Abstract
Spark discharge plasma is an efficient means to activate methane. In this work methane was converted to olefins via a two stage recycle-plasma-catalyst reactor. A recycle spark discharge plasma reactor was employed in the first stage to actively convert methane to a mixture comprising mainly acetylene and hydrogen. The mass transfer in the pulsed spark discharge channel was strengthened by recycling part of plasma effluent to the entrance, which improved the reactivity and energy efficiency of the plasma reactor. Pd and Ni based MgAl2O4 supported catalysts were employed in the second stage to selectively hydrogenate acetylene in the plasma reactor effluent. With the conversion of methane of 73 % the integration of recycle plasma and catalyst reactor may obtain a ethylene yield of 55 and 35 % on 0.3 wt% Pd-0.6 wt% Ag/MgAl2O4 and 2.5 wt% Ni-7.5 wt%-Zn/MgAl2O4 catalysts, respectively. Other than ethylene about 23 % of methane was transformed to C3–C5 light olefins over cheap 2.5 wt% Ni-7.5 wt% Zn/MgAl2O4 catalysts.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With abundant reserves natural gas comprising mainly methane will substitute for diminishing petroleum as a dominant energy source in the near future. However, due to its chemical inertness, efficient utilization of natural gas, i.e. conversion of methane into useful chemicals and transportation fuels, is still a challenge. In this field many research work and accomplishments in both industry and academia have been fulfilled. Gas to liquid process via a syngas intermediate generated by reforming of methane by various means such as partial oxidation, steam reforming and autothermal reforming have been established in industry [1–3]. This process needs to operate at a large scale to gain economic benefits. It was estimated that the synthesis of syngas accounts for about 50–60 % of the total investment cost. Various means to activate methane efficiently have stimulated researchers much interest worldwide. Direct activation of methane has been reported by means of pyrolysis, oxidative coupling, plasma, chlorination/oxychlorination, etc. [4–6]. Pyrolysis of methane subjects methane to very high temperature up to 2500 K to crack it into acetylene and hydrogen. The drawbacks of the process are highly energy intensive and problems associated with coke formation due to high temperature in the cracker. Oxidative coupling of methane to ethylene suffers from low yield of ethylene due to low conversion of methane. The single pass conversion of methane is around 20 % with the selectivity for ethylene of about 70 %. Oxychlorination employs chloride to activate methane. In the first step methane is oxychlorinated to methyl chloride and the resulting methyl chloride is converted in the second stage to gasoline range hydrocarbons over ZSM-5 catalyst. This process suffers from a number of disadvantages such as multiple reaction processes, formation of unwanted polychloromethanes as well as corrosion problems associated with HCl and chloromethanes. Recently, methane activation by non-thermal plasmas such as corona discharge, spark discharges, dielectric barrier discharges (DBD) have been investigated at atmospheric pressure and ambient temperature [7–9]. It was reported that plasma discharge techniques have a great effect on methane conversion and product selectivities. Jeong et al. studied methane conversion by pulsed DC DBD [7]. The maximum methane conversion was about 25 % and ethane selectivity was about 70–80 %. The effect of alumina pellets filled in gas gap was also discussed and it was found that they played a role in enhancing ethane selectivity. Zhu et al. studied methane conversion to C2 hydrocarbons and hydrogen by different discharge techniques at atmospheric pressure and ambient temperature [8]. In the streamer discharge and pulsed spark discharge processes, acetylene is the dominant C2 product. The highest yields of acetylene and H2 reach up to 54 and 51 % respectively at methane conversion of 69 % on a needle-to-plate reactor under pulsed spark discharges. In the DBD processes, ethane is the major C2 products and the pulsed DC DBD process provides the highest ethane yield. Recently, Zhu et al. reported the conversion of methane to ethylene in a plasma followed by catalyst reactor [9]. A packed bed of Pd-Ag/SiO2 catalyst was used after plasma reactor to hydrogenate acetylene to ethylene. Kado et al. investigated the activation of methane using low temperature plasmas such as DBD, corona and spark discharge. The energy efficiency in spark discharge was much higher than that in DBD and corona discharge. In point to point spark discharge, acetylene was produced with the selectivity higher than 85 % and small amount of deposited carbon [10]. Light olefins such as ethylene, propylene and butene are major building block of petrochemical industry. Transformation of methane to these light olefins is highly desirable.
