Abstract
Three different bimetallic zinc-cobalt zeolitic imidazolate frameworks (ZIFs) were successfully synthesized at room temperature in water through a green and simple procedure and tested as catalysts for the Knoevenagel condensation reaction between p-Br-benzaldehyde and malononitrile as model reaction. The materials were characterized with various techniques (XRD, ICP-AES, SEM, N2 adsorption–desorption, TGA) and displayed excellent activities and selectivities. Moreover, the crystalline structure was preserved and the catalysts could be reused at least four times with negligible loss of activity.
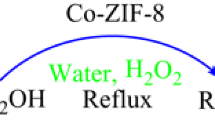
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Knoevenagel condensation reaction between a carbonyl group and a methylene-activated group is known as a powerful tool in organic chemistry to form new C–C bonds and applied widely in pharmaceutical intermediates synthesis [1]. One of the major drawbacks of the aforementioned reaction is the employment of conventional homogeneous catalyst such alkali metal hydroxides and organic bases [2, 3], with consequent difficulties in separation and recycling and production of large amounts of waste. For this reason, many efforts have been done towards the implementation of heterogeneous catalysts for this reaction with the explicit advantages in terms of ease of separation, recycling, cleaner products and minimum waste.
Many examples can be found of solid heterogeneous catalysts for the Knoevenagel condensation which include amino-functionalized mesoporous silica [4], diamine-functionalized mesopolymers [5], amine-functionalized mesoporous zirconia [6], superparamagnetic mesoporous Mg–Fe bi-metal oxides [7], mesoporous titanosilicate [8], basic MCM-41 silica [9], acid–base bifunctional mesoporous MCM-41 silica [10] and zeolites [11]. In the past few years, a new class of ordered materials has emerged. Metal organic frameworks (MOFs) have been identified as crystalline structures and widely employed for many different applications such as gas separation and storage [12,13,14,15], sensors [16], drug delivery and catalysis [17,18,19,20]. Compared to the conventional microporous and mesoporous materials, MOFs exhibited a flexible rational design, which can be controlled trough the size and functionalization of the organic linker [21, 22]. Metal organic frameworks have also been reported as active catalysts for the Knoevenagel condensation [23,24,25] Recently, a new subclass of MOFs called zeolitic imidazolate frameworks (ZIFs) have attracted considerable attention since, resembling the typical channel structure of zeolites, ZIFs can combine both properties of MOFs and zeolites in one material. Being also environmentally friendly, since their synthesis can easily be carried out in water [26, 27], these new crystalline materials are potential candidates as catalyst for industry, in which nowadays the minimization of the chemical waste is a utmost important feature to achieve.
Only limited literature employing ZIFs as active catalyst for the Knoevenagel condensation has been provided till now [28,29,30,31]. Tran et al. for example, employed the ZIF-8 for the same reaction and found that the catalytic system provided high conversion, although after the first cycle it decreased dramatically [31]. Another recent report showed the catalytic activity of ZIF-8 in the synthesis of cyanoacrylates and 3-cyanocoumarins, achieving excellent yields although higher reaction times and temperatures had been used [32]. We believe that having two different metal sites with different coordination behaviour may enhance the reaction rate, since the synergistic effect of the two metals plays a key role on the activation of the substrates. This idea is also supported by a recently published article in which a bimetallic ZIF (ZnCo-ZIF) was successfully employed for the synthesis of cyclic carbonates, starting from epoxides and carbon dioxide as substrates, outperforming all the catalysts already reported for the reaction [33]. Hence our goal was to evaluate the activity and the selectivity of three different new bimetallic Zn@ZIF-67. Excellent conversions were generated at room temperature and the Zn@ZIF-67 catalysts retained their crystalline structure and were able to be reused at least four times without significant loss of activity.
2 Results and Discussion
2.1 Characterization
The synthesis of the catalysts was achieved using cobalt nitrate and zinc nitrate as metal sources and 2-methylimidazole as ligand, following the procedure reported by Qin et al. [34] The textural properties of the three Zn@ZIF-67 were analyzed by X-ray diffraction (XRD), thermal analysis (TGA), Fourier transform infrared (FTIR), N2 adsorption–desorption and scanning electron microscopy (SEM). The metal content of Zn@ZIF-67 samples (before and after reaction) was analyzed by inductively coupled plasma atomic emission analyses spectroscopy (ICP-AES). The XRD patterns are shown in Fig. 1.
