Abstract
In this study, we have successfully synthesized bimetallic MOFs of MIL-100(Fe-Mn) for SCR of NOx with NH3 via a hydrothermal method The catalyst were characterized by XRD, EDX, N2 adsorption(BET), SEM, in situ FT-IR and XPS. MIL-100(Fe-Mn) catalyst exhibited higher NOx conversion than either of its monometallic counterparts of MIL-100(Fe) and MIL-100(Mn). MIL-100(Fe-Mn) displayed excellent low-temperature activity, which achieved a maximum NOx conversion of 96 % at 260 °C. Moreover, MIL-100(Fe-Mn) showed a satisfactory stability and a good capacity of resisting SO2 and H2O. During NH3-SCR process, a slightly raised NO x conversion (approximately 7 %) was observed in the presence of H2O and SO2, which could be attributed to the formed sulfate species that act as new acid sites on the surface of the catalyst. Combined with the in situ FT-IR results, it was concluded that the SCR reaction of the MIL-100(Fe-Mn) followed Eley–Rideal (E–R) mechanism.
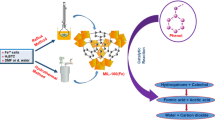
Graphical Abstract

Schematic diagram of SCR reaction
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Selective catalytic reduction (SCR) of NOx by NH3 (NH3-SCR) is one of the most effcient technologies to control stationary source NOx emissions [1–3]. The widely used commercial catalyst is based on TiO2-supported V2O5–WO3 system with high activity and resistance to SO2 poisoning [4, 5]. However, such SCR catalyst is effective only in a high temperature window ranging from 300 to 400 °C. In addition, the V2O5–WO3/TiO2 catalyst is unfavorable for SCR reaction due to vanadium toxicity and the high activity for oxidation of SO2 to SO3. Hence, there is still an urgent requirement for the development of superior vanadium-free catalysts with high activities at low temperature (<300 °C).
Porous metal–organic frameworks (MOFs) are known to be useful porous crystalline materials for catalysis and adsorption [6–8]. For example, iron-based MIL-100(Fe) MOFs (MIL: Materials of Institute Lavoisier) have been reported as a novel heterogeneous catalyst with good NH3-SCR activity [9], not only because it has highly-dispersed active sites as catalytic components, but also because some MOFs could keep stable structure with the existence of humid air (H2O), acid gasses (NOx, SO2) and NH3 [10–12]. Recently, researchers have reported that several bimetallic MOFs exhibited superior catalytic activity compared with that of their monometallic counterparts, ascribed to the improvement of electronic structures. Usually, hybrid bimetallic MOFs contains two kinds of active sites that may enhance its catalysis performance and framework stability [14, 23]. Because iron and manganese-based catalysts, such as Fe-Mn oxide have been proved to be highly active and environmentally friendly for NH3-SCR [5], bimetallic MOFs contained both iron and manganese could be an alternative low-temperature SCR catalyst with high catalytic activity.
In this work, we report the hydrothermal syntheses of MIL-100(Fe-Mn) by the incorporation of Mn into MIL-100(Fe). Compared with its counterparts MIL-100(Fe) and MIL-100(Mn), the feasibility and effectiveness of bimetallic MIL-100(Fe-Mn) for selective catalysis reduction of NOx by NH3 were investigated. The catalyst was also tested for durability in the presence of SO2 and H2O. As far as we know, this is the first work that demonstrates bimetallic MIL-100(Fe-Mn) to be an efficient SCR catalyst with good resistance to H2O and SO2 poisoning.
2 Experiment
2.1 Catalyst Preparation
MIL-100(Fe-Mn) was prepared from the hydrothermal reaction of 3.9 mmol of trimesic acid with 1 mmol of Fe0, 1 mmol of Mn(NO3)2 and 200µL of HF, 25 mL of deionized water. The reactant solution was transferred to a Teflon autoclave and maintained at 120 °C for 2 h, then maintained at 120 °C for another 4 h. After cooling to room temperature, the solution was filtrated and then the obtained powder was washed with deionized water. The as-synthesized MIL-100(Fe-Mn) need to be further purified by treatment with ethanol for 5 times. Finally, the resulting power was dried at 70 °C overnight. Before catalytic performance test, specific procedure was used to activate all prepared MOFs via vacuum-drying process (100 °C, 10 h). For comparison, MIL-100(Fe) and MIL-100(Mn) were also prepared by the similar hydrothermal method [13, 16]. The composition of reaction mixture for MIL-100(Fe) is 1.0:Fe0:0.66:1,3,5-BTC:2.0 HF:1.2 HNO3:277 H2O (1,3,5-BTC = benzene tricarboxylic acid). And the reaction mixture for MIL-100(Mn) was 2 mmol of Mn(NO3)2·4 H2O, 1.9 mmol H3BTC and 18mL ethanol.
