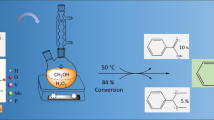
Abstract
Various V/P mole ratios of vanadium substituted Keggin-type phosphomolybdic acids were synthesized by the hydrothermal method. These materials were characterized using several physico-chemical techniques such as X-ray diffraction, FT-IR, N2-sorption, Raman spectroscopy, 31P MAS NMR, SEM and NH3-TPD. FT-IR, Raman spectroscopy and 31P NMR results confirm the formation of the primary structure of the Keggin ion and its crystalline nature is shown clearly by XRD. NH3-TPD results reveal that the acidity of the materials systematically decreases with increasing vanadium content. The Knoevenagel reaction carried out over vanadium substituted phosphomolybdic acid with various V/P mole ratios indicate that the higher V/P mole ratio exhibits better catalytic performance under solvent free conditions. The catalytic properties correlate with the structural properties and the acidity of the materials.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, there has been increasing emphasis on the use and design of environment-friendly solid acid catalysts to reduce the amount of toxic waste and by-products arising from chemical processes prompted by stringent environment protection laws. The challenge is to perform heterogeneous catalysis reactions leading to C–C bond formation which are widely employed in organic synthesis in a solvent free system [1]. The Knoevenagel reaction is an important C–C bond forming reaction that is widely used in the synthesis of important intermediates or end-products for perfumes, pharmaceuticals, calcium antagonists and polymers [2, 3]. The reaction is catalyzed by bases, acids or materials containing both acidic and basic sites [4]. Bases such as ammonia, the primary amines, secondary amines and their salts, Lewis acids such as CuCl2, ZnCl2 and SmI2 and the ZnY zeolites are some of the examples that have been employed in the Knoevenagel condensation reaction [5–9]. Numerous types of heterogeneous catalysts containing both acidic and basic sites have been reported in the literature [10–15]. Activated Mg–Al hydrotalcite was used for the first time in condensation reactions producing excellent yields. However, all the reactions were carried out using toluene and DMF as solvents [16]. Joshi et al. [17] studied the effect of incorporating other alkali metals in NaX- type zeolites in deciding the activity in the Knoevenagel condensation reaction. They found that an increase in the cation kinetic diameter improved activity by 50 %. In a number of studies conducted by Corma and co-workers [18–21], solid base catalysts such as zeolites, sepiolites and hydrotalcites have been used to prepare prepolymers of malononitrile with a series of ketones. In all the condensation reactions studied, they proposed that the controlling step was not the proton abstraction as assumed, but the attack of the carbonyl group by the carbanion. In the studies mentioned, although some reactions are carried out in heterogenous medium, all of the catalytic reactions required the presence of solvents. To avoid the use of organic solvents, water as a desirable solvent was investigated [22, 23]. However, in the bulk phase production of fine chemicals, large amounts of water are required which needs purification post reaction for environmental reasons. Thus, it has become imperative to develop catalytic systems that are efficient in organic reactions without the use of a solvent. Metzger [24] and Tanaka and Toda [25] emphasized very emphatically in their paper and review, respectively, the importance in carrying out solvent free reactions. Pillai et al. [26] studied the Knoevenagel reaction using iridium and platinum hydroxyapatites. Hydroxyapatites (HAp) are bifunctional materials with both acidic and basic sites in the crystal lattice. Their results showed that the IrHAp catalyzed reactions gave higher yields than PtHAp catalyzed reactions, but both IrHAp and PtHAp catalyzed reactions were very efficient in the Knoevenagel reaction without the use of a solvent, with the overall production of excellent yields.
In our work, we report the solvent free liquid phase Knoevenagel condensation of benzaldehyde and substituted benzaldehydes with malononitrile over vanadium substituted phosphomolybdic acid catalysts with various V/P mole ratios. The aim of this investigation is to study the effect of V/P mole ratio of the catalyst during the Knoevenagel condensation reaction and the effect of substituent on the benzaldehyde on the catalysis.
2 Experimental
2.1 Catalyst Synthesis
The solid acid catalysts containing vanadium, H4PMo11VO40 (PMoV1), H5PMo10V2O40 (PMoV2) and H6PMo9V3O40 (PMoV3) were prepared using V2O5 (Sigma Aldrich), H3PMo12O40 (Sigma Aldrich), MoO3 (Fluka) and H3PO4 (S.D Fine-Chem. Ltd.) according to the method reported previously [27]. For the detailed synthesis of PMoV1, 14.4 g of MoO3 and 0.91 g of V2O5 were placed in 250 mL distilled water, refluxed at 100 °C with stirring, followed by the drop wise addition of 1.15 g of 85 % H3PO4. The resulting slurry was maintained at this temperature, under reflux, for 24 h. The solid was collected after evaporation of the slurry in a vacuum heater set at 60 °C. A similar procedure was followed for the synthesis of PMoV2 and PMoV3.