The two sharp peaks below and above 10 degrees confirms the crystallinity of the structure suggesting that the synthesized frameworks possess the same sodalite topology of ZIF-8. Furthermore, the material was homogeneously distributed and no segregation of cobalt or zinc clusters was observed, proving the fact that Zn ions can substitute Co ions in the lattice, in which the two metals are likely to interact well, since the ionic radii of Zn-ions and Co-ions are comparable (0.74 and 0.72 Å respectively). Moreover, no alteration or damage was found for the as-synthesized Zn@ZIF-67, proving the homogeneous nature of the network. The FT-IR (Fig. 2) analysis also confirmed the structural similarity between the bimetallic and monometallic ZIFs (ZIF-8 and ZIF-67).
The spectra report typical bands at 1350–1500 cm−1 for all samples that are assigned to the stretching vibration of the imidazole ring, while the bands observed in the region of 1000–1350 cm−1 are attributed to the plane vibration of the imidazole ring. The bands below 800 cm−1 are attributed to the out-of-plane vibration of the imidazole ring, whereas the bands at 400 cm−1 are assigned to the metal-N stretching.
Consistent with the XRD patterns, SEM images, depicted in Fig. 3, also proved the crystallinity of the as-synthesized ZIFs.
The resulting particles adopted a well-defined truncated rhombic dodecahedral morphology. The SEM images clearly demonstrate that the nanoparticles are homogeneously dispersed and that the crystalline morphology of the recycled samples is preserved. Preservation of the morphology and thus the composition is also confirmed by ICP-AES, showing that negligible leaching occurred during the reaction and proving that the desired metal molar ratios were successfully achieved (Table 1).
TGA-DSC analysis disclosed no substantial differences between the three Zn@ZIF-67 materials, suggesting the similarity of the networks and indicating that their crystalline structures are stable up to 400°, with a slight mass loss for the 25Zn@ZIF-67 and 50Zn@ZIF-67 due to the evaporation of the solvent molecules and possible unreacted 2-methylimidazole molecules (see Supporting information).
As expected for the N2 adsorption–desorption analysis, the properties of the three Zn@ZIF-67 materials are similar and comparable to the isotherms of ZIF-8 and ZIF-67 (Table 2).
All three as-synthesized Zn@ZIF-67 materials exposed a type I isotherm (see Supporting information), which can be related to the microporous nature of the materials. At relative high pressures very small loops are present, suggesting that the three Zn@ZIF-67 materials possess a small percentage of mesoporous domains. Interestingly, the surface area decreases with the increasing of the Zinc content. This effect may be due to the different growth of the particles in the as-synthesized materials: bigger particles lead to smaller surface areas. The highest surface area was found for 10Zn@ZIF-67, with 1881 and 2021 m2/g for BET and Langmuir respectively, which is consistent with a recent paper in which a series of bimetallic ZIFs have been synthesized [35], although the materials in this study (especially the 10 and 25%) present an higher surface area.
2.2 Catalytic Studies
In order to assess the activity of three Zn@ZIF-67 materials, the Knoevenagel condensation reaction between p-Br-benzaldehyde and malononitrile was selected as model reaction (Fig. 4).
Compared with the previous reports the substrate amount used in this study was twice the amount as has been reported [30]. The reaction was studied at room temperature and the kinetics of the reaction were monitored by withdrawing aliquots from the reaction mixture at the desired time intervals (50 µL) and analyzed by GC. The results are presented in Fig. 5 and Table 3.