In this work methane was converted by a recycle spark discharge plasma reactor to a gas mixture comprising mainly acetylene and hydrogen. To obtain highly desirable olefins a selective hydrogenation reactor was combined with methane plasma activation. Selective hydrogenation of acetylene in a C2 cut from a thermal cracking unit to ethylene is an established process in industry [11]. Pd/alumina eggshell catalysts are widely used in industry. In view of the high price of Pd, in this work Ni was also investigated. A Ni-containing hydrogenation catalyst, promoted with a second metal Zn, was synthesized in this work. Without providing extra hydrogen acetylene could be selectively hydrogenated on both Pd-Ag/MgAl2O4 and Ni-Zn/MgAl2O4 catalyst to olefins by using hydrogen generated by plasma reactor. It was observed that the integration of plasma activation and selective catalytic hydrogenation is a good means to achieve highly efficient conversion of methane to olefins.
2 Experimental
2.1 Catalyst Synthesis
Methane (99.99 %) was purchased from Xinyuan Gas Company. Magnesium aluminate spinel, MgAl2O4, was prepared through the thermal decomposition of hydroxides co-precipated from aqueous solutions of magnesium and aluminum nitrates [12]. The starting materials used were magnesium nitrate hexahydrate, Mg(NO3)2 6H2O (Sinopharm 99.9+ %) and aluminum nitrate nonahydrate, Al2(NO3)3 6H2O (Sinopharm 99.0+ %). In a typical preparation, stoichiometric amount of magnesium nitrate and aluminum nitrate were dissolved in deionized water to make a mixture solution. The precipitant, a 25 wt% NH4OH solution, was then added dropwise to the nitrate solution under continuous stirring, and pH value of the final suspension was adjusted to 9. After precipitation, the slurry was stirred for another 30 min and then refluxed at 80 °C for 24 h. The mixture was then cooled to room temperature, filtered and washed with de-ionized water. The final product was dried at 110 °C for 12 h and calcined at 600 °C for 5 h.
MgAl2O4 supported metal catalysts, 0.3 wt% Pd-0.6 wt% Ag/MgAl2O4 and 2.5 wt% Ni-7.5 wt% Zn/MgAl2O4, were prepared by incipient wetness impregnation method. The impregnation solution contained both metal precursors such that the metals were preferably applied to the support MgAl2O4 together at the same time. The impregnated samples were dried at 110 °C overnight before calcination in static air at 450 °C for 4 h.
The structure properties of the samples were investigated by X-ray diffraction (XRD, X’Pert Pro MPD) using a CuKa monochromatized radiation source and a Ni filter in the range of 2θ = 5–75°. The content of crystalline phases was evaluated from the relative intensity of the strongest diffraction peaks of standard pure materials. The surface areas (BET) were determined by nitrogen adsorption at −196 °C using an automated gas adsorption analyzer (ASAP2020-M). The pore size distribution was calculated from the adsorption branch of the isotherm by the Barrett, Joyner and Halenda (BJH) method.
2.2 Reactor Setup
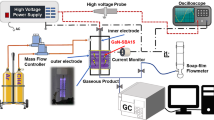
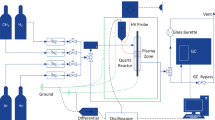
Figure 1 shows the plasma-catalyst reactor setup, which consists of gas feed, recycle plasma-catalyst two stage reactor and product analysis sections. The gas feeds (CH4, H2, N2 and air) were controlled by mass flow controllers. H2, N2 and Air were used for the regeneration of the deactivated catalysts. The plasma reactor was made of a quartz tube of 22 mm o.d. × 18 mm i.d. × 120 mm long. A 1.5 mm o.d. × 1.0 mm i.d. ss tube were used as the high voltage electrode. A rotary ss plate with a diameter of 16 mm and a thickness of 3 mm was used as the ground electrode. Multiple grooves were made on the plate to improve mixing of reactant in the discharge channel. The distance from the needle to the plate was fixed at 7 mm. The high-voltage power supply source, model CTP-2000 of Corona Lab, was used to generate pulsed spark discharges between the two electrodes. The discharge power was measured via the area of the voltage/charge Lissajous figures on an oscilloscope (TDS1012B-SC, Tektronix). A recycle pump was installed at the outlet to provide a means to recycle part of the effluent back to the entrance of the plasma reactor. The recycle ratio can be adjusted by a control valve mounted at the pump outlet. The plasma reactor effluent was directed to the downstream catalytic reactor, which was a 15 mm o.d. × 12 cm i.d. × 400 mm long ss tube reactor packed with catalyst. The reactor axial temperature profile was monitored by a sliding thermocouple inserted into the catalyst bed.