2.2 Catalyst Characterizations
Powder X-ray diffraction (XRD) patterns were recorded on Empyrean X-ray diffractometer using Cu Ka radiation in the range of 3°–30°. The morphology and surface structure were studied by field-emission scanning electron microscopy (SEM, Hitachi SU8010). The element analysis was measured with energy dispersive X-ray (EDX, X-Max Oxford Instruments). The Brunauer–Emmet–Teller (BET) specific surface area was determined from a N2 adsorption isotherm (BET model in Quanta chrome SI). Thermo gravimetric analysis (TGA) and differential thermal analysis (DTA) was carried on a TGA SDTA850 thermogravimetric analyzer in a nitrogen flow from 30 to 600 °C. H2-TPR and NH3-TPD were carried out by Chembet PULSAR TPR/TPD (p/n02139-1). The chemical states of the electrodes were analyzed by X-ray photoelectron spectroscopy (XPS) (ESCA Lab 250, U.K.). All the binding energies were referenced to C 1 s at 285.6 eV. FT-IR (Fourier transform infrared spectroscopy) spectrums were recorded on a Bruker VERTEX 70 FTIR spectrometer in the range of 4000–650 cm−1 with the resolution of 4 cm−1.
2.3 Catalytic Performance Test
NH3-SCR reaction was carried out in fixed-bed quartz flow reactor (inner diameter 8 mm). Catalytic activity measurement was performed from 150 to 340 °C with a heating rate of 10 °C min−1. The reaction conditions were as follows: 500 ppm NH3, 500 ppm NO, 5 vol% O2, 0 or 250 ppm of SO2, 0 or 5 % of H2O and N2 as balance gas. The total flow of inlet gas rate was kept at 100 mL min−1 to achieve a GHSV of 15 000 h−1.The concentration for inlet and outlet gases was continually monitored by a flue gas analyzer (Testo 350). The activity data was collected and recorded when the NH3-SCR reaction reached a steady-state condition at each temperature. NO x conversion was calculated by the following equation:
3 Results and Discussion
3.1 Catalytic Performance
Figure 1a shows the NO x conversion to N2 on MIL-100(Fe-Mn), MIL-100(Fe), and MIL-100(Mn) catalyst in the temperature range of 140–340 °C. For MIL-100(Fe-Mn), the NO x conversion increased steadily with reaction temperature to a maximum values (more than 96 %) and exhibits more than 90 % NO x conversation within a temperature range from 260 to 330 °C. While for the classic V2O5–WO3/TiO2 catalyst, the NO x conversion of was more than 90 % above 300 °C [9]. Accordingly, the maximum NO x conversions over the MIL-100(Fe) and MIL-100(Mn) catalysts only reached to 78 and 53 %, respectively. This result demonstrated that bimetallic MIL-100(Fe-Mn) was more effective than MIL-100(Fe) and MIL-100(Mn) catalyst, which could be attributed to promotion effect of Mn at low-temperature and the synergetic effects between Fe and Mn on the catalytic activity of MIL-100(Fe-Mn) catalyst for NH3-SCR of NOx [5].
a NO x conversions over MIL-100(Fe-Mn), MIL-100(Fe) and MIL-100(Mn). b The stability tests without SO2 and H2O (A) and stability tests with SO2 and H2O (B) of SCR reaction over MIL-100(Fe-Mn) at 280 °C; the stability tests without SO2 and H2O (C) and stability tests with SO2 and H2O (D) of SCR reaction over MIL-100(Fe) at 260 °C; the stability tests without SO2 and H2O (E) and stability tests with SO2 and H2O (F) of SCR reaction over MIL-100(Mn) at 240 °C
However, the catalytic behavior of MIL-100(Fe-Mn) decreased significantly when the temperature was higher than 330 °C. This decrease in NOx conversion could be ascribed to the decomposition of MIL-100(Fe-Mn) structure when the react temperature was relatively high.
