Abstract
Development of non-noble metal catalysts for oxidation of CO is an important subject for reducing the automotive emissions. Recently, shape-controlled synthesis of CeO2 has increasingly attracted the attention of researchers due to its size- and morphology-dependent unique properties. Following this line of thinking, herein, we successfully report the synthesis of Cr-doped CeO2 (Ce1−xCrxO2−δ; X = 0.05, 0.1, and 0.15) nanorods with various Cr contents by a facile hydrothermal method. Structural, surface, optical, and redox properties of the Cr-doped CeO2 nanorods were investigated by various techniques, namely, ICP-OES, TEM-HRTEM, FE-SEM/EDX/EDS, XRD, BET, Raman, UV–vis DRS, PL, XPS, H2-TPR, and O2-TPD. The catalytic performance was evaluated for CO oxidation. For comparison, the efficiency of Cr2O3 was also studied for CO oxidation under identical conditions. As revealed by various characterization results, the chromium ions were doped into the ceria lattice (formation of Ce–O–Cr solid solution), which enhanced the intrinsic properties such as oxygen vacancy concentration and surface area. It was found that the Cr-doped CeO2 nanorods show superior CO oxidation activity than the pristine counterparts (CeO2 nanorods and Cr2O3). The highest CO oxidation efficiency was achieved with the light-off temperature of T50 = 261 °C, when the Cr doping amount was 10% (Ce0.9Cr0.1O2−δ). A high specific surface area, more number of surface oxygen vacancies, a high concentration of Ce3+, and enhanced oxygen reducibility of Ce0.9Cr0.1O2−δ nanorods were found to be responsible for its superior catalytic performance. Further, the Ce0.9Cr0.1O2−δ nanorods exhibited a steady CO conversion over a period of 55 h investigated. The obtained results are expected to have a significant impact on the use of non-noble metal based Cr-doped CeO2 nanorods in environmental applications.
Graphic Abstract
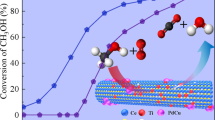
The Cr-doped CeO2 nanorods with Ce0.9Cr0.1O2−δ composition showed enhanced CO oxidation performance at a lower temperature (~ 261 °C) than that of pristine CeO2 nanorods (338 °C) and Cr2O3 (361 °C) catalyst. This behaviour is a result of enhancement of oxygen vacancies, surface Ce3+ species, low-temperature reducibility, and high surface area.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The emission of carbon monoxide (CO) due to incomplete combustion of fossil fuels is highly harmful to human beings and the environment [1, 2]. CO emissions can be controlled via catalytic oxidation approach and this process has immense importance in industrial catalysis and environmental related applications [3]. Various metal oxide supported noble-metal catalysts have been widely investigated for low-temperature CO oxidation [4]. For instance, ceria (CeO2) based Pd/Rh was already proven to be the successful commercial catalyst in the vehicle exhaust pollution control area [5]. Key reason is that the CeO2 has wonderful property to act as an oxygen buffer in the redox catalysis. At elevated temperatures and reducing environment it forms a range of oxygen-deficient non-stoichiometric CeO2-x oxides (0 < x ≤ 0.5), whereas, at a lower temperature it forms a series of discrete compositions [6]. During this process, CeO2 loses considerable amounts of oxygen from its lattice and leads to the formation of many oxygen vacancies. The oxygen vacancies generated in the ceria have been reported to enhance the oxygen mobility, reduction/oxidation capability, and they can, therefore be considered as critical for the generation of catalytic active centres [7]. The oxygen-deficient non-stoichiometric oxides are re-oxidized to CeO2 on exposure to an oxidizing environment. Thus, CeO2 can store and release oxygen in fluctuating oxidizing and reducing atmospheres and this property is known as oxygen storage capacity (OSC). However, there are two drawbacks such as poor thermal stability of CeO2 and higher cost of the noble metals, which are restraining their practical usage in the exhaust emission control applications [8].
Generally, the disadvantages of CeO2 could be overcome by doping with other metals [9]. Doping of transition metals can easily form solid solutions with CeO2 without altering its fluorite structure. The formation of solid solutions may lead to low temperature activation and an increase of OSC. The intrinsic redox nature of transition metal-ion dopant influences the Ce–O bond [10]. From a chemical perspective, weakening of the Ce–O–Ce bond increases the reducibility of ceria, thus, facilitating oxidation catalysis. The modification of CeO2 structure by transition metal ions have been extensively investigated in the oxidation catalysis. Among various transition metals, primarily, Zr was recognized as an efficient dopant for CeO2-based vehicle exhaust catalysts and other important applications [11,12,13,14]. Mn-doped CeO2 has also drawn considerable attention in the literature for catalytic oxidation reactions. In our previous works, we observed an excellent CO and soot oxidation performance at low-temperatures (T50 = 117 °C and 392 °C for CO and soot respectively) over Mn-doped CeO2 catalyst as compared to pure ceria [15, 16]. Likewise, chromium oxide (CrOx) has also been frequently applied as oxidation catalyst because of its Cr6+/Cr3+ redox couple [17]. Doping of Cr ions into the ceria lattice is expected to distort the ceria lattice which leads to the modification of structural properties due to their mixed valence nature of Ce4+/Ce3+ and Cr+6/Cr3+ ions. Singh and co-workers reported that Cr-doped CeO2 catalysts show a higher OSC than that of CeO2–ZrO2, which suggests that Cr–CeO2 might be the promising catalyst for soot oxidation [18]. Harrison and Daniell studied the promotional role of CeO2 by Cr doping towards the oxidation of CO and propane [19]. Further, some recent studies revealed the beneficial role of Cr-doping in enhancing oxygen release of CeO2 [20]. However, to the best of our knowledge, the investigation of Cr-doped ceria catalysts for low-temperature CO oxidation has been scarcely reported.
On the other hand, several investigations have been carried out for enhancing the catalytic efficiency of CeO2 by altering its morphology, surface structure–properties, and composition [21]. Particularly, the morphology effect on the catalytic CO oxidation has been well documented in ceria-based nanomaterials [22]. For example, CeO2 in different shapes such as nanorods, nanocubes, and nanooctahedra were synthesized and studied the morphology effect on CO oxidation [23, 24]. Wu and co-workers have examined the activity of CeO2 with different morphologies for CO oxidation, which followed the activity sequence of: nanorods > nanocubes > nano-octahedra [24]. Chen et al. reported that the Zr-doped CeO2 nanorods show a superior redox property and much higher activity towards CO oxidation than the CeO2 nanoparticles [25]. It was explained that the superior catalytic performance of nanorod-structured CeO2 were due to the presence of preferentially exposed highly active (110) and (100) crystal planes, improved reducibility, and high OSC [26,27,28].