2.2 Catalyst Characterization
Powder X-ray diffraction patterns of samples were obtained with Bruker D8 Advance diffractometer, using Cu Kα radiation (1.5406 Å) at 40 kV and 30 mA. The measurements were recorded in steps of 0.045° with a count time of 0.5 s in the range of 2–40°. BET surface area and NH3 temperature programmed studies of the catalysts were conducted on a Micromeritics Auto Chem 2910 instrument. For TPD studies, about 50 mg of sample was pretreated at 200 °C for 2 h by passing through helium at 50 mL/min). After pretreatment, the sample was saturated with a mixture of 10 % NH3 in helium at 80 °C for 1 h and subsequently flushed with helium flowing at 50 mL/min to remove physisorbed ammonia. The entire TPD analysis was carried out from ambient temperature to 700 °C at a ramp rate of 10 °C/min. Ex-situ pyridine adsorbed FT-IR experiments spectra of samples were carried out to investigate the nature of acidity, such as Brønsted and Lewis acid sites present on the catalyst. The spectra were recorded on a Perkin-Elmer ATR spectrometer at room temperature. Prior to analysis, pyridine adsorption experiments were carried out by placing a drop of pyridine on a small amount of the catalyst followed by evacuation in air for 1 h to remove the reversibly adsorbed pyridine. FT-IR spectra of catalysts were recorded on the Nicolet 670 spectrometer by the KBr disc method, whereas The Raman spectra were collected using a Horbia–Jobin–Yvon LabRam high resolution spectrometer equipped with a confocal microscope with 2400/900 grooves/mm gratings and a notch filter. The visible laser excitation at 532 nm (visible/green) was supplied by a Yag double diode pumped laser (20mW). The surface morphology of materials was examined by scanning electron microscopy (SEM) with a Hitachi S-4800 microscope. Solid state 31P MAS NMR spectra were recorded at ambient temperature using 4 mm diameter zirconia rotors with a spinning rate of 7 kHz on a Jeol ECA-500 NMR spectrometer.
2.3 Catalytic Activity
The Knoevenagel condensation of benzaldehyde and its derivatives was carried out in a 25 mL reaction flask, equipped with a condenser, at 70 °C without solvent (Scheme 1). In typical reaction, 10 mmol of benzaldehyde, 10 mmol of malononitrile and 2 mol % of the catalyst were placed in the flask and refluxed at 70 °C. The reaction was constantly monitored by thin layer chromatography (TLC) and after completion of reaction, the resulting mixture was analysed by GC–MS using a HP 6890 Series Gas Chromatograph coupled with a mass selective detector. The catalyst was then recycled and used for the same reaction.
3 Results and Discussion
3.1 Characterization Results
The XRD profiles the vanadium substituted phosphomolybdic acid catalysts are shown in Fig. 1. The Bragg diffraction wide angle reflections at 2θ = 8.3°, 9.0°, 27.8° and 29.1° are observed and these peaks correspond to the crystalline nature of the Keggin type heteropolyacid [27]. The characteristic diffraction peaks are retained with increasing V/P mole ratios [28].
The pure and vanadium substituted PMA catalysts were characterized by FT-IR spectroscopy in the range of 400–1500 cm−1 and the spectra are shown in Fig. 2. The catalysts exhibit well defined IR bands in the range of 1100–700 cm−1. The bands are assigned to P–O, Mo=O and Mo–O–Mo vibrations and are located at 1080–1060, 990–960 and 900–760 cm−1, respectively [29, 30]. There is a slight shift in IR bands with increase of the V/P mole ratio of V substituted PMA catalysts, indicating that vanadium is incorporated in the Keggin ion structure of PMA catalyst. These results agree well with the XRD patterns obtained for the catalysts.
The structure of the Keggin ion in vanadium containing PMA catalysts with various V/P mole ratios, characterized by Raman spectroscopy, in the range 200–1200 cm−1, is shown in Fig. 3. All the samples possess a Raman band at 1010 cm−1 which is characteristic for the Keggin structure. Other bands at 849, 817, 656 and 286 cm−1 are assigned to Mo–Ot, Mo–Ob–Mo, Mo–Oc–Mo and Mo–Oa, respectively [31]. There is a significant shift in the characteristic Keggin ion Raman bands towards the higher region with an increase in the V/P mole ratio, suggesting that vanadium is incorporated into the PMA catalysts.