3.2 Effect of SO2 and H2O
As water vapor and SO2 are inevitable component in industrial flue, it is essential to further inspect the effects of these species on the catalytic performance of MIL-100(Fe-Mn) catalyst. Generally, it is concluded that H2O and SO2 can inhibit the SCR reaction owing to the formation of ammonia sulfate and the competitive adsorption between NO and SO2 on catalyst surface [13]. However, as shown in Fig. 1b, it is interestingly observed that the NO x conversion over MIL-100(Fe-Mn) was slightly increased (approximatly 6 %) with the introduction of 250 ppm of SO2 and 5 % H2O under 280 °C. Then the conversion of NO x was retained at above 96 % during such stability test. This enhancement of catalytic activity observed with SO2 and H2O could be attributed to the formation of SO4 2−, which leads to an increase of the acid sites on the surface of catalyst [15]. The stability test of MIl-100(Fe) and MIL-100(Mn) has also been investigated. As shown in Fig. 1b, Both MIL-100(Fe) and MIL-100(Mn) can maintain good catalytic activity after SO2 exposure.
To determine the various forms of reactive species on catalyst surface, the IR analysis for MIL-100(Fe-Mn) samples before (curved a) and after (curved b) durability tests was displayed in Fig. 2. For the used catalyst, v(C–O) peaks observed at 1620 and 1368 cm−1, and v(C=C) peaks at 1590 and 1428 cm−1 [16] (curve b) was in accord with the fresh catalyst MIL-100(Fe-Mn) (curved a). This result indicated that MIL-100(Fe-Mn) catalyst could keep its stable structure after durability test. Moreover, a new characteristic peak in curve b appears at 1085 cm−1 could be assigned to v(S-O) [22], which confirmed the formation of SO4 2− which could act as acid sites that was responsible for the improvement of MIL-100(Fe-Mn) catalytic activity.
While the SO2 and H2O vapor were switched off, the conversion of NOx was still maintained at a high level of 95 %. This observation confirmed that MIL-100(Fe-Mn) catalyst exhibits a good capacity of resisting SO2 and H2O.
3.3 Structural Property of Catalyst
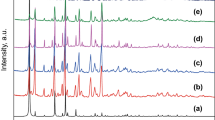
The XRD patterns of MIL-100(Fe-Mn), MIL-100(Fe) and MIL-100(Mn) are plotted in Fig. 3. The main diffraction peaks positions for all samples showed high similarity in the range of 3°–30°. This results showed that the XRD diffraction peak of MIL-100(Fe-Mn) matched well with those of MIL-100(Fe) and MIL-100(Mn) [9, 16]. In addition, the strong peak intensities of MIL-100(Fe-Mn) indicates high crystallinity of this sample. As shown in Fig. 4a, SEM of the as-synthesized MIL-100(Fe-Mn) exhibits stable cubic crystal structure with the size of approximately 1 µm. Figure 4b showed the SEM images of MIL-100(Fe-Mn) sample after NH3-SCR activity test above 330 °C. While after SCR reaction, the sample was found to be irreversible cracking. This evidence proves that the catalyst was callapsed when the temperature above 330 °C during activity test. Furthermore, the SEM image of the MIL-100(Fe-Mn) catalyst used in durability test of the resistance of SO2 and H2O is shown in Fig. 4c. This image indicates that the main structure of the catalyst still remain intact after such durability test. This observation demonstrates that the catalyst can keep stable during the durability test and further explained the catalyst can maintains an excellent capacity of resisting SO2 and H2O.
The TG curve of MIL-100(Fe-Mn),MIL-100(Fe) and MIL-100(Mn) in a temperature gradient from 25 to 550 °C were presented in Fig. 5. Three weight losses are apparently observed between 25 and 600 °C for MIL-100(Fe-Mn). At first step, the weight loss is attributed to the departure of the ethanol inside the pore, which is about 7 %. For the second, the weight loss between 98 and 400 °C (20.4 %) comes from residual water inside the pore and coordinated water molecules. The final weight loss, between 400 and 500 °C (31.63 %) is related to the decomposition of trimesic acid. This thermal stability result demonstrates the stability and applicability of the MIL-100(Fe-Mn) catalyst during SCR in the temperature range from 140 to 330 °C. Moreover, because the test gas condition is more complex than an N2 atmosphere, it resultes that the MIL-100(Fe-Mn) began to decompose when the temperature is far below 400 °C during activity test. The TG result indicates the stability of MIL-100(Fe-Mn) is superior than the other two simples, not only because MIL-100(Fe-Mn) can keep stable until 400 °C, but also because its TG curve exhibits a more gentle decrease with the increase of temperature from 25 to 400 °C.