The present investigation was undertaken against the aforesaid background. Accordingly, in the present study a series of Cr-doped CeO2 nanorods with different Cr loadings (5, 10, and 15%) were synthesized by a facile hydrothermal method. The as-prepared materials were evaluated for CO oxidation and characterized with the help of several techniques including, ICP-OES, TEM-HRTEM, FE-SEM/EDX/EDS, XRD, BET, Raman, UV–vis DRS, PL, XPS, H2-TPR, and O2-TPD to elucidate the information about structure–activity relationship.
2 Experimental Section
2.1 Materials and Catalyst Preparation
Cerium (III) nitrate hexahydrate (Ce(NO3)3·6H2O, 99.9%, AR), chromium (III) nitrate nonahydrate (Cr(NO3)3·9H2O, 99%, AR), and sodium hydroxide (NaOH, 99%, AR) were purchased from Sigma-Aldrich and used without further purification. Milli-Q water (18.2 Mega ohm (M Ω) cm) was used throughout the experiments.
Cr-doped CeO2 nanorods (Ce1−xCrxO2−δ; X = 0.05, 0.1, and 0.15; X is the molar ratio of Cr/(Cr + Ce)) with different Cr contents were synthesized by a hydrothermal process [29]. Firstly, a NaOH solution with a concentration of 8 M was prepared. In a typical experiment, totally 4 mmol of Ce(NO3)3·6H2O and Cr(NO3)3·9H2O with different mole ratios were dissolved in 30 mL Milli-Q water under vigorous stirring until the corresponding salts were completely dissolved. There after an aqueous NaOH (60 mL) was added to the above solution at one time and kept stirring for 30 min. Subsequently, the formed mixed slurry was transferred to a 100 mL Teflon lined autoclave reactor and kept in the oven for 24 h at 100 °C. After cooling down to room temperature naturally, the precipitates were collected and washed with Milli-Q water/ethanol several times until free from ion impurities (Na+ and NO3− ions). Then, the obtained product was dried at 100 °C for 12 h in an electric oven and ground in a pestle and mortar to give the fine powder. Finally, the powder was calcined under static air at 350 °C (at a ramping rate of 5 °C/min) for 2.3 h in a muffle furnace to obtain Ce1−xCrxO2−δ nanorods. For convenience, the 5, 10, and 15% Cr-doped CeO2 nanorod catalysts were designated as 5CrCe, 10CrCe, and 15CrCe, respectively. Pure ceria nanorods were also synthesized under similar conditions and designated as CeO2.
2.2 Catalyst Characterization
The actual metal contents of Ce and Cr in various catalysts were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES) with Thermo Jarrel Ash model IRIS Intrepid II XDL instrument. Prior to analysis, the solids were dissolved in a solution of 25 mL aqua regia and 475 mL distilled water. Afterwards, 10 mL of the above solution was taken and diluted to 250 mL to perform the elemental analysis measurements.
The TEM-HRTEM studies were made on a TECNAIG2 TEM microscope equipped with a slow-scan CCD camera and at an accelerating voltage of 200 kV. Before TEM analysis, the samples were well dispersed in isopropanol by sonication and dispersed on a carbon grid. Field emission-scanning electron microscopy (FE-SEM) images and energy dispersive X-ray spectroscopy (EDS) were taken on a Carl Zeiss model Ultra 55 electron microscope. For imaging, powder samples were well mixed with ethanol and then deposited onto super-thin carbon films. The XRD patterns were acquired with a MiniFlex 600 X-ray diffractometer using Cu-Kα radiation (λ = 1.5406 Ǻ), operating at 40 kV and 15 mA. A continuous mode was used to collect the data in the 2θ range of 10°–80° at a scanning rate of 4° min−1 with a step size of 0.02°. Phase confirmation was carried out by comparison with JCPDF database. The XRD patterns were subjected to Rietveld analysis using the Fullprof.2k program. The BET specific surface areas and pore size distribution of the samples were determined by N2-sorption isotherms at 77 K using a Micromeritics (ASAP 2000) analyzer. The specific surface area and pore size distribution were calculated by BET and BJH methods, respectively. Before the measurements, the samples were degassed at 300 °C for 4 h under vacuum. Raman spectra were measured in the 200–800 cm−1 range with a 1 cm−1 spectral resolution using a Raman spectrometer (Renishaw inVia Raman Microscope). The excitation source was an Ar+ ion laser (λ = 514 nm) and the laser power was 20 mW. UV–vis DRS were recorded using a JASCO V650 UV–Vis spectrophotometer in the range between 200–800 nm with a step size of 1 nm and by using barium sulfate (BaSO4) as the reference. The Kubelka–Munk function was used to calculate the absorbance of the samples. Photoluminescence (PL) emission spectra were recorded using a Horiba Scientific Fluoromax‒4 luminescence spectrometer at an excitation wavelength of 250 nm at room temperature. XPS measurements were performed on Thermo Scientific, equipped with a monochromatic Al–Kα source (Kα = 1,486.6 eV) at room temperature under ultra-high vacuum (10‒8 Pa). The peak positions were corrected by using the containment carbon 1s peak (284.6 eV).
Hydrogen-temperature programmed reduction (H2-TPR) was carried out using a Micromeritics AutoChem 2920 apparatus with a thermal conductivity detector (TCD). In each procedure, 50 mg of the sample was first pretreated with He (10 mL/min) at 300 °C for 1 h, and then cooled down to room temperature. Then, the temperature was raised to 900 °C at a constant rate of 10 °C/min under a gas flow of 10 vol % H2/Ar stream (10 mL/min). Oxygen-temperature programmed desorption (O2-TPD) was performed by using the same apparatus. The sample (50 mg) was placed in an U‒shape quartz reactor tube and was heated up to 500 °C and evacuated for 1 h under a He atmosphere. After O2 (10 mL/min) was introduced, the sample was held at 500 °C for 1 h. Afterward, the sample was cooled down to room temperature under He atmosphere. The sample was then heated from 50 to 900 °C at a constant heating rate (10 °C/min), and the signal of desorbed oxygen was monitored with a TCD.
