magnetic Nanosupport. Catal Lett 152, 2434–2444 (2022). https://doi.org/10.1007/s10562-021-03826-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03826-y