N2 gas adsorption and desorption isotherms for as-synthesized MIL-100(Fe-Mn) catalyst were measured at 77 K (Fig. 6a). MIL-100(Fe-Mn) sample presents the type I isotherm characteristic of microporous solid. This material exhibits wide pore size distribution with size range 0.5–0.8 nm, which could be observed in Fig. 5b. The BET surface areas and pore volumes of the catalyst is estimated to be 958.6 m2g−1 and 0.612 cm3g−1. For MIL-100(Fe-Mn) catalyst, such high surface area would promote efficient gas transfer between the reactants and active sites and improve the dispersion of active components.
3.4 Surface Component
The chemical composition of MIL-100(Fe-Mn) was analyzed by energy dispersive X-ray spectroscopy (EDX), as shown in Fig. 4d.The MIL-100(Fe-Mn) contained 5.13 % Fe atoms, 1.14 % Mn atoms, 51.78 % C atoms and 38.8 % O atoms, respectively. Because the detected Fe/Mn molar ratio did not vary significantly with the probing position, this EDX result of the sample not only demonstrate the existence of Fe and Mn element in the catalyst, but also suggests the sample is homogeneous.
The as-prepared MIL-100(Fe-Mn) catalyst was further investigated by X-ray photoelectron spectroscopy (XPS) for determining chemical states on the surface of the sample. In Fig. 7a, full-range XPS survey spectrum confirms that MIL-100(Fe-Mn) catalyst is composed of Fe, Mn, O elements., The high-resolution spectra of Fe2p given in Fig. 7b shows two peaks centered at 712.2 and 725.8 eV, which can be assigned to Fe 2p3/2 and Fe 2p1/2 binding energies, respectively. By performing peak-fitting deconvolution, the Fe 2p3/2 peak could be separated into two peaks at 711.2 and 714.1 eV, which corresponding to Fe3+ and Fe2+, respectively [17]. Similarly, in Fig. 7c, the Mn 2p peaks could be deconvoluted to three peaks. The peaks at 641.4, 642.4 and 644.1 eV represented Mn2+, Mn3+and Mn4+, respectively [18]. The existence of Mn3+ ions on SCR catalyst surface is believed to significantly promote low-temperature SCR activity [3]. Moreover, the XPS spectrum of O 1s could be fitted by one peak corresponding to 531.7 eV binding energies (Fig. 7d). The presence of oxygen vacancy in the catalyst play a major role in the NO decomposition activity [19]. These result means the existence of two kinds of metal active sites on the catalyst surface which can give positive contribution to low temperature NO reduction.
3.5 NH3-TPD and H2-TPR
The acid-site distribution in various temperature ranges of as-synthesized catalyst was studied by NH3-TPD, as shown in Fig. 8a. The sample exhibits NH3 desorption peaks caused by the combination of physical adsorption and chemical adsorption on Brønsted or Lewis acid sites. The broad peaks range from 200 to 340 °C demonstrates the existence of medium acid sites which are favorable active site for SCR on the surface of catalyst. With the temperature rises above 400 °C, the structure of the catalyst was collapsed. The narrow peaks appeared between 400 and 600 °C are very likely caused by components decomposed from MIL-100 (Fe-Mn).
In addition, H2-TPR profiles were used to investigate the reducibility of MIL-100(Fe-Mn). As shown in Fig. 8b, the TPR profiles of the catalyst exhibits three reduction peaks. The first peak at 304 °C could be attributed to the reduction of Mn4+ to Mn3+ [20]. Then after structure decomposition of MIL-100(Fe-Mn), two peaks appear in the temperature 473 and 563 °C, which may be correspond to the reduction of Fe3+ to Fe2+ and Fe2+ to Fe0, respectively [21]. This results, which are agreed with above EDX and XPS analysis, also proved that Mn has been incorporated into MIL-100(Fe) framework.