2.3 Catalytic Activity Evaluation
The CO catalytic oxidation activity was evaluated in a continuous flow fixed-bed quartz reactor under atmospheric pressure. The required amount (100 mg) of the catalyst was put into the reactor and fixed by quartz wool on both ends. Before each test, the catalyst was pretreated at 300 °C for 1 h in 10% O2/N2 (100 mL/min) to remove moisture from the catalyst surface. Then, the reactant gas mixture (1% CO, 4% O2, and balanced with 95% He) was passed through the reactor at a space velocity of 60,000 h−1. The temperature was increased from 20 to 250 °C with a heating rate of 10 °C/min. The outlet gas composition was monitored by gas chromatography equipped with a thermal conductivity detector (GC, Aglien7890A). The conversion of CO was determined based on CO consumption. The kinetic study was performed with CO conversion less than 20%. The reactant gas consisted of 1% CO, 4% O2, and balanced with He. The reaction rates were obtained when the reaction reached steady-state conditions. The pretreatment and temperature ramping test were performed twice for each catalyst. Preliminary tests were performed without the catalyst and no evident CO conversion has been observed in these experiments at the typical reaction temperature.
3 Results and Discussion
3.1 Characterization Studies
ICP-OES analysis was performed to determine the presence of Cr content in Cr-doped CeO2 samples and the data were shown in Table S1. As per the data from Table S1, it can be noted that the measured contents of Cr in 5CrCe, 10CrCe, and 15CrCe catalysts are 4.96, 9.94, and 14.97, respectively. Thus, the present results demonstrated that the Cr doping with different contents was fulfilled greatly in this study.
The morphology and microstructure of the CeO2 and Cr-doped CeO2 nanorods were studied by TEM and the obtained results are shown in Fig. 1. As shown in Fig. 1, all the catalysts exhibited rod-like morphology. The average diameter and length of CeO2 nanorods (Fig. 1a) are around ~ 10–15 nm and 100 nm. Doping of Cr with various contents slightly altered the CeO2 nanorods size, suggesting that doped Cr ions play an important role in the growth process of CeO2 nanorods. Figure 1b–d show the TEM images of the 5CrCe, 10CrCe and 15CrCe catalysts, respectively. For these catalysts, the measured size is approximately with ~ 5 nm in diameter and 100–200 nm in length. The morphology of the three Cr-doped CeO2 nanorods is nearly the same, so we have focused mainly on CeO2 and 10CrCe nanorods as the representative for further characterization and discussion.
Figure 2 shows the HRTEM images of CeO2 and 10CrCe nanorods, which reveal the crystalline nature of both samples. The distance between the adjacent lattice fringes for CeO2 nanorods (Fig. 2a) was found to be 0.311 and 0.272 nm, which is associated with the (111) and (200) planes of cubic ceria, respectively [30]. While, the 10CrCe nanorods (Fig. 2c) showed the interplanar spacing of 0.309 and 0.27 nm, which correspond to the (111) and (200) planes of cubic ceria, respectively. It is noteworthy to mention that the interplanar distance of 10CrCe nanorods is slightly smaller than that of CeO2 nanorods. This result could be due to Cr doping into the CeO2 lattice and smaller ionic radius of Cr (Cr6+ (0.44 Å)/Cr3+ (0.62 Å)). Recently, Ta et al. and Agarwal et al. have reported the synthesis of CeO2 nanorods with mostly exposed (111) surfaces with various surface defects [31, 32]. From the HRTEM image shown in Fig. 2c, the 10CrCe nanorods exhibited various surface defects (lattice disorder and dislocation) compared with that of CeO2 nanorods. The corresponding selected area electron diffraction (SAED) patterns, shown in Fig. 2b and d, exhibit four well-defined diffraction rings, which are in support for the cubic ceria with (111), (200), (220), and (311) planes.
The morphology of the samples was further examined with FE-SEM analysis (Fig. S1). It was confirmed from FE-SEM images that the catalysts show rod-like morphology, which is consistent with TEM results. To confirm the chemical composition, the CeO2 and Cr-doped CeO2 nanorods were investigated by EDX analysis. As shown in Fig. S1, the EDX results confirmed the presence of Ce and O, and Ce, O and Cr elements in the CeO2 and Cr-doped CeO2 nanorods, respectively. Besides, the elemental mappings of CeO2 and 10CrCe nanorods were obtained by EDS and the results are shown in Fig. S2. Clearly, the Cr ions were uniformly distributed throughout the ceria matrix in 10CrCe nanorods, highlighting the formation of Ce–O–Cr solid solution without second phases. From the above results, it can be concluded that the as-synthesized Cr-doped CeO2 nanorods were in cubic phase and chromium ions were successfully doped into the CeO2 lattice, which are further evidenced by XRD and Raman analysis.
Figure 3 shows the XRD patterns of CeO2 and Cr-doped CeO2 nanorods with different Cr contents. For comparison, the reflections of ceria with the standard phase are also presented in the figure. As shown in Fig. 3, all four catalysts displayed 8 typical peaks corresponding to (111), (200), (220), (311), (222), (400), (331), and (420) crystal facets, which are in agreement with the cubic fluorite structure of CeO2, further consistent with the standard data (JCPDS 34-0394) [33]. Surprisingly, the peaks related to Cr-oxides are not detected even with increase of Cr content up to 15%, implying that Cr-ions were successfully doped into the ceria lattice to generate a solid solution (Ce–O–Cr) while maintaining the fluorite structure. The CeO2 nanorods show very sharp diffraction peaks, demonstrating that it is well-crystallized. In contrast, the diffraction peaks of 5CrCe, 10CrCe, and 15CrCe nanorods are broadened, indicating that the incorporation of Cr ions suppress the CeO2 growth and crystallization. To quantify this observation, the crystallite sizes of all the catalysts were calculated based on the ceria (111) plane by applying Scherrer formulae [34]. Obviously, all the Cr-doped CeO2 nanorods have smaller crystal sizes than that of the CeO2 nanorods. Usually substitution of smaller ionic radii ions can shift the characteristic peak position towards higher angles. Accordingly, in our case, a slight displacement of the diffraction maximums to higher angles (2θ) can be observed in the Cr-doped CeO2 nanorods in comparison to CeO2 nanorods. This fact is visible when zooming the region 26º and 36º, where the differences can be clearly seen compared to CeO2 nanorods, Fig. 3b. As the Cr doping concentration increases from 5 to 10%, the diffraction peak (111) is shifted towards higher angle side, whereas, at higher doping concentration (15%) the peak is again shifted towards lower angle side. The shifting of (111) peak towards higher and lower angle side is attributed to the lattice distortion, which is caused by doping of Cr3+ ions in the ceria lattice. A similar observation was also noted by Dalela et al. for their Gd-doped ceria samples [35]. The calculated lattice parameter values, Å, for CeO2, 5CrCe, 10 CrCe, and 15CrCe nanorods are ~ 5.46, 5.39, 5.38, and 5.42 Å, respectively. The decrease of the lattice parameter in Cr-doped CeO2 nanorods was due to the substitution of the bigger Ce4+/Ce3+ (0.97 Å)/(1.143 Å) ions with smaller Cr6+/Cr3+ (0.44 Å)/(0.62 Å) ions [29]. Besides that, Phokha et al. have demonstrated that a decrease in the lattice parameter with doping of Cr reflect an increase in Ce3+ ions into the crystal lattice and the subsequent increase in the oxygen vacancies [36]. This conclusion was further verified by other characterization results, which are discussed in the following sections. The size variation in Ce4+ and Ce3+ ions and oxygen vacancies distort the lattice (a change in the Ce–O–Ce bond length) and the overall lattice parameter [37].
