Abstract
2,4,6-Trimethylbenzoyldipenylphosphine oxide (TPO) is an important photoinitiator, nevertheless some drawbacks exist in its synthesis. So V/MCM-41 catalysts were designed. Compared with commonly used VO(acac)2, V/MCM-41 catalysts exhibited excellent TPO yield (92.5%), which makes the usage of inorganic vanadium possible and is benefit both in academic and in industry.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Monoacylphosphine oxides (MAPOs) are highly effective radical photoinitiators, which are widely used in the curing of white pigmented coating [1, 2] and biomedicalmaterials [3,4,5], especially dental [6,7,8,9,10,11] and orthopedic resin composites [12,13,14]. However, only two methods are known for the synthesis of MAPOs. Method I is one-step reaction of acyl chloride and alkoxyphosphine. Method II is two-step reaction: (1) aldehyde reacts with diphenylphosphine oxide to form α-hydroxyphosphine oxide; (2) followed by oxidation of α-hydroxyphosphine oxide to MAPOs. Comparatively, Method I has limited substrate tolerance and forms by-product of alkyl chloride (difficult to handle) [15]; Method II has the advantage of mild reaction condition and broad substrate scope. Thus, Method II is commonly used in MAPOs synthesis.
It is known that the oxidation of alcohols to the corresponding carbonyl compounds is of great importance both in academia and in industry. Thus, plenty of studies were carried out to obtain the optimized and environmental friendly oxidation system [16,17,18,19]. Based on these, many oxidants (such as chromium trioxide, potassium permanganate, iodobenzenediaceate, vanadyl acetylacetonate, manganese dioxide, pyridinium dichromate, etc.) have been used for the oxidation of α-hydroxyphosphine oxide [20]. However, the instability of α-hydroxyphosphine oxide under common oxidation conditions makes all the aforementioned experiments fail [20, 21]. Up to now, only two oxidation systems could give satisfactory yield of MAPOs: (1) active MnO2 [20]; (2) combination of vanadyl acetylacetonate (VO(acac)2) and tert-butylhydroperoxide (TBHP) [22]. For the first oxidation system, huge amounts (20 times to the substrate) of active MnO2 are needed [20]. For the second system, large usage (ca. 35 times to the substrate) of VO(acac)2 causes environmental pollution [22]. Taking account of the importance of MAPOs in light-curing and the drawbacks of the two oxidation systems, we decided to explore highly effective and environmental friendly synthesis using Method II method for MAPOs. 2,4,6-trimethylbenzoyldipenylphosphine oxide (TPO, Fig. 1) is chosen as the target MAPOs in this study and Method II is used in the synthesis. Initially, Mn, Mo, W and Fe were chosen as active metal for the oxidation synthesis of TPO. Both zeolites and metal oxides were used as support. However, the experiments failed: in most cases no TPO was detected after reaction (Table S1). So MCM-41 supported vanadium (V/MCM-41) was designed as the oxidation catalyst for the synthesis of TPO. Inorganic vanadium compounds (NH4VO3 and V2O5) were used as precursors for catalyst preparation. For comparison, the performance of VO(acac)2 + TBHP system was tested.
2 Experimental
2.1 Catalyst Preparation
MCM-41 supported vanadium (V/MCM-41) catalysts were prepared by solid state impregnation, similar as the procedure in literature [23]. Typically, vanadium precursor (NH4VO3 or V2O5, Aladdin) and MCM-41 (JCNANO Tech Co., Ltd) were added to an agate mortar and manually ground for 0.5 h. The ground mixture was then transferred to a crucible and calcined in air at 550 °C for 4 h. The theoretical V loading was set as 7 wt%, which was then analyzed by ICP (Table S2). Obtained samples were denoted as V1/M (NH4VO3 as precursor) and V2/M (V2O5 as precursor).
2.2 Catalyst Characterization
Crystalline structures of the catalysts were investigated by X-ray diffraction (XRD) measurements (D/Max250pc). The morphologies of samples were recorded using SEM (Nova Nano SEM450) and TEM (Tecnai G2 20). The textural properties of samples were analyzed by low temperature N2 adsorption/desorption using an Autosorb-IQ gas absorption analyzer and the specific surface areas were calculated using the BET equation. The vanadium loading was determined by ICP analysis (Optima 7300 DV). Chemical states of vanadium in the catalysts were determined by UV–Vis (UV-3031, Shimadzu).