The results of the BET surface area of the vanadium substituted phosphomolybdic acid catalyst with different V/P mole ratios are summarized in Table 1. From the results, the surface area decreases as V/P mole ratio of the catalyst is increased, probably due to bulk crystallite formation. These catalysts appear to be more crystalline.
Figure 4 shows the ammonia TPD profile of the PMoV1, PMoV2 and PMoV3 samples. All the samples exhibit three types of acid sites, located between 50 and 150 °C, 150 and 300 °C and 300 and 500 °C. The peak observed between 50 and 150 °C is attributed to weak acidic sites, the peak located between 150 and 300 °C, to moderate acidic sites and the peak between 300 and 500 °C, exclusively to strong acidic sites (Table 1). An increase in V/P mole ratio in the vanadium substituted phosphomolybdic acid catalysts shows a significant decrease in acidity.
The nature of the acidic sites was evaluated by pyridine adsorbed FT-IR spectroscopy on the PMoV3 catalyst (Fig. 5). It is shown that most are Brønsted acidic sites with a few Lewis acidic sites.
Scanning electron microscopy images of pure PMA and the vanadium substituted phosphomolybdic acid catalysts are shown in Fig. 6. The surface morphology of the PMA and PMoV1 compounds appears as cylindrical particles, whereas PMoV2 and PMoV3 have a cubic-like morphology. In addition, a bulk-like structure formation is observed for the PMoV3.
To understand the coordination and the chemical environment of P in the catalysts, 31P MAS NMR was studied. The results are shown in Fig. 7. With an increase in the V/P mole ratio, a change in the chemical shift from −3.9 δ for PMoV1 catalyst to −2.5 δ for PMoV3 is observed, attributed to the change in the local environment of 31P with an increase in the V/P mole ratio of the catalyst [32–34]. When these catalysts were compared to the acidity of PMA, PMoV1 was found to be more acidic than PMA, whereas at higher loadings of vanadium, the catalysts were less acidic than the pure PMA catalyst.
3.2 Catalytic Activity Studies
The catalytic properties of pure PMA and vanadium substituted phosphomolybdic acid samples with different V/P mole ratios was tested for the liquid phase Knoevenagel condensation reaction, without solvent at 70 °C. The blank reactions gave up to 5 to 10 % yield towards the desired product in 50 min. Aldehyde conversion increased significantly with increasing vanadium content in the heteropolyacid (Table 2). The higher aromatic aldehyde conversion and yield towards the desired condensation product are observed in the case of higher V/P mole ratio of the PMoV3 catalyst. These results suggest that conversion of a particular aldehyde and yield towards the desired product can be controlled by changing the vanadium content, resulting in altering the acidity of the catalyst. The results clearly show that the catalyst with a lower acidity exhibits superior catalytic performance.
The Knoevenagel reaction was further studied with compounds containing activating and deactivating groups substituted on the benzaldehyde. The results, given in Table 2, clearly show that higher conversions and yields are obtained with benzaldehyde and 4-nitro benzaldehyde than the methoxy and chloro substituted benzaldehyde. This is mainly due to the withdrawing group decreasing the electron density around the carbonyl group and therefore enhancing the conversion in presence of the heteropolyacid.
There was evidence no leaching of vanadium during the reaction. This was confirmed by ICP-AES analysis on the fresh and used catalyst. In both cases, the P/Mo/V atomic ratio was determined to be 1.00/9.01/3.05. The used catalyst was regenerated and studied for 3 cycles and the results are reported in Table 3. There was a slight difference in the activity and selectivity towards the desired products for all 3 cycles.
From Table 4, Ogiwara et al. [35] reported that combination of a catalytic amount of InCl3 in acetic anhydride remarkably promotes the Knoevenagel condensation of an aldehyde with an activated methylene group, producing a 94 % yield. Oskooie et al. [22], on the other hand, showed 91 % yield for the condensation reaction using phosphotungstic acid in water. Both these processes use solvents and require higher reaction temperatures to attain the yields mentioned. In our study, the PMoV3 catalyst consistently produces a yield of about 96 % of the condensation product in a solvent free environment at a reaction temperature of 70 °C. It is assumed that due to the presence of largely Brønsted acidic sites, as shown by pyridine adsorbed FT-IR spectroscopy, the reaction is understood to proceed via Brønsted acidic sites or protons present in the catalyst (Fig. 5).