A blank test without catalyst was performed to understand the reactivity of the substrates however no product could be detected. Subsequently, homogeneously catalyzed reactions with the metal salts were screened to test their activity. A conversion of 11% after 90 min was achieved when a mixture of the metal salts, Co(NO3)2·6H2O and Zn(NO3)2·4H2O was applied. Thereafter, ZIF-8 and ZIF-67 were tested in order to assess the activity of the single metal ZIFs, see Table 3. While ZIF-8 reached a conversion of 37% after 90 min, the cobalt counterpart achieved 91% conversion within the same time window. The difference in activity between ZIF-8 ad ZIF-67 can be observed already from the beginning of the reaction, at 5 and 10 min the conversion of p-Br-benzaldehyde is twice as high for ZIF-67 compared to ZIF-8. Comparing the three different Zn@ZIF-67, the best performing Zn@ZIF-67 corresponds to the molar ratio Co:Zn of 9:1, Fig. 5. Introducing the catalyst to the reaction mixture, high conversions are achieved, e.g. after the first minute 33% conversion is obtained. Within 45 min complete conversion was obtained, tackling all other systems. Considering the other two bimetallic ZIF systems, 25Zn@ZIF-67 and 50Zn@ZIF-67, the increment of the zinc content in the lattice did not cause any beneficial effect to the reaction rate, proceeding slower even though after 90 min the conversion of 90 and 83% respectively were obtained which are comparable with the 10Zn@ZIF-67. The reason of this behavior could be surmised by taking in consideration the two different electronic environments that the two metal atoms, Zn and Co, posses. It is known that Zn(II) species possess strong Lewis acid properties, while for cobalt, originating its acidity from Co(II) and Co(III) species, exerts a milder acidic behavior than the counterpart, as supported by TPD analysis reported by Chizallet et al. [36]. Yang et al. as well proved this behaviour [37] as reported for the synthesis of ethylmethylcarbonate. Exhibiting different CO2 and NH3 desorption properties, zinc showed a broader desorption peak at 440 °C, while for cobalt a sharp peak at 330° was observed. Hence, the substrate mildly coordinates to Co-species and is more apt to desorb at the end of the catalytic cycle. For this reason, increasing the percentage of zinc in the as-synthesized ZIFs decreases the rate of reaction and consequently the overall activity of the catalyst.
2.3 Effect of the Solvent
The influence of the solvent was also investigated since heterogeneous catalyzed reactions can be the extremely sensitive to the solvent [38, 39]. Four different solvents with increasing polarity were selected [toluene, tetrahydrofuran (THF), methanol (MeOH), dimethylsulfoxide (DMSO)]. The obtained results are displayed in Fig. 6.
Different results are reported in literature regarding the solvent influence on the condensation reaction. For example, MacQuarrie and co-workers reported that non-polar solvents resulted in the optimum catalytic performance [40], while Gaston et al. reported an enhanced reaction rate using polar solvents, while non-polar solvents had a negative effect on the overall activity of the catalyst [41, 42]. In these work the obtained results indicate that a higher reaction rate is attained when the polarity of the solvent increased with the following observed trend in catalytic activity: toluene < THF < MeOH ≅ DMSO. This effect can be rationalized taking in consideration the nature of the catalyst and the mechanism of the reaction. Since Zn@ZIF-67 is hydrophobic, increasing the polarity of the reaction medium may encourage the polar substrates to leave the catalyst and transfer into the reaction media. On the other hand, the reaction mechanism is based on a nucleophilic addition–elimination following the SN1 path in which the carbocation and the nucleophile are stabilized by the solvent hydrogen bonds. For this reason, since these two effects are synergistic, the reaction readily occurs in MeOH and DMSO, achieving quantitative conversion after 15 min. It is noteworthy to mention that even though the conversions are comparable, a higher reaction rate was achieved using DMSO (75% conversion after 1 min) and could be due to the solvatation of the substrate, which in MeOH is much more sterically hindered and therefore less reactive, see Fig. 7.
From the comparison of the obtained TOFs using different solvents, it is obvious that a higher reaction rate is achieved applying DMSO. Therefore, a polar solvent could stabilizes the carbocation and enhances the nucleophilicity of the malononitrile. However, the issue is still under further investigations to confirm the trend.
2.4 Effect of the Substrate
Kinetic plots for the Knoevenagel condensation of different substrates in toluene catalyzed by 10Zn@ZIF-67. Having determined the influence of the polarity of different solvents, the catalytic performance of Zn@ZIF-67 applying different substrates bearing both electron-withdrawing (activating) and electron-donating (deactivating) groups was investigated. A series of different substrates in combination with malononitrile were employed for the Knoevenagel condensation and the obtained results are given in Table 4.