2.3 Oxidation Synthesis of 2,4,6-Trimethylbenzoyldipenylphosphine Oxide (TPO)
TPO was synthesized through the oxidation of α-hydroxy-(2,4,6-trimethylbenzyl)-diphenyl phosphine oxide (α-HDPO, Scheme 1). α-HDPO was obtained by the reaction of diphenylphosphine oxide (DPO) and 2,4,6-trimethylbenzaldehyde (TMBA). The structure of product was confirmed by 1H NMR, 31P NMR and 13C NMR (shown in Supporting Information). Typical procedure was as follows.
Firstly, TMBA (0.1 mol) and DPO (0.1 mol) were dissolved in 150 mL 1,2-dichloroethane (DCM). The mixture was stirred at room temperature for 6 h (TLC analysis). The solvent was evaporated in vacuo, and crude product of α-HDPO was purified by washing with EtOAc (3 × 80 mL).
Secondly, TBHP (10.28 mmol) was added to a stirred solution of α-HDPO (8.57 mmol) and catalyst (1 mol% V as α-HDPO) in 30 mL DCM at 5 °C. After 30 min, the mixture was then stirred at room temperature for another 6 h. Reaction products were analyzed using HPLC (Shimadzu) equipped with UV detector and C-18 column (Anasil AQ-C18, 5 µm). The investigated catalysts were V1/M, V2/M, VO(acac)2, NH4VO3 and V2O5. Catalytic activity was described in terms of α-HDPO conversion and TPO yield.
3 Results and Discussion
Figure 2 depicted the XRD patterns of MCM-41 and V/MCM-41 catalysts. Typical diffraction peaks of MCM-41 [24] could be clearly observed on V1/M and V2/M, indicating that the hexagonally order structure is well preserved after the introduction of V species by solid state impregnation. Such maintenance could also be confirmed by the results of SEM (Fig. S1) and TEM (Fig. S2). On the other hand, peaks due to V2O5 species (JCPDS card no. 41-1426) could be clearly observed on these two catalysts. But the diffraction peaks on V2/M were more intense than those on V1/M (Fig. 2b). Because the amount of V was the same, this result suggested that vanadium species on V1/M dispersed better than those on V2/M.
Band at ca. 450 nm (due to crystalline V2O5 [25]) could be observed on the UV–Vis spectra of V/MCM-41 catalysts (Fig. 3), which confirms the existence of V2O5 species. Moreover, strong peaks around 260 nm (with a shoulder at ca. 213 nm) and medium peak around 370 nm could be detected. The former peak corresponded to tetrahedrally coordinated vanadium in terminal position (V=O), while the shoulder was due to tetrahedrally coordinated vanadium in the framework (V–O–Si) [26]. The peak around 370 nm could be assigned to vanadium in octahedral coordination [27]. The results reveal that parts of vanadium species impregnated to MCM-41 existed in the framework. It is in accordance with the XRD results (intensity decrease of diffraction peaks of MCM-41 structure).
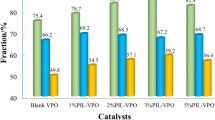
The catalytic performance of V1/M and V2/M in the oxidation of α-HDPO to TPO was investigated and results were depicted in Fig. 4. For comparison, VO(acac)2 + TBHP system was also tested. After 6.5 h reaction, α-HDPO was almost fully converted in all cases. However, the yield of TPO over V/MCM-41 catalysts were much higher than that on VO(acac)2, especially for V1/M (92.5%). It suggested that the trend towards oxidation reaction was much greater on V/MCM-41 catalysts than that on VO(acac)2. Because α-HDPO is easily decomposed under oxidation conditions, the results also reveal that the usage of V/MCM-41 catalysts could partially inhibit the decomposition of α-HDPO. It should be noticed that inorganic vanadium compounds were used for the preparation of oxidation catalysts. The substitution of organic VO(acac)2 and the inhibition of α-HDPO decomposition is of great importance both in academia and in industry.
For the two V/MCM-41 catalysts, with similar α-HDPO conversion, TPO yield on V1/M is ca. 14% higher than that on V2/M. Taking account of results in Figs. 2 and 3, it could be speculated that the highly dispersed vanadium species on V1/M were much more benefit for α-HDPO oxidation.
Since V2O5 species existed on the two catalysts which shown high activity towards α-HDPO oxidation. Moreover, the catalyst resulting from NH4VO3 exhibited the highest TPO yield. We wonder the performance of commercial V2O5 and NH4VO3 in this reaction. The conversion of α-HDPO and yield of TPO over V2O5 and NH4VO3 were presented in Table 1. Obviously, the activity of V2O5 and NH4VO3 for the aimed reaction was much lower. Especially for NH4VO3, TPO could not be detected after reaction. Comparing the catalytic results in Fig. 4 and Table 1, it could be concluded that V2O5 plays important roles in the oxidation of α-HDPO. Moreover, the enhanced activity of V2O5 after solid state impregnation could be due to the dispersion of V species onto MCM-41, which increased the amounts of active sites.