Further, structural characterization was carried out for all the samples by Rietveld refinement using FULLPROF suit program and the obtained results are shown in Fig. S3. As shown in Fig. S3, the refined XRD patterns are in good agreement with the measured data. No additional diffraction peaks related to possible impurity phases of Cr and oxides of Cr were observed in Cr-doped samples, which revealed the primary indication that the Cr ions are incorporated into the ceria lattice giving rise to the formation of a single phase Ce–O–Cr solid solution. The lattice parameters determined by the observed XRD profiles of CeO2, 5CrCe, 10CrCe and 15CrCe nanorods were found to be ~ 5.45, 5.38, 5.37, and 5.42 Å, respectively. These values are very close to the calculated lattice parameters.
Generally, the surface physicochemical properties of the materials have a great influence on the CO oxidation performance. Therefore, BET analysis was performed for all the materials to investigate such properties. The N2-sorption isotherms and pore size distribution curves are shown in Fig. 4a. According to the IUPAC classification, the isotherms of all the catalysts were classified as type-IV with the characteristic H3-hysteresis loop, indicating the existence of mesopores in the catalysts. Interestingly, this loop is located at higher volume adsorbed (Vads) values for 10CrCe nanorods than for other samples, indicating the higher surface area. Further, as shown in Fig. 4b, all the samples exhibited unimodal pore size distribution curves with the pore sizes in the range of 6.83–7.52 nm, suggesting that the samples are typical mesoporous nature. The textural properties of the samples, including the BET surface area, pore volume and pore size, are listed in Table 1. The surface area of the CeO2 nanorods was measured to be 100.3 m2/g. For the Cr-doped ceria samples, the surface area was significantly increased with increasing in Cr amount in the ceria up to 10% and this behavior could be related to the synergistic effects between Ce and Cr oxides in Ce–O–Cr solid solution, which drastically suppresses the crystallite growth of ceria. While a further increase in Cr amount (i.e., 15%) resulted in a decreased surface area, which may be due to the aggregation of Cr on the surface of the ceria thereby blocking some of the pores. Similar results have been reported in the case of Cr-doped CeO2 as well as other transition metal ions doped ceria catalysts [29, 38,39,40]. Moreover, the pore volume of CeO2 (0.17 cm3/g) nanorods was altered after Cr-doping. On increasing Cr doping from 5 to 10%, the pore volume of catalyst was increased from 0.19 to 0.21 cm3/g, whereas after the 15% Cr doping, the pore volume slightly decreased to 0.16 cm3/g. Thus 10CrCe nanorods exhibited a larger surface area and pore volume compared to other catalysts, which can provide more active sites thereby allowing attaining the higher catalytic activity.
Figure 5 presents the Raman spectra of Cr-doped CeO2 nanorods along with the pristine CeO2 nanorods. The CeO2 nanorods present one prominent band (F2g) at approximately ~ 464 cm−1, it is attributed to the symmetric breathing mode of the oxygen atoms neighboring each Ce4+ cations [41, 42]. It is also observed that the prominent band (F2g) is shifted to a lower frequency (red-shift) for 5CrCe (452.8 cm−1), 10CrCe (449.4 cm−1), and 15CrCe (456.6 cm−1) nanorods compared to CeO2 nanorods. This shift implies that a considerable alteration of the Ce–O bond, which may be a significance of integration of Cr ions in the fluorite lattice. Basically, this vibrational mode is dependent on the oxygen movement. Therefore, the peak characteristics such as peak width and peak positions are enormously sensible to the disorder induced in the oxygen-ion sub-lattice of the oxide. With the introduction of Cr ions into the ceria lattice, crystal defects are observed, and it has an effect on the oxygen surroundings in the region of the metal ion. As an outcome, the changes in the peak positions and peak widths take place. According to the previous reports, the crystallite size can also affect the F2g peak position. Most probably decrease in the crystallite size is responsible for the red-shift. Hence, it is fairly supported from the XRD and Raman results that the lower crystallite sizes could be the reason for the shift of the F2g peak position towards lower frequency [43, 44]. Along with the F2g band, CeO2 nanorods demonstrate another broad peak (shown in inset and denoted as Ov) at maxima ~ 600 cm−1, which can be assigned to structural defects, such as Ce3+ ions and oxygen vacancies, arising from lattice distortion owing to the disparity among the ionic radius of the host and guest metal oxides [45]. The augment in the intensity of this peak is identified with the doping of Cr, implying that Cr-doped CeO2 nanorods might have higher oxygen vacancy intensity than CeO2. Interestingly, the relative highest intensity of Ov peak in 10CrCe nanorods further indicated that it possessed higher amount of oxygen vacancies when compared to 5CrCe and 15CrCe nanorods. This might be a good reason to explain the difference in the activity. However, no peaks were observed associated with the CrOx, which specifies the formation of a Ce–O–Cr solid solution.