The kinetic plots given in Fig. 8 demonstrate an unexpected behavior for the different substrates that can be explained based on the different substituents present on the phenyl ring, see Table 5.
Since electron-withdrawing and electron-donating groups are present on the applied substrates the different effect these moieties exert on the carbonylic carbon has to be considered. Firstly the electronegativity plays an important role on the activation of the substrate. A more electronegative substituent present on the phenyl ring of the substrate will result in a higher polarization of the electronic cloud and consequently drained the electron density from the aldehyde carbon, which becomes more electrophilic and apt to react with the activated methylene group of the malononitrile. Secondly, the presence of a hydroxyl group e.g. salicylaldehyde affects the reaction rate since the uncoordinated metal sides present in the framework can coordinate with the oxygen atoms and hinders the nucleophile attack on the carbonyl group. A comparison of 10Zn@ZIF-67 with other reported heterogeneous catalysts clearly demonstrates that nearly all of them require higher temperature and all the reported systems necessitate a longer reaction time to achieve good yields. A more detailed study of Table 4 reveals that the reported metal organic frameworks all demand the presence of basic moieties such as primary amines to be able to activate the methylene nucleophile, while for the new catalyst, 10Zn@ZIF-67 the synergistic role played by the uncoordinated metal nodes (defects) and the uncoordinated imidazole ligands on the activation of the malononitrile requires milder basic conditions to exert the catalytic activity.
2.5 Recyclability Studies
When employing a solid catalyst, a crucial point that needs to be taken in consideration is whether possible species could migrate into the reaction medium and affects the reaction rate by homogeneous contributions. Hence, the catalyst was recovered and reused to test its stability. 10Zn@ZIF-67 can catalyze the Knoevenagel condensation reaction at least four times without significant loss of activity. This recycling study demonstrates that high conversion can still be achieved and no homogeneous leached species contributes to the catalytic performance. Furthermore, the crystalline structure of 10Zn@ZIF-67 was retained, as confirmed by the XRD (see Supporting information), SEM and ICP analysis. The results of the recycling study are depicted in Fig. 9.
2.6 Mechanism
To interpret the mechanism of the Knoevenagel condensation between p-Br-benzaldehyde and malononitrile, the external active sites are to be taken in consideration, as already reported by Chizallet and coworkers. [36] They found that at ambient temperature and pressure, the active sites located on the external surface are likely to be Zn(II) and Zn(III) together with OH and NH groups. Therefor in this study, it is reasonable to believe that the reaction occurs on the uncoordinated surface species, which are readily available to react in the presence of the substrate. Consequently, a plausible mechanism could involve the interaction between the Co and the Zn species in a synergistic adsorption of the aldehyde compound through the coordination of the oxygen, which attracts the electronic density and activates the carbonyl carbon making it partially positively charged and apt it to the nucleophilic attack of the malononitrile. The dicyano derivative, on the other hand, is activated by the coordination of the metal ions with the N atoms of the malononitrile, inducing the deprotonation of the –CH2– by non-coordinated 2-methylimidazole ligands, in which the α-H of the methylene group is much more positively charged and exhibit stronger acidity [43]. The coordination of the aldehyde would occur most likely on the zinc atoms, since the coordination bond that is generated between the d orbitals and the p orbitals of the substrates is much stronger than with the cobalt, as proved by the study of Gao and coworkers [37]. Finally the last step occurs, and while losing a molecule of water, simultaneously the C=C bond is formed, releasing the product and closing the catalytic cycle (Fig. 10).
3 Experimental
All chemicals were purchased from Aladdin Chemical Co. and Sigma-Aldrich and used without any further purification unless otherwise noted.
3.1 Zn-ZIF-67 Synthesis
Zn@ZIF-67 was synthesized at room temperature according to the procedure reported for ZIF-67 [34] using zinc nitrate tetrahydrate [Zn(NO3)2·4H2O] cobalt nitrate hexahydrate [Co(NO3)2·6H2O], and 2-methylimidazole as ligand. The desired amount of cobalt nitrate was dissolved in 8 mL of ultrapure H2O and mixed with a solution of Zn(NO3)2·4H2O in 8 mL of ultrapure H2O, obtaining three different final molar ratios Zn:Co of 1:9; 2.5:7.5; 5:5 respectively. Another solution of 67 mmol of 2-methyl imidazole in 8 mL of water was prepared and subsequently added to the metal solution resulting in a instant precipitation of the product. The mixture was then stirred vigorously for 4 h. Afterwards, the precipitates were separated by centrifugation at 8000 rpm and washed with methanol three times. The solid crystals were dried at room temperature in a vacuum oven for 12 h. The catalyst was activated at 150 °C, for 3 h under vacuum before use.