3.6 In Situ FT-IR
FT-IR analysis of the adsorbed species on the catalyst surface during the SCR reaction was carried out to investigate the chemical mechanisms. NH3 adsorbed species on MIL-100(Fe-Mn) at various temperatures were investigated by FTIR spectroscopy. Before NH3 adsorption, the sample were treated with He for 40 min at 100 °C. Figure 9a showed the FT-IR spectra of as-prepared catalyst after being exposed to NH3/He in the temperature range of 50–250 °C. The bands at 1074 and 1191 cm−1 could be assigned to coordinated NH3 on Lewis.acid sites [24]. The bands at 1366 and 1759 cm−1 could be attributed to intermediate species formed due to ammonia oxidation and stretching vibration of NH3 interacting with open Brønsted acid sites in the framework, implying the formation of ionic NH4 + [2, 26]. Furthermore, IR spectra of NH3 adsorption over MOFs is closely related with the amount of unsaturated metal active sites [11, 34]. Figure 9a exhibits weak ammonia adsorption peaks at 1074 cm−1 because there is less metal active sites at 50 °C. Then with the increase of temperature, the peak become larger indicating the increase of metal active sites for ammonia adsorption. In addition, with the increase of reaction temperature, the intensity of these bands became stronger, indicating the increase of the quantity of coordinated NH3 and ionic NH4 + which would improve the SCR performance by enhancing relationships between adsorbed NH3 species and accessible iron and manganese sites. Especially, both the bands at 1508 and 1560 cm−1 might be attributed to –NH2 species [27], which is a typical intermediate of oxidation of ammonia.
a In situ FTIR spectra of MIL-100(Fe-Mn) pre-adsorbed 500 ppm NH3 species at various temperatures. b In situ FTIR spectra at 250 °C upon passing 500 ppm NO + 5 %O2 over the NH3 pre-adsorbed and purged with He for 40 min. c In situ FTIR spectra at 250 °C upon passing 500 ppm NH3 over the NO + 5 %O2 pre-adsorbed and purged with He for 40 min
The samples were first treated with NH3/He for 40 min and then purged with He for 30 min at 250 °C. When NO + O2 was introduced, the spectra were recorded as a function of time. After NO + O2 was introduced into the system, all ammonia ad-species such as coordinated NH3 (1074 and 1191 cm−1), NH4+ (1759 cm−1) and –NH2 species (1508, 1560 cm−1) decreased quickly, as show in Fig. 9b. After 10 min reaction, some new bands appeared at 1600, 1628, 1845 and 1907 cm−1, which could be attributed to surface nitrate species [28, 29]. These results suggest that NOx react readily with the adsorbed ammonia species on catalyst surface and both the ionic NH4+ and NH3 species are capable of participating in SCR reaction. Moreover, the observed –NH2 species, which could immediately react with gaseous NO on the surface of catalyst to generate N2 and H2O, has been considered as an important intermediate in Eley–Rideal (E–R) mechanism [30].
Figure 9c shows IR spectra of NH3 adsorption after NO + O2 pre-adsorb. When pre-adsorbed with NO + O2, the catalyst was mainly covered by monodentate nitrate (1600 cm−1) [5], bidentate nitrate (1020 cm−1) [32], nitrites (1360 cm−1) and NO2 disproportionation (1628 cm−1) [31]. Then purged with He, the peak at 1600 cm−1 for monodentate nitrate decreases rapidly, while the peak at 1020 cm−1 for bidentate nitrate keep constant. Further, after NH3 was introduced for 10 min, the peak at 1600 cm−1 can still be clear observed, which indicating that monodentate nitrite may not participate as real reactive species in the denitrification process following Langmuir–Hinshelwood (L–H) mechanism [35].
Therefore, we tentatively propose that the SCR reaction on MIL-100(Fe-Mn) is in accordance of E–R mechanism. The SCR reaction through the Eley–Rideal mechanism can be described as [5, 27, 33]:
Reaction (1) was the adsorption of gaseous ammonia on the acid sites to form adsorbed ammonia species. And Reactions (2) and (3) were the dehydrogenation of adsorbed ammonia species to form intermediate species (–NH2) by Fe3+ and Mn4+, respectively. Then, as the reaction (4) shows, gaseous NO was reduced by –NH2 to form N2 and H2O. At last, the reduced metal sites (Fe3+ and Mn4+) were regenerated by O2 oxidation (Reactions (5)and (6)).
4 Conclusion
The bimetallic MIL-100(Fe-Mn) catalyst was successfully synthesized via hydrothermal method and proved to be an excellent catalyst in NH3-SCR at the temperatures ranging from 260 to 330 °C. The SCR catalytic activity of MIL-100(Fe-Mn) was superior to that of the MIL-100(Fe) and MIL-100(Mn) catalyst. Furthermore, the MIL-100(Fe-Mn) catalyst exhibited an enhanced SCR catalytic performance in the presence of H2O and SO2, which could be ascribed to the formation of SO4 2− that increase the number of acid sites on the surface of catalyst. The in situ FTIR results discovered that the adsorbed ammonia species and –NH2 species as intermediate, and thus proved the SCR reaction over MIL-100(Fe-Mn) may follow an E–R mechanism. This study demonstrated the feasibility of using bimetallic MOFs as a novel catalyst for SCR reaction.