As shown in Fig. 6a, a change in the color from bright yellow to light grey indicated that Cr ions were successfully doped into the lattice of CeO2. Thus, we thought that Cr doping alters the optical and surface chemical properties of CeO2 nanorods. The optical properties of the CeO2 and Cr-doped CeO2 nanorods were investigated by UV–vis DRS in the range of 200–800 nm at room temperature and the results are shown in Fig. 6b. Generally, the UV absorbance bands (Fig. 6b) at around ~ 255, 290 and 345 nm are originating from the O2− → Ce3+, O2− → Ce4+ charge transfer (CT) and interband (IBT) transitions, respectively [16, 46]. These results confirm the presence of Ce4+ and Ce3+ ions. The formed Ce3+ can be distributed around oxygen vacancies in the CeO2. This implies the occurrence of oxygen vacancy defects in the catalysts; Raman results also corroborate this result. Atla et al. also reported such absorbance peaks in the UV–vis DRS studies [47]. Doping of CeO2 with Cr ions obviously affects its optical absorption characteristics. As shown in Fig. 6b, some differences in the optical absorption were observed for Cr-doped ceria nanorods as a function of Cr-content. Compared to CeO2 nanorods, it is observed that the absorption of all Cr-doped CeO2 nanorods extends into the visible region (i.e. red-shift), indicating the substitution of Ce4+ ions with Cr ions forming Ce3+ ions, thus results in the increase of oxygen vacancies of ceria due to the charge compensation mechanism [38, 48]. The indirect band gaps of the investigated catalysts were estimated using the Kubelka–Munk (KM) function [(αhϑ)n = k(hϑ − Eg)]. According to the KM plots in Fig. 6c, the bandgap for CeO2 nanorods is approximately ~ 2.94 eV. Surprisingly, the bandgap of our CeO2 nanorods is lower than that of bulk ceria i.e., 3.19 eV [49]. Further, the band gaps of 5CrCe, 10CrCe, and 15CrCe nanorods were calculated to be ~ 2.73, 2.64, and 2.82 eV, respectively. The bandgap values of the nanomaterials strongly affected by the lattice defects and interface effects, and it is found that the bandgap decreases due to increase in the lattice defects [50, 51]. In our work, the reduction in the bandgap values in Cr-doped CeO2 nanorods compared to CeO2 nanorods revealed the formation of defects, namely, Ce3+ ions and the initiation of related oxygen vacancies in the ceria lattice. Significantly, 10CrCe nanorods showed the best absorption ability and lower bandgap which reflected higher lattice defects such as oxygen vacancies among all the samples. Also, the intensity of CT and IBT peaks is increased when 5% and 10% Cr doped into the ceria, while, it decreased with a further increase of 15% Cr content.
PL analysis is a useful technique to investigate the presence of defects in the nanomaterials. Figure 7 shows PL spectra of all the catalysts measured using 250 nm as an excitation wavelength. The PL spectra of all the catalysts showed almost the same emission peaks and exhibit three strong photoluminescence peaks at ~ 420, 450, and 465 nm and one shoulder weak peak at 490 nm, which is also observed in the other transition metal doped ceria systems [52]. According to the previous reports, the emission bands ranging from ~ 350 to 500 nm for the CeO2-based catalysts are attributed to the presence of structural defects including oxygen vacancies between the Ce4+ conduction band and O2− valence band, as supported by the DRS and Raman results [52, 53]. Additionally, the PL intensity of Cr-doped CeO2 nanorods is lower than that of CeO2 nanorods, which is due to the formation of higher oxygen vacancy defects in the CeO2 lattice. Among all the samples, 10CrCe nanorods exhibited very low intensity PL emission peaks, thereby indicating the generation of relatively more oxygen vacancy defects in the structure.
The surface chemical state of Ce, O, and Cr in the prepared samples was examined by XPS. The XPS survey-scan spectra results, as shown in Fig. S4, confirm the existence of Ce, O, and Cr elements at their characteristic binding energies in Cr-doped CeO2 nanorods. The deconvoluted Ce 3d XP spectrum, shown in Fig. 8a, was fitted into two sets of spin-orbital multiplets, corresponding to the 3d3/2 and 3d5/2 contributions that are labeled as U and V, respectively. The spectral lines denoted as v/u, v′′/u′′, v′′′/u′′′ were characteristic of the Ce+4 (3d104f0) state, while v′/u′ were assigned to the Ce3+ 3d104f1 state [54]. This finding revealed that cerium is present in both 4+ and 3+ oxidation states on the surface of the catalysts, which is consistent with the previous reports [38]. The relative amount of surface Ce3+ to total Ce (Cetotal) can be calculated from the XP spectra and the results are listed in Table S2. Noticeably, the Cr-doped CeO2 nanorods exhibited a higher content of Ce3+ compared to the CeO2 nanorods. This indicates that the doping of Cr into the CeO2 promotes the formation of higher amount of Ce3+ on CeO2 surface. According to the literature, the existence of Ce3+ ions could produce oxygen vacancies on the catalyst surface [55]. Thus, the oxygen vacancies increase with the increase in the proportion of Ce3+. The 10CrCe nanorods have the highest Ce3+/Cetotal within the Cr-doped CeO2 nanorods; consequently, it has the highest number of oxygen vacancies. It has been proven that the exposed Ce3+ ions associated with the oxygen vacancies on the CeO2 surface is considered as potentially effective surface sites in the CO oxidation reaction. As a result, it is expected that 10CrCe nanorods would possess higher CO conversion. The O 1s XPS peaks of CeO2, 5CrCe, 10CrCe, and 15CrCe nanorods are shown in Fig. 8b, where, two peaks can be clearly resolved according to the curve fitting method. The peak with lower binding energy ~ 529.6 eV corresponding to the lattice oxygen in the CeO2 (as Ce–O–Cr, denoted as Oα), and the next peak at higher binding energy ~ 529.8 eV is assigned to the chemisorbed oxygen such as O2− and O− and/or weakly-bonded oxygen (denoted as Oβ). The previous reports concluded that “Oα” peak belongs to the oxygen in the O–Ce4+ bond, whereas the “Oβ” peak originates from the oxygen in the O–Ce3+ bond [56, 57]. Therefore, the higher binding energy peak can be related with the Ce3+ ions and oxygen vacancies. It can be noted that the amount of crystal lattice oxygen decreased with increasing Cr doping amount, while the number of oxygen vacancies increased. The increased oxygen vacancies concentration (Oβ) in the surface region can be semi-quantitatively calculated through the peak area ratio of Oα and Oβ. As shown in Table S2, the concentration of oxygen vacancies in 10CrCe nanorods is 45.88% and this value is higher than others. This observation agrees with Raman, UV–vis DRS, and PL results. The Cr 2p XP spectra of Cr2O3, 5CrCe, 10CrCe, and 15CrCe nanorods are shown in Fig. 8c. The spectra consist of two main spin-orbital lines, 2p3/2 and 2p1/2, separated by about ~ 10.2 eV. The two main peaks at approximately ~ 576.5 eV and 586.4 eV can be attributed to the Cr 2p3/2 and Cr 2p1/2 of Cr3+ species, respectively. The peaks at approximately ~ 578.6 eV and 587.5 eV can be attributed to the Cr 2p3/2 and Cr 2p1/2 of Cr6+ species. This confirms the presence of Cr ion in both 3+ and 6+ oxidation states, which is also supported by previous reports [58]. Further, with the increasing amount of Cr, the photoelectron peak positions of Cr3+ and Cr6+ were slightly shifted to lower binding energies, owing to the presence of Ce–O–Cr species, implying that Cr ions were successfully doped into the CeO2 lattice.