3.2 Catalytic Studies
In a typical procedure reported by Phan’s research group [30] (although the quantities have been doubled in order to overcome aliquots withdrawn problems during the reaction), 3.8 mmol of p-Br-benzaldehyde was transferred in a two-neck round bottom flask and dissolved in 5 mL of toluene. Another solution containing 7.6 mmol of malononitrile in 5 mL of toluene was prepared and transferred in the previous solution achieving a total volume of 10 mL. After the introduction of 2.5 mol% of catalyst based on the aldehydes substrates (the initial time for the reaction) the mixture was let to react for 90 min while aliquots were withdrawn at different time intervals and analyzed them by GC. At the end of the reaction the catalyst was then separated by centrifugation and washed several times with methanol and dried at 80 °C under vacuum.
3.3 Characterization Methods
The characterization of Zn@ZIF-67 was performed by XRD (Bruker D8 advance diffractometer, Bragg–Brentano geometry) using a Cu Kα radiation source (λ = 1.54056 Å) at 40 kV and 45 Ma. The data were collected at a 5° s−1 scanning speed. Gas adsorption–desorption isotherms and pore size distributions of products were measured using the volumetric method on a Micrometrics instrument (ASAP 2020 analyzer). N2 and CO2 gas with purity of 99.999% was applied for the measurements. For degassing of the samples, the samples were evacuated at 200 °C under vacuum for 200 min. The micropore surfaces were analyzed using the Brunauer–Emmett–Teller (BET) and Langmuir methods. The linearized BET and Langmuir equations were fit to data within the range 0.003 < P/P0 < 0.05. The morphology of Zn-ZIF-67 was measured using a FE-SEM (FEI Nova 600). Thermal analysis (TGA) was performed on a Netzsch STA 409 thermal analyzer. A certain amount of compound was placed inside a crucible and heated from room temperature to 800 °C under a flow of nitrogen gas. The FI-IR was performed with a Bruker Vertex 80 V FT-IR spectrometer. Substrate conversions were determined by GC (Agilent) with a HP-5 column and a flame ionization detector; the temperature program for the GC analysis was as follow: 60–150 °C at 20° min−1, at 150 °C the T was held for 1 min and then heated from 150 to 160 °C at 1° min−1 and held for 1 min.
4 Conclusions
In summary, it is demonstrated in this study that three different bimetallic Zn@ZIF-67 are highly potent heterogeneous catalyst for the Knoevenagel condensation between benzaldehyde derivatives and malononitrile. The catalysts were tested using p-Br-benzaldehyde as model substrate and displayed excellent conversion and selectivity. Furthermore, the catalytic materials could be recycled up to at least four times without considerable loss of activity while maintaining their crystalline structure. In comparison with other different reported catalysts, the Zn@ZIF-67 materials exhibited higher reaction rates and excellent activities, which make them potent candidates to be employed in organic chemistry to achieve the synthesis of new carbon–carbon bonds.