References
Shi XY, Liu FD, Xie LJ, Shan WP, He H (2013) Environ Sci Technol 47:3293
Chen L, Li JH, Ge MF (2010) Environ Sci Technol 44:9590
Jiang BQ, Deng BY, Zhang ZQ et al (2014) J Phy Chem C 118:14866
Jin RB, Liu Y, Wang Y et al (2014) Appl Catal B 148:582
Yang SJ, Wang CZ, Li JH, Yan NQ, Ma L, Chang HZ (2011) Appl Catal B 110:71
Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen SBT, Hupp JT (2009) Chem Soc Rev 38:1450
Zhang M, Guan J, Zhang B, D Williams Su, Liang C (2012) Catal Lett 142:313
Stock N, Biswas S (2012) Chem Rev 112:933
Wang P, Zhao H, Sun H, Yu H, Quan X (2014) RSC Adv 4: 48912
Petit C, Bandosz TJ (2011) Adv Funct Mater 21:2108
Wuttke S, Bazin P, Vimont A, Serre C, Seo Y, Hwang YK, Chang J et al (2012) Chem Eur J 18:11959
Han S, Huang Y G, Taku. W et al (2013) Micro Meso Mater 173:86
Tan FC, Liu M, Li KY et al (2015) Chem Eng J 281:360
Song X, Oh M, Lah MS (2013) Inorg Chem 52:10869
Qu ZP, Miao L, Wang H, Fu Q (2015) Chem Commun 51:956
Reinsch H, Stock N (2013) Cryst Eng Comm 15: 544
Bukhtiyarova MV, Ivanova AS, Plyasova LM et al (2009) Appl Catal A 357:193
Yang SJ, Wang CZ, Ma L et al (2013) Catal Sci Technol 3:161
Yang SJ, Yan NQ, Guo YF et al (2011) Environ.Sci.Thchnol 45:540
Yang SJ, Wang CZ, Li JH, Yan NQ, Ma L, Chang HZ (2011) Appl.CataB 110:71
Wang CZ., Yang SJ, Chang HZ, Yue P, Li JH (2013) J Mol Catal A Chem 376:13
Yamaguchi T, Jin T, Tanabe K J (1986) Phy Chem 90:3148
Vu TA, Le GH, Dao CD et al (2014) RSC Adv 4:41185
Yue P, Li KZ, Li JH (2013) Appl Catal B: Environ 140–141:483
Jiang BQ, Deng BY, Zhang ZQ, Wu ZL (2014) J Phys Chem C 118:14866
Jiang BQ, Li ZG, Lee S (2013) Chem Eng J 225:52
Qi GS, Yang RT, Chang R (2004) Appl Catal B 51:93
Wu ZB, Jiang BQ, Liu Y, Wang HQ, Jin RB (2007) Environ Sci Technol 41:5812
Liu ZM, Zhu JZ, Li JH, Ma L, woo S (2014) ACS Appl Mater Interfaces 6:14500
Anstrom M, Topsoe NY, Dumesic JA (2003) J Catal 213:115
Machidam, Uto M, Kurogid et al (2001) J. Mater Chem 11:900
Yeom YH, Wen B, Sachtler WMH, Weitz E.(2004) J Phys Chem B 108:5386
Busca G, Lietti L, Ramis G, Berti F (1998) Appl Catal B 18:1
Horike S, Dinca M, Tamaki K et al (2008) J ACS 130: 5854
Karami A, Salehi V (2012) J Catal 292: 32
Acknowledgments
This work is supported by the National Basic Research Program of China (No. 2011CB936002), National Natural Science Foundation of China (No. 51178076) and the Fundamental Research Funds for the Central Universities (No. DUT14LK17).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Shi, Y., Li, C. et al. Synthesis of Bimetallic MOFs MIL-100(Fe-Mn) as an Efficient Catalyst for Selective Catalytic Reduction of NO x with NH3 . Catal Lett 146, 1956–1964 (2016). https://doi.org/10.1007/s10562-016-1840-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1840-4