A N2 flow added into the catalytic reactor effluent was used as an internal standard for methane and hydrocarbon products quantification. The reaction products were analyzed by two online gas chromatographs (GC). One HP5890 GC equipped with a flame ionization detector (HP PLOT/A12O3 capillary column) and a thermal conductivity detector (5A packed column, N2 carrier gas) was used to analyze hydrocarbon components up to C12 and hydrogen. Another GC equipped with a thermal conductivity detector (13X column, H2 carrier gas) was used to analyze methane and nitrogen. The combination of a 6-way and an 8-way valves were used to accomplish the work of sampling, injection and back flushing off C2+ components during TCD analysis. For accurate gas chromatography analysis, the transfer line from the reactor exit to the gas chromatograph and the sampling valve were heat traced to keep the reactor effluent in gas state.
Based on mass balance of carbon and hydrogen, the conversion of methane (Xm) and the selectivities of individual hydrocarbon products (SHC) were calculated using the internal standard method as follows.
Hydrogen was quantified by the external standard method.
In the above equations F in and F out denote the molar flow rate at the inlet and outlet of the reactor, respectively, and C represents molar fraction.
As the hydrogenation reaction is highly exothermic, 0.5 g catalyst was diluted with 5 g inert α-Al2O3 to keep the packed bed isothermal during the reaction. Prior to reaction, catalysts were activated by first removal of moisture at 500 °C for 4 h, and then was reduced by a mixture of hydrogen and nitrogen at molar ratio of 1:9 at 500 °C for 4 h.
3 Results and Discussion
3.1 Plasma Activation of Methane
3.1.1 Effect of Spark Discharge Power
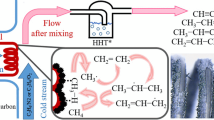
The effect of spark discharge power on methane activation was investigated by varying the plasma power input at pure methane flow rate of 40 ml/min. As shown in Fig. 2, the conversions of methane increases from 27 to 72 % with increasing spark discharge power input from 8.0 to 24.0 W. With the increase of input energy more methane molecule may be activated and decomposed to radicals, resulting in the increased conversion. It is observed that acetylene is the dominant product with selectivity up to 85 % at 8.0 W. With the increase of plasma power input the selectivity for acetylene decreases slightly. High discharge power generates a strong electric field in the plasma reactor, which may accelerate electrons to a high energy in a short distance. Methane molecule is thus activated by collision with high energy electrons, which sequentially decomposes to CH3 ·, CH2 ·, CH· and hydrogen radicals as follows [13]:
The rates of these dehydrogenation reactions are very fast, which leads to the high concentration of carbon. The concentration of CH formed by hydrogenation of atomic carbon and C2 formed by coupling of atomic carbon are considered to be high. Both them are the precursor of acetylene, which give high selectivity for C2H2. It is shown that other than acetylene small amounts of C3–C5 light hydrocarbons are formed. Along with the formation of unsaturated hydrocarbons, e.g. acetylene, ethylene and propylene, hydrogen is also produced. It is shown in Fig. 3 that the hydrogen yield at methane conversion of 72 % reaches up to 51 %. The co-product, hydrogen free of oxygen, in this process is highly desirable for proton exchange membrane fuel cell [14, 15].
3.1.2 Effect of Recycle Ratio
The effluent from the plasma reactor was returned back to the entrance at different recycle ratio, which was defined as the ratio of the volumetric flow rate of the stream returned to the reactor entrance to the stream leaving the system. Although the feed rate of methane to the plasma reactor was fixed at 40 ml/min, the increase of the recycle ratio strengthened the mass transfer of reactants and improved the contacting between reactants and discharge channel. The reactor may be model as a continuously stirred tank reactor. The larger the recycle ratio, the better is the mixing ability. Methane was activated by spark discharge between high voltage needle and ground plate. It was observed that the maximum diameter of the discharge channel was estimated to be 1.5–2.5 mm. This indicated that some of methane might pass through the discharge region without contacting the discharge channel. Therefore, the improvement of the contacting between reactants and discharge channel increased the reacting ability of methane. As shown in Fig. 4, the conversion of methane increases from 66 to 74 % with increasing the recycle ratio from 0 to 4, then starts to decrease to 69 % with further increasing the recycle ratio to 6. The activation of methane by pulse spark discharge plasma consists of a complex reaction network, involving various carbon-containing radicals, hydrogen radical and short-chain hydrocarbons. The maximum formation of C2H2, a intermediate, has an optimum recycle ratio for this type of reaction network. The energy cost for methane conversion decreases from 10.6 ev/molecule with no effluent recycle to 9.3 ev/molecule with optimal recycle ratio of 4. The energy efficiency of pulsed spark discharge plasma reactor was improved by the means of recycling effluent to the entrance of the reactor.