As known, the reducibility of the catalysts plays an important role in CO oxidation. Based on that, H2-TPR was performed to evaluate the redox properties of the catalysts and the results are shown in Fig. 9. Generally, pure ceria shows two notable reduction peaks, corresponding to surface reduction (< 500 °C) and bulk reduction at > 800 °C [33]. Interestingly, in our case, CeO2 nanorods exhibit one broad reduction peak ranging from 260 to 765 °C with the peak maxima at 580 °C. We attribute this peak to the concurrent reduction of surface and bulk lattice oxygen of CeO2 [59]. The TPR profiles of Cr-doped CeO2 nanorods are very different from that of CeO2 nanorods, suggesting a different redox behavior which might be due to the interaction between CeO2 and the Cr dopant. For the Cr-doped CeO2 nanorods, according to the literature, the high intense reduction peak in the low temperature region 240–450 °C could be attributed to the reduction of surface ceria involving the surface-active oxygen removal, including Ce–O–Cr solid solution redox sites. While a small shoulder peak in the high temperature region 440–540 °C was ascribed to the reduction of Cr6+ (Cr6+ to Cr3+), suggesting that there are small nanocrystalline Cr2O3 species, which cannot be distinguished by XRD, Raman, and TEM [60, 61]. It is noteworthy that the reduction of the ceria surface oxygen in Cr-doped CeO2 nanorods shifts toward lower temperatures as compared to CeO2 nanorods, which suggests that the presence of Cr ions could easier the reduction of the Ce–O–Ce species. Among all the catalysts, 10CrCe nanorods possess the lowest reduction temperature with the highest reduction peak area, indicating that there exists strong interaction between the Ce and Cr species. The higher surface area and smaller crystallite size are favorable for the formation of easily reducible sites. Thus, the surface ceria reduction of 10CrCe nanorods at a lower temperature is also consistent with its higher surface area and smaller crystal size. In addition, the higher amount of oxygen vacancies in the 10CrCe nanorods, as inferred from Raman, UV–vis DRS, PL and XPS results, also improves oxygen mobility from lattice to the surface which contributes to the easiest surface reduction.
The mobility of oxygen species has shown to be crucial in applications such as catalyzing the oxygen involved redox reactions. As is reported, the O2-TPD analysis is considered as an effective way to verify the mobility of oxygen species; therefore, these catalysts are subsequently characterized by O2-TPD analysis. Three main desorption peaks were observed in O2-TPD profiles, as shown in Fig. S5. Generally, the desorption peaks below 350 °C were ascribed to the physical and weak chemical adsorbed oxygen (α), mainly referring to surface gaseous oxygen (O2). The desorption peaks appeared at temperatures higher than 600 °C result from the removal of bulk lattice oxygen from Ce–O–Cr solid solution (γ). The desorption peak (β) in the range of 350–600 °C could be attributed to the surface chemisorbed oxygen species and these oxygen species regularly are linked to surface oxygen defects [59]. It is well acknowledged in the literature that the characteristics of surface chemisorbed oxygen species (in terms of position and area) make a great difference in the CO oxidation [62]. When chromium ions were incorporated into the CeO2 lattice, however, the desorption peaks are different. It can be observed that the β-desorption peak was shifted to lower temperature along with peak intensifying in Cr-doped CeO2 nanorods compared to that of CeO2 nanorods. By comparison, the 10CrCe nanorods showed the “β” peak at lower temperature compared to other two loadings. Further, the peak area of the β-desorption for 10CrCe nanorods was larger than other samples, so the 10CrCe nanorods might show higher CO oxidation ability. These results were in line with the results of H2-TPR.
3.2 Catalytic Activity Studies
The catalytic oxidation of CO to CO2 has aroused broad interest from the research community owing to its industrial relevance and relative simplicity. Further, it is a widely used reference model system for oxidation catalysis [63]. Thus, in the present investigation CO oxidation was used as a probe reaction to evaluate the catalytic performance of all-synthesized catalysts. As shown in Fig. 10a, the CO oxidation increases for all catalysts with reaction temperature, while the curves have an S-shaped type. The light-off temperature values at 50% and 90% CO conversion i.e., T50 and T90 were taken as a reference to conveniently compare the catalytic activities of the studied catalysts. As shown in Fig. 10a and Table S2, Cr2O3 exhibits the lowest activity with T50 and T90 of 361 and 394 °C, respectively. Besides, CeO2 nanorods exhibit T50 and T90 values of 338 and 379 °C, respectively. This is mainly due to the presence of Ce3+ ions and oxygen vacancies. Apparently, compared with the CeO2 nanorods and Cr2O3, doping of Cr ions considerably improved the catalytic activity of CeO2 nanorods, which might be due to the synergistic effect of Cr and Ce on the reactivity. The activity of Cr-doped CeO2 nanorods increases with an increase in Cr amount from 5 to 10%, and then it slightly decreased with further increase in Cr amount to 15%. These results indicate that the catalytic activities of the Cr-doped CeO2 nanorods are affected obviously by the Ce:Cr molar ratio, and the sequence of catalytic activity of the Cr-doped CeO2 nanorods is as follows: 10CrCe > 5CrCe > 15CrCe > CeO2 > Cr2O3. Among the investigated catalysts, the 10CrCe nanorods had the lowest oxidation temperature with the T50 and T90 values of ~ 261 and 280 °C, which are 77 and 99 °C lower than those over CeO2 nanorods, so the optimum catalyst composition was Ce0.9Cr0.1O2−δ. According to various characterization results, we believe that the notable performance of the 10CrCe nanorods could be related to the special composition, structural, and textural properties as well as reduction and desorption behavior, which account for various beneficial properties. Firstly, the BET results indicated that the 10CrCe nanorods possess a larger specific surface area and pore volume with mesoporous structure, resulting in the creation of more available active sites, which could be in favor of the adsorption and diffusion of reactant and product gases. From the XPS results, 10CrCe nanorods have an abundance of Ce3+ species and surface oxygen vacancies (Oβ), which can actively take part in the CO oxidation process and greatly contribute to the catalytic activity. This conclusion was also supported by the Raman, UV–vis DRS, and PL results. Also, it can be concluded from HR-TEM study that an abundance of surface defects (lattice disorder and dislocation) were found on 10CrCe nanorods, thereby resulting in the improved catalytic activity. The H2-TPR results indicated that easily available surface oxygen that gets reduced at lower temperature was observed in 10CrCe nanorods, due to the strong interaction between Ce and Cr oxides (weakened the Ce–O bond length), resulting in its better reducibility, which consequently facilitated the redox cycle and leads to the enhanced CO oxidation. Moreover, 10CrCe nanorods exhibit the great quantity of surface desorbed oxygen species at lower temperatures (from O2-TPD) compared to other catalysts that correlate with its highest catalytic activity.