The reusability of V1/M catalyst was also investigated (Table 2). After the first round of reaction, the catalyst was recycled by centrifuging several times. After careful washing, drying and calcining, the recycled catalyst was reused for the next round. According to the data in Table 2, V1/M catalyst kept a good catalytic activity in the first two cycles: both α-HDPO conversion and TPO yield could be well preserved. As VO(acac)2 is unrecyclable after oxidation synthesis of TPO, these results reveal that V1/M would be a good substitute for VO(acac)2. However, an obvious drop of TPO yield in the third round could not be ignored. It might be due to the leaching of vanadium species during repeated washing. Further studies on preserving the activity of V1/M catalyst are carried out.
All catalytic results revealed that the incorporation of V species onto MCM-41 is an effective way to enhance the catalytic performance of inorganic vanadium compound in the oxidation of α-HDPO. Since VO(acac)2 is used in the synthesis of TPO in industry, the above results would give a new, inorganic and more effective catalyst for the production of TPO, which would benefit for the field of photoinitator.
4 Conclusions
In this study, the important and difficult step in synthesis of MAPOs is investigated. Vanadium based MCM-41 catalysts were used in the oxidation of α-HDPO to TPO. Catalytic results indicated that the incorporation of V onto MCM-41 is benefit for the aimed reaction and vanadium oxide might be the active site for the reaction. Based on these, inorganic vanadium compound could be used in the preparation of TPO, which is favorable in industry.
References
Scherzer T (2002) Appl Spectrosc 56:1403
Schneider LFJ, Cavalcante LM, Prahl SA et al (2012) Dent Mater 28:392
Cooke MN, Fisher JP, Dean D (2003) J Biomed Mater Res B 64B:65
Fisher JP, Holland TA, Dean D (2001) J Biomater Sci Polym Ed 12:673
Manojlovic D, Dramićanin MD, Milosevic M et al (2016) Mater Sci Eng C 58:487
Miletic V, Pongprueksa P, Brooks NR (2013) J Dent 41:918
Meereis CTW, Leal FB, Lima SG (2014) Dent Mater 30:945
Price RBT, Labrie D, Rueggeberg FA et al (2014) Dent Mater 30:1345
Liu Y, Bai X, Liu YW et al (2016) J Dent Res 95:334
Pongprueksa P, Miletic V, Janssens H et al (2014) Dent Mater 30:695
Miletic V, Pongprueksa P, Munck JD et al (2013) J Dent 41:918
Hayki N, Lecamp L, Lebaudy P (2010) Macromolecules 43:177
Oliveira DCRS., Rocha MG, Gatti A et al (2015) J Dent 43:1565
Oliveira DCRS., Rocha MG, Correa IC et al (2016) Dent Mater 32:1209
Miller RC, Miller CD, Rogers W et al (1957) J Am Chem Soc 79:424
Naghdi Z, Farzaeli R, Aliyan H (2015) Russ J Appl Chem 88:1343
Burange AS, Kale SR, Zboril R et al (2014) RSC Adv 4:6597
Atashin H, Malakooti R, Reihaneh (2014) J Chin Chem Soc 61:1039
Shaikh M, Sahu M, Gavel PK et al (2015) Catal Commun 64:18
Nazir R, Danilevicius P, Gray D et al (2013) Macromolecules 46:7239
Miletic V, Santini A (2012) J Dent 40:106
Fischer M, Hickmann E, KroppR et al (1997) US Patent 5679863A
Sun JF, Zhang L, Ge CY et al (2014) Chin J Catal 35:1347
Kim BS, Jeong CS, Kim JM et al (2016) Catal Today 265:184
Nguyen VH, Lin SD, Wu JCS (2015) J Catal 331:217
Shylesh S, Singh AP (2005) J Catal 233:359
Solsona B, Blasco T, Nieto JML et al (2001) J Catal 203:443
Acknowledgements
This work was founded by National Natural Science Foundation of China (Grant No. 21103099), Basic Research Projects in Science and Technology Program of Qingdao (13-1-4-242-jch) and Research Foundation of Qingdao Fusilin Chemical Science &Technology Co., Ltd. (FSL-RF 2016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Wang, Z., Zhao, J. et al. Effective Catalyst for Oxidation Synthesis of 2,4,6-Trimethylbenzoyldipenylphosphine Oxide: V/MCM-41. Catal Lett 148, 953–957 (2018). https://doi.org/10.1007/s10562-018-2299-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2299-2