3.2 Selective Hydrogenation of Acetylene
Acetylene and hydrogen were the dominant product in the recycle spark discharge plasma reactor. In view of the co-existence of acetylene and hydrogen in the product it is possible to transform acetylene and hydrogen to value-added olefins via selective hydrogenation reaction on a hydrogenation catalyst without any extra addition of hydrogen from outside sources. So, the integration of plasma and selective hydrogenation reactors are investigated in this work.
As acid sites could catalyze oligomerization of alkyne and alkene, α-alumina support, free of acid sites, is widely used in selective hydrogenation to avoid oligomerization of olefins. MgAl2O4 spinel has gained much interest as support due to its chemical inertness, high thermal stability and mechanical resistance in heterogeneous catalysis. In this work MgAl2O4 was synthesized by co-precipitation of a mixed solution of MgSO4 and Al(NO3)3 with fixed Mg2+/Al3+ ratio using diluted NH4OH solution. It was found that the contacting pattern of these two solutions has certain effect on the purity of MgAl2O4 phases synthesized. As shown in Fig. 5, samples a and b synthesized by addition of precipitant NH4OH solution to the mixed solution of MgSO4 and Al(NO3)3 gave pure MgAl2O4 spinel phase only. Whereas, besides MgAl2O4 phase, trace amounts of MgO phase was also generated when the addition sequence was reversed in the case of sample c. So sample a was chosen as support for the preparation of selective hydrogenation catalyst. It was characterized by BET that sample a has a surface area of 37.02 m2/g and a pore volume of 0.25 ml/g, respectively.
3.2.1 Selective Hydrogenation on Pd-Ag/MgAl2O4 Catalysts
The operating conditions were fixed for recycle plasma reactor, whereas, the effluent was directed to a second catalytic reactor, which was packed with selective hydrogenation catalyst to convert acetylene to valuable olefins selectively. The selective hydrogenation of acetylene in the effluent of the recycle plasma reactor was investigated firstly on 0.3 wt% Pd-0.6 wt% Ag/MgAl2O4 catalyst under temperature in the range from 75 to 150 °C. It is observed in Fig. 6 that the conversion of methane remains almost constant with temperature. With the combination of hydrogenation reaction at 75 °C the selectivity for acetylene decreases dramatically from 83 % with plasma alone to 36 %. In the meantime, the selectivity for ethylene increases from 1 to 36 %. The selectivity for acetylene decreases further from 36 % to 0 with increasing temperature from 75 to 125 °C, then levels off. Whereas, the selectivity for ethylene increases from 43 to 76 %. With the increase of temperature, the selectivity for ethane only slightly increased from 3 to 4 %. The catalyst is highly selective with most acetylene converted to ethylene. The integration of plasma and hydrogenation reactor is very efficient to convert methane to ethylene. The yield of ethylene can reach 55 % at methane conversion of 73 %.
3.2.2 Selective Hydrogenation on Ni-Zn/MgAl2O4 Catalysts
The disadvantage of Pd-containing catalysts is the price of Pd. There is a need for a commercially attractive and efficient selective catalyst. Ni has been widely used as hydrogenation catalyst, and it has been reported in literature that significant improvements in the selectivity to ethylene over Ni/MgAl2O4 resulted from the addition of promoters, Zn [16]. In this work we investigated MgAl2O4 supported Ni catalyst and adjusted its hydrogenation performance by alloying with a second metal, Zn. The catalyst was changed from 0.3 wt% Pd-0.6 wt% Ag/MgAl2O4 to 2.5 wt% Ni-7.5 wt% Zn/MgAl2O4 catalyst, whereas the feed to the catalytic reactor was the same as before. Figure 7 shows the effect of reaction temperature on the selective hydrogenation of acetylene in the effluent of recycle plasma reactor. The selectivity for acetylene decreases dramatically from 83 % in the recycle plasma reactor to 37 % with the combination of hydrogenation reaction at 125 °C. In the meantime, the selectivity for ethylene increases from 1 to 24 %. The selectivity for acetylene decreases further from 37 to 1 % with increasing temperature from 125 to 175 °C, while the selectivity for ethylene increases from 24 to 48 %. Other than selective formation of ethylene, selectivity for C3–C5 hydrocarbons increases from 7 to 39 %, probably due to the dimerization of ethylene and propylene. The optimal hydrogenation temperature for 2.5 wt% Ni-7.5 wt% Zn/MgAl2O4 catalyst is 175 °C, about 25 °C higher than that on Pd-Ag/MgAl2O4. It is shown in Fig. 8 that the selectivities for C3, C4 and C5 olefins at 175 °C are 4, 23 and 4 %, respectively, leading to the sum of light olefin selectivity in C3–C5 up to 31 %. In sum, the integration of recycle plasma reactor and hydrogenation catalyst MgAl2O4 leads to the yields of ethylene and C3–C5 olefins of 35 and 23 %, respectively.