a Conversion of CO (%) versus temperature (°C) for CeO2, 5CrCe, 10CrCe and 15CrCe nanorods and Cr2O3 catalyst. b Arrhenius plots for CO oxidation over CeO2 nanorods, 10CrCe nanorods and Cr2O3 catalyst. c Stability test of 10CrCe nanorods. d CO conversion as a function of reaction temperature for four successive runs of 10CrCe nanorods
The apparent activation energy of CO oxidation over CeO2 nanorods, 10CrCe nanorods, and Cr2O3 were derived using Arrhenius plot, as shown in Fig. 10b. The activation energy (Ea) values for CeO2 nanorods, 10CrCe nanorods, and Cr2O3 were ~ 78.3, 65.4, and 86.8 kJ/mol, respectively. Surprisingly, CeO2 nanorods displayed a considerably lower Ea than the Cr2O3 catalyst, thereby suggesting that the CeO2 nanorods contain more active sites than Cr2O3 for CO oxidation. Regardless, the lowest Ea value for 10CrCe nanorods indicates that CO oxidation proceeds more readily than for the other two samples, which confirmed the promoting role of chromium. In addition, to evaluate the potential CO oxidation performance in practical applications, the stability test was carried over 10CrCe nanorods. Figure 10c shows CO conversion as a function of time‐on‐stream at 275 °C (where 85% CO conversion) for 55 h under similar conditions. The results revealed that the CO conversion could remain above 81% after 55 h, which strongly indicates that the 10CrCe nanorods can function as a durable catalyst for CO oxidation. The textural stability of the catalysts is also studied since it is the key requirement for the application of these materials in three-way catalytic applications. Taking the example of the highly active 10CrCe nanorods, the recycling tests were performed with three more catalytic runs and compared in Fig. 10d. After each cycle, the catalyst was washed and dried completely and then subjected to the next cycle. As shown in Fig. 10d, the 10CrCe nanorods exhibit an insignificant deactivation after three cycles with a small decrease in T50 (around 14 °C) of CO conversion, indicating the high chemical stability of the 10CrCe nanorods.
Further, the textural stability of the used catalyst was characterized by XRD and TEM. The XRD results confirm that the phase and structure of the used catalyst was basically unchanged (Fig. S6A). The TEM image (Fig. S6B) shows that the nanorod structure of the used catalyst was well maintained. The BET surface area of the used 10CrCe nanorods was 132.5 m2/g, which was close to that of fresh catalyst (137.5 m2/g). From these results, the 10CrCe nanorods possess significant features, particularly, high catalytic activity, excellent stability and practicability, making it a suitable catalyst for CO oxidation.
4 Conclusions
In this work, pure and Cr-doped ceria (Ce1−xCrxO2−δ; X = 0.05, 0.1, and 0.15) nanorods with different Cr contents were successfully synthesized by a hydrothermal method. Then, we applied these catalysts for CO oxidation, with special attention to the effects of doped Cr contents on the CO oxidation activity. XRD patterns demonstrate that the CeO2 nanorods are highly crystalline and Cr ions were successfully incorporated into the CeO2 lattice. The presence of Cr doping with different concentration was confirmed by ICP-OES analysis. The XRD and ICP-OES results were also consistent with the UV–vis DRS, Raman, and XPS. The Cr-doped CeO2 nanorods exhibited higher activity than CeO2 nanorods and Cr2O3 catalysts. Further, the catalytic activity of the Cr-doped CeO2 nanorods was closely related to the Cr content and was higher at 10%. The superior activity of 10CrCe nanorods is attributed to the enhanced redox properties together with the high surface area, provided by the formation of Ce–O–Cr solid solution. Through Raman UV–vis DRS, PL, XPS and O2-TPD measurements, we provided solid evidence that the 10CrCe nanorods possess a higher number of Ce3+ species and oxygen vacancies, which were also found to be correlated with its highest activity. Further, the higher Ce3+ species and Oβ ratios in 10CrCe nanorods enhanced the adsorption capacity of reactant molecules, thus promoting the CO oxidation efficiency at low temperature. Additionally, 10CrCe nanorods maintained excellent long-term stability at least up to 55 h. By taking into consideration, the excellent activity with the lowest activation energy and long-term stability, it can be stated that the Cr-doped CeO2 nanorods should be a competitive solution to the removal of hazardous CO as well as other environmental pollutants.
5 Supporting Information
Detailed information about the elemental compositions, the surface atomic concentrations, light-off temperatures, FE-SEM/EDX, EDS elemental mapping, Rietveld refinement XRD data, survey-scan XP spectra, O2-TPD profiles, and characterization of the used catalysts (XRD and TEM) are provided.
References
Valechha D, Suresh-Kumar M, Al-Fatesh A, Jiang H, Labhasetwar N (2019) Catal Lett 149:127–140
Taira K, Nakao K, Suzuki K, Einaga H (2016) Environ Sci Technol 50:9773–9780
Antonaroli S, Crociani B, Natale CD, Nardis S, Stefanelli M, Paolesse R (2015) Sens Actuator B Chem 208:334–338
Punde SS, Tatarchuk BJ (2017) Chin J Catal 38:475–488
Ho PH, Ambrosetti M, Groppi G, Tronconi E, Jaroszewicz J, Ospitali F, Rodríguez-Castellón E, Fornasari G, Vaccari A, Benito P (2018) Catal Sci Technol 8:4678–4689
Körner R, Ricken M, Nölting J (1989) J Solid State Chem 78:136–147