References
Simon C, Constantieux T, Rodriguez J (2004) Utilisation of 1,3-dicarbonyl derivatives in multicomponent reactions. Eur J Org Chem 2004:4957–4980
Balalaie S, Bararjanian M (2006) Tetra-n-butylammonium hydroxide (TBAH)–catalyzed Knoevenagel condensation: a facile synthesis of α-cyanoacrylates, α-cyanoacrylonitriles, and α-cyanoacrylamides. Synth Commun 36:533–539
Dalessandro EV, Collin HP, Valle MS, Pliego JR (2016) Mechanism and free energy profile of base-catalyzed Knoevenagel condensation reaction. RSC Adv 6:57803–57810
Mondal J, Modak A, Bhaumik A (2011) Highly efficient mesoporous base catalyzed Knoevenagel condensation of different aromatic aldehydes with malononitrile and subsequent noncatalytic Diels–Alder reactions. J Mol Catal A 335:236–241
Xing R, Wu H, Li X, Zhao Z, Liu Y, Chen L, Wu P (2009) Mesopolymer solid base catalysts with variable basicity: preparation and catalytic properties. J Mater Chem 19:4004–4011
Parida KM, Mallick S, Sahoo PC, Rana SK (2010) A facile method for synthesis of amine-functionalized mesoporous zirconia and its catalytic evaluation in Knoevenagel condensation. Appl Catal A 381:226–232
Gao Z, Zhou J, Cui F, Zhu Y, Hua Z, Shi J (2010) Superparamagnetic mesoporous Mg–Fe bi-metal oxides as efficient magnetic solid-base catalysts for Knoevenagel condensations. Dalton Trans 39:11132–11135
Karmakar B, Chowdhury B, Banerji J (2010) Mesoporous titanosilicate Ti-TUD-1 catalyzed Knoevenagel reaction: an efficient green synthesis of trisubstituted electrophilic olefins. Catal Commun 11:601–605
Parida KM, Rath D (2009) Amine functionalized MCM-41: an active and reusable catalyst for Knoevenagel condensation reaction. J Mol Catal A 310:93–100
Shang F, Sun J, Wu S, Yang Y, Kan Q, Guan J (2010) Direct synthesis of acid–base bifunctional mesoporous MCM-41 silica and its catalytic reactivity in deacetalization–Knoevenagel reactions. Microporous Mesoporous Mater 134:44–50
Chaves TF, Carvalho KTG, Urquieta-González EA, Cardoso D (2016) One-step synthesis of functionalized ZSM-12 zeolite as a hybrid basic catalyst. Catal Lett 146:2200–2213
Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, Yazaydin AÖ, Snurr RQ, O’Keeffe M, Kim J, Yaghi OM (2010) Ultrahigh porosity in metal-organic frameworks. Science 329:424–428
Kaye SS, Dailly A, Yaghi OM, Long JR (2007) Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3 (MOF-5). J Am Chem Soc 129:14176–14177
Chae HK, Siberio-Perez DY, Kim J, Go Y, Eddaoudi M, Matzger AJ, O’Keeffe M, Yaghi OM (2004) A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427:523–527
Tranchemontagne DJ, Ni Z, O’Keeffe M, Yaghi OM (2008) Reticular chemistry of metal–organic polyhedra. Angew Chem Int Ed 47:5136–5147
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C, Chang J-S, Hwang YK, Marsaud V, Bories P-N, Cynober L, Gil S, Ferey G, Couvreur P, Gref R (2010) Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater 9:172–178
Yoon M, Srirambalaji R, Kim K (2012) Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem Rev 112:1196–1231
Valvekens P, Vermoortele F, De Vos D (2013) Metal-organic frameworks as catalysts: the role of metal active sites. Catal Sci Technol 3:1435–1445
Dhakshinamoorthy A, Opanasenko M, Cejka J, Garcia H (2013) Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal Sci Technol 3:2509–2540
Corma A, García H, Llabrés i Xamena FX (2010) Engineering metal organic frameworks for heterogeneous catalysis. Chem Rev 110:4606–4655
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276–279
Rowsell JLC, Yaghi OM (2004) Metal–organic frameworks: a new class of porous materials. Microporous Mesoporous Mater 73:3–14
Hartmann M, Fischer M (2012) Amino-functionalized basic catalysts with MIL-101 structure. Microporous Mesoporous Mater 164:38–43
Luan Y, Qi Y, Gao H, Andriamitantsoa RS, Zheng N, Wang G (2015) A general post-synthetic modification approach of amino-tagged metal-organic frameworks to access efficient catalysts for the Knoevenagel condensation reaction. J Mater Chem A 3:17320–17331