4 Conclusion
It was observed that pulsed spark discharge was an efficient means to convert methane into acetylene and hydrogen. A recycle plasma reactor was employed to activate methane in this work. The recycling of part of spark discharge reactor effluent to the entrance strengthened mass transfer and improved contacting between reactants and pulsed spark discharge channel, which improved the reactivity of methane. It was found that both spark discharge power input and recycle ratio affected the conversion of methane. The conversion of methane increased from 66 % with no effluent recycle to 74 % at the optimal recycle ratio of 4.0. With a selectivity of acetylene of around 85 %, the conversion of methane varied from 27 to 74 %. The yield of hydrogen free of COx, varied from 20 to 52 % depending on conversion, was also highly desirable for PEM fuel cell.
Without extra addition of hydrogen acetylene in the effluent from recycle plasma reactor was hydrogenated selectively to olefins over two kinds of Pd and Ni based MgAl2O4 supported catalyst. It was observed that 0.3 wt% Pd-0.6 wt% Ag/MgAl2O4 exhibited superior selectivity for ethylene in the hydrogenation of acetylene. The yield of ethylene in the integrated recycle plasma catalyst reactor reached up to 55 % at a conversion of methane of 73 %. A much cheaper 2.5 wt% Ni-7.5 wt% Zn/MgAl2O4 catalyst showed good selectivity for ethylene and other C3–C5 light olefins in the hydrogenation of acetylene in the effluent of recycle plasma reactor. The yield of ethylene and C3–C5 light olefins were 35 and 23 %, respectively. In sum the integration of recycle spark discharge plasma and catalyst reactor is an efficient means to transform methane to highly desirable olefins.
References
Musa AA, Shabunya S, Martynenko V, Enazy KA (2014) J Power Sources 246:4731
Alamdari A (2015) J Nat Gas Sci Eng 27:934
Ciambelli P, Palma V, Palo E (2010) Catal Today 155:92
Fau G, Gascoin N, Gillard P, Steelant J (2013) J Anal Appl Pyrol 104:1
Lunsford JH (1995) Angew Chem Int Ed 34:970
Shalygin A, Pauksht E, Kovalyov E, Zhinimae B (2013) Front Chem Sci Eng 7:279
Jeong HK, Kim SC, Na BK (2001) Korean J Chem Eng 18:196
Li XS, Zhu AM, Wang KJ, Xu Y, Song ZM (2004) Catal Today 98:617
Wang KJ, Li XS, Wang H, Zhu AM (2008) Plasma Sci Tech 10:600
Kado S, Sekine Y, Nozaki T, Okazaki K (2004) Catal Today 89:47
Studt F, Norskov JK, Christensen CH, Bligaard T. US 2011/006174 A1
Gabelkov SV, Tarasov RV, Ledovskaya EG, Belkin FV (2007) Inorg Mater 43:398
Kado S, Urasaki K, Sekine Y, Fujimoto K, Nozaki T, Okazaki K (2003) Fuel 82:2291
Wang B, Froment GF, Goodman DW (2008) J Catal 253:239
Ghenciu AF (2002) Curr Opin Solid St M 6:389
Wang B, Froment GF (2005) I&EC Res 44:9860
Acknowledgments
The support of this work by the Fundamental Research Funds for the Central Universities (Grant No. 24720094028) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Guan, H.M. Highly Efficient Conversion of Methane to Olefins via a Recycle-Plasma-Catalyst Reactor. Catal Lett 146, 2193–2199 (2016). https://doi.org/10.1007/s10562-016-1846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1846-y