Li W, Zhang C, Li X, Tan P, Zhou A, Fang Q, Chen G (2018) Chin J Catal 39:1653–1663
Devaiah D, Tsuzuki T, Aniz CU, Reddy BM (2015) Catal Lett 145:1206–1216
Fornasiero P, Dimonte R, Rao GR, Kaspar J, Meriani S, Trovarelli A, Graziani M (1995) J Catal 151:168–177
Puigdollers AR, Schlexer P, Tosoni S, Pacchioni G (2017) ACS Catal 7:6493–6513
Reddy BM, Bharali P, Saikia P, Thrimurthulu G, Yamada Y, Kobayashi T (2009) Ind Eng Chem Res 48:453–462
Reddy BM, Bharali P, Thrimurthulu G, Saikia P, Katta L, Park S-E (2008) Catal Lett 123:327–333
Alammar T, Chow Y-K, Mudring A-V (2015) New J Chem 39:1339–1347
Muhich CL, Blaser S, Hoes MC, Steinfeld A (2018) Int J Hydrogen Energy 43:18814–18831
Venkataswamy P, Jampaiah D, Rao KN, Reddy BM (2014) Appl Catal A 488:1–10
Venkataswamy P, Rao KN, Jampaiah D, Reddy BM (2015) Appl Catal B 162:122–132
Liu H, Wei L, Yue R, Chen Y (2010) Catal Commun 11:829–833
Singh P, Hegde MS, Gopalakrishnan J (2008) Chem Mater 20:7268–7273
Harrison PG, Daniell W (2001) Chem Mater 13:1708–1719
Mostrou S, Büchel R, Pratsinis SE, van Bokhoven JA (2017) Appl Catal A 537:40–49
Wu J, Zeng L, Cheng D, Chen F, Zhan X, Gong J (2016) Chin J Catal 37:83–90
Li H, Dong Q, Li Y, Guo T, Zhang J (2018) React Kinet Mech Catal 125:663–673
Huang X-S, Sun H, Wang L-C, Liu Y-M, Fan K-N, Cao Y (2009) Appl Catal B 90:224–232
Wu Z, Li M, Overbury SH (2012) J Catal 285:61–73
Chen A, Zhou Y, Ta N, Li Y, Shen W (2015) Catal Sci Technol 5:4184–4192
Zhou K, Wang X, Sun X, Peng Q, Li Y (2005) J Catal 229:206–212
Si R, Flytzani-Stephanopoulos M (2008) Angew Chem Int Ed 47:2884–2887
Si R, Flytzani-Stephanopoulos M (2008) Angew Chem Int Ed 120:2926–2929
Huang X, Zhao G, Wang P, Zheng H, Dong W, Wang G (2018) Chem Cat Chem 10:1406–1413
López JM, Gilbank AL, García T, Solsona B, Agouram S, Torrente-Murciano L (2015) Appl Catal B 174–175:403–412
Ta N, Liu J, Chenna S, Crozier PA, Li Y, Chen A, Shen W (2012) J Am Chem Soc 134:20585–20588
Agarwal S, Lefferts L, Mojet BL, Ligthart DAJ, Hensen EJM, Mitchell DRG, Erasmus WJ, Anderson BG, Olivier EJ, Neethling JH, Datye AK (2013) Chem Sus Chem 6:1898–1906
Rao BG, Jampaiah D, Venkataswamy P, Reddy BM (2016) Chem Select 1:6681–6691
Cullity BD, Weymouth JW (1957) Am J Phys 25:394–395
Soni S, Chouhan N, Meena RK, Kumar S, Dalela B, Mishra M, Meena RS, Gupta G, Kumar S, Alvi PA, Dalela S (2019) Glob Chall 3:1800090–1800109
Phokha S, Prabhakaran D, Boothroyd A, Pinitsoontorn S, Maensiri S (2014) Microelectron Eng 126:93–98
Deshpande S, Patil S, Kuchibhatla SVNT, Seal S (2005) Appl Phys Lett 87:133113–133115
Wang Y, Bai X, Wang F, Kang S, Yin C, Li X (2019) J Hazard Mater 372:69–76
Li B, Raj A, Croiset E, Wen JZ (2019) Catalysts 9:805–830
Liu X, Ding J, Lin X, Gao R, Li Z, Dai W-L (2015) Appl Catal A Gen 503:117–123
Venkataswamy P, Jampaiah D, Mukherjee D, Aniz CU, Reddy BM (2016) Catal Lett 146:2105–2188
Ferreira NS, Abracado LG, Macedo MA (2012) Physica B 407:3218–3221
Jampaiah D, Reddy TS, Kandjani AE, Selvakannan PR, Sabri YM, Coyle VE, Ravi S, Bhargava SK (2016) J Mater Chem B 4:3874–3885
Spanier JP, Robinson RD, Zhang F, Chan S-W, Herman IP (2001) Phys Rev B 64:245407–245414
Neelapala SD, Harshini D (2018) Mater Sci Eng Technol 1:155–159
Zhang YW, Si R, Liao CS, Yan CH (2003) J Phys Chem B 107:10159–10167
Atla SB, Wu M-N, Pan W, Hsiao YT, Sun A-C, Tseng M-J, Chen Y-J, Chen C-Y (2014) Mater Charact 98:202–208
Arul NS, Mangalaraj D, Han JI (2015) J Mater Sci Mater Electron 26:1441–1448
Ma Q, Izu N, Masuda Y (2018) ACS Appl Nano Mater 1:2112–2119
Ansari SA, Khan MM, Ansari MO, Kalathil S, Lee J, Cho MH (2014) RSC Adv 4:16782–16791
Barreca D, Bruno G, Gasparotto A, Losurdo M, Tondello E (2003) Mater Sci Eng C 23:1013–1016
Bharathi RN, Shankar S (2018) J Supercond Nov Magn 31:2603–2615
Lu XH, Huang X, Xie SL, Zheng DZ, Liu ZQ, Liang CL, Tong YX (2010) Langmuir 26:7569–7573
Gang Y, Wang J, Liu J, Cheng X, Ma H, Wu H, Yang Z, Zhang G, Sun X (2019) Catal Lett. https://doi.org/10.1007/s10562-019-02891-8
Liu P, Niu R, Li W, Wang S, Li J (2019) Catal Lett 149:1007–1016
Paunovic N, Mitrovic ZD, Scurtu R, Askrabic S, Prekajski M, Matovic B, Popovic ZV (2012) Nanoscale 4:5469–5476
Liu B, Li C, Zhang G, Yao X, Chuang SSC, Li Z (2018) ACS Catal 8:10446–10456
Zhu L, Lu Y, Li F (2018) Int J Hydrogen Energy 43:13754–13763
Jampaiah D, Venkataswamy P, Coyle VE, Reddy BM, Bhargava SK (2016) RSC Adv 6:80541–80548
Cai W, Zhao Y, Chen M, Jiang X, Wang H, Ou M, Wan S, Zhong Q (2018) Chem Eng J 333:414–422
Takehira K, Ohishi Y, Shishido T, Kawabata T, Takaki K, Zhang Q, Wang Y (2004) J Catal 224:404–416
Venkataswamy P, Devaiah D, Kuntaiah K, Vithal M, Reddy BM (2017) Catal Lett 147:2028–2044
Cao F, Zhang S, Gao W, Cao T, Qu Y (2018) Catal Sci Technol 8:3233–3237
Acknowledgements
PV is thankful to the Science and Engineering Research Board (SERB), New Delhi for financial support in the form of Research Associate (Grant No: EMR/2016/001533). MV thanks the University Grants Commission (UGC), New Delhi for BSR fellowship. BMR thanks the Department of Atomic Energy (DAE), Mumbai for the award of the Raja Ramanna Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Venkataswamy, P., Damma, D., Jampaiah, D. et al. Cr-Doped CeO2 Nanorods for CO Oxidation: Insights into Promotional Effect of Cr on Structure and Catalytic Performance. Catal Lett 150, 948–962 (2020). https://doi.org/10.1007/s10562-019-03014-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-03014-z