4 Conclusion
Keggin-type vanadium substituted phosphomolybdic acid catalysts have been synthesized with different vanadium to phosphorous mole ratios and characterized successfully by powder XRD, FT-IR and Raman spectroscopy. NH3-TPD results confirmed that the acidity of the catalysts decreases with an increase in the V/P mole ratio and these results are supported by 31P NMR analysis. The catalytic properties strongly depend on the amount of vanadium incorporated into PMA, the higher the V/P mole ratio, the higher the catalytic activity. The vanadium containing PMA catalysts are competitive, in most cases superior for the Knoevenagel reaction under a solvent free condition when compared to most catalytic systems even employing solvents.
References
Trost BM (1991) Comprehensive organic synthesis. Elsevier, Oxford, p 133
Knoevenagel L (1898) Ber 31:258
Enders D, Muller S, Demir AS (1988) Tetrahedron Lett 29:6437
Reeves RL, Patai S (1996) The chemistry of carbonyl compounds. Interscience Publishers, New York 567
Jones G (1967) Org React 15:204
Attanasi O, Fillippone P, Mei A (1983) Syn Commun 13:1203
Shanthan Rao P, Venkatratnam RV (1991) Tetrahedron Lett 32:5821
Bao W, Zhang Y, Wang J (1996) Syn Commun 26:3025
Saravanamurugan S (2006) Appl Catal A Gen 298:8
Boullet FT, Focucad A (1982) Tetrahedron Lett 23:4927
Macquarrie DJ, Clark JH, Lambert A, Gmode JE, Priest A (1997) React Funct Polym 35:153
Brunel D (1993) Micropor Mesopor Mater 27:329
Hein RW, Astle MJ, Shelton JR (1961) J Org Chem 26:4874
Moison H, Boullet FT, Focaud A (1987) Tetrahedron 43:537
Bigi F, Chesini L, Maggi R, Sartori G (1999) J Org Chem 64:1033
Kantam ML, Choudary BM, Reddy CV, Rao KK, Figueras F (1998) Chem Commun 1033
Joshi UD, Joshi PN, Tamhankar SS, Joshi VV, Rode CV, Shiralkar VP (2003) Appl Catal A Gen 239:209
Climent MJ, Corma A, Forens V, Frau A, Lopez RG, Iborra S, Primo J (1996) J Catal 163:392
Corma A, Fornes V, Aranda RMM, Garcia H, Primo J (1990) Appl Catal 59:237
Corma A, Aranda RMM, Sanchez F, Guinst M, Barrault J, Bouchoule C, Duprez D, Maurel R, Montassier C (1991) Stud Surf Sci Catal 62:503
Corma A, Aranda RMM (1993) Appl Catal A 105:271
Oskooie HA, Heravi MM, Derikvand F, Khorasani M (2006) Syn Commu 36:2819
Bhunia S, Saha D, Koner S (2011) Langmuir 27:15322
Metzger JO (1998) Ang Chem Inter Ed 37:2975
Tanaka K, Toda F (2000) Chem Rev 100:1025
Pillai MK, Singh S, Jonnalagadda SB (2011) Kinet Catal 52:536
Fumin Z, Maiping G, Hanqing G, Jun W (2007) Front Chem Eng China 1:296
Sen R, Bera R, Ashis B, Gutlich P, Ghosh S, Mukherjee AK, Koner S (2008) Langmuir 24:5970
Ilkenhans T, Herzag B, Braun T, Schlogl R (1995) J Catal 153:275
Zhang J, Tang Y, Li G, Hu C (2005) Appl Catal A Gen 278:251
Predoeva A, Damyanova S, Gaigneaux EM, Petrov L (2007) Appl Catal A Gen 319:14
Raj NKK, Deshpande SS, Ingle RH, Raja T, Manikandan P (2004) Catal Lett 24:1001
Yue ZY, Bao B, Liu RL, Xi HS, Yong H (2006) Chin J Chem 24:1001
Tang Y, Zhang J (2006) J Serb Chem Soc 71:111
Ogiwara Y, Takahashi K, Kitazawa T, Sakai N (2015) J Org Chem 80:3101
Acknowledgments
B Viswanadham thanks to the University of KwaZulu-Natal for the award of the AES Postdoctoral Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viswanadham, B., Jhansi, P., Chary, K.V.R. et al. Efficient Solvent Free Knoevenagel Condensation Over Vanadium Containing Heteropolyacid Catalysts. Catal Lett 146, 364–372 (2016). https://doi.org/10.1007/s10562-015-1646-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1646-9