Burgoyne AR, Meijboom R (2013) Knoevenagel condensation reactions catalysed by metal-organic frameworks. Catal Lett 143:563–571
Kida K, Okita M, Fujita K, Tanaka S, Miyake Y (2013) Formation of high crystalline ZIF-8 in an aqueous solution. CrystEngComm 15:1794–1801
Jian M, Liu B, Liu R, Qu J, Wang H, Zhang X (2015) Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv 5:48433–48441
Zhang G, Zhang T, Zhang X, Yeung KL (2015) Continuous flow ZIF-8/NaA composite membrane microreactor for efficient Knoevenagel condensation. Catal Commun 68:93–96
Li Q, Jiang S, Ji S, Ammar M, Zhang Q, Yan J (2015) Synthesis of magnetically recyclable ZIF-8@SiO2@Fe3O4 catalysts and their catalytic performance for Knoevenagel reaction. J Solid State Chem 223:65–72
Nguyen LTL, Le KKA, Truong HX, Phan NTS (2012) Metal-organic frameworks for catalysis: the Knoevenagel reaction using zeolite imidazolate framework ZIF-9 as an efficient heterogeneous catalyst. Catal Sci Technol 2:521–528
Tran UPN, Le KKA, Phan NTS (2011) Expanding applications of metal–organic frameworks: zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the Knoevenagel reaction. ACS Catal 1:120–127
Kolmykov O, Chebbat N, Commenge J-M, Medjahdi G, Schneider R (2016) ZIF-8 nanoparticles as an efficient and reusable catalyst for the Knoevenagel synthesis of cyanoacrylates and 3-cyanocoumarins. Tetrahedron Lett 57:5885–5888
Zanon A, Chaemchuen S, Mousavi B, Verpoort F (2017) Zn-doped ZIF-67 as catalyst for the CO2 fixation into cyclic carbonates. J CO2 Util 20:282–291
Qian J, Sun F, Qin L (2012) Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater Lett 82:220–223
Zaręba JK, Nyk M, Samoć M (2016) Co/ZIF-8 heterometallic nanoparticles: control of nanocrystal size and properties by a mixed-metal approach. Crystal Growth Design 16:6419–6425
Chizallet C, Lazare S, Bazer-Bachi D, Bonnier F, Lecocq V, Soyer E, Quoineaud A-A, Bats N (2010) Catalysis of transesterification by a nonfunctionalized metal–organic framework: acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J Am Chem Soc 132:12365–12377
Yang L, Yu L, Sun M, Gao C (2014) Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the synthesis of ethyl methyl carbonate. Catal Commun 54:86–90
Langhendries G, De Vos DE, Baron GV, Jacobs PA (1999) Quantitative sorption experiments on Ti-zeolites and relation with α-olefin oxidation by H2O2. J Catal 187:453–463
Phan NTS, Jones CW (2006) Highly accessible catalytic sites on recyclable organosilane-functionalized magnetic nanoparticles: an alternative to functionalized porous silica catalysts. J Mol Catal A 253:123–131
Macquarrie DJ, Jackson DB (1997) Aminopropylated MCMs as base catalysts: a comparison with aminopropylated silica. Chem Commun 1781–1782
Gascon J, Aktay U, Hernandez-Alonso MD, van Klink GPM, Kapteijn F (2009) Amino-based metal-organic frameworks as stable, highly active basic catalysts. J Catal 261:75–87
Juan-Alcañiz J, Ramos-Fernandez EV, Lafont U, Gascon J, Kapteijn F (2010) Building MOF bottles around phosphotungstic acid ships: one-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts. J Catal 269:229–241
Položij M, Rubeš M, Čejka J, Nachtigall P (2014) Catalysis by dynamically formed defects in a metal–organic framework structure: Knoevenagel reaction catalyzed by copper benzene-1,3,5-tricarboxylate. ChemCatChem 6:2821–2824
Acknowledgements
The authors are grateful to the State Key Lab of Advanced Technology for Materials Synthesis and Processing for financial support (Wuhan University of technology). S. C. appreciates the National Natural Science Foundation of China (No. 21502146), The Fundamental Research Funds for the Central Universities (WUT: 2016IVA092) and the Research Fund for the Doctoral Program of Higher Education of China (471-40120222).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zanon, A., Chaemchuen, S. & Verpoort, F. Zn@ZIF-67 as Catalysts for the Knoevenagel Condensation of Aldehyde Derivatives with Malononitrile. Catal Lett 147, 2410–2420 (2017). https://doi.org/10.1007/s10562-017-2153-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2153-y