Abstract
4 Å molecular sieve modified with titanium(IV) is an efficient heterogeneous catalyst for the tetrahydropyranylation of alcohols under mild, slightly basic reaction conditions. The catalyst can be reused with good yield after a simple treatment with dichloromethane.
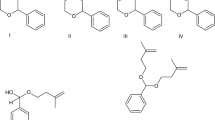
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tetrahydropyranylation is a widely used and common method to protect hydroxy compounds (Fig. 1). According to the literature it is a classical acid catalysed reaction [1]. Numerous catalysts have been used for the tetrahydropyranylation of hydroxyl groups such as conventional mineral or organic acids like concentrated hydrochloric acid [2], p-toluenesulphonic acid [3] and Lewis acids (e.g. boron trifluoride etherate [4] or zirconium tetrachloride [5]). Most of these catalysts are dangerous and harmful to the environment, and a homogenous catalyst is difficult to remove from the reaction mixture. Thus, various heterogeneous solid acid systems have also been used. i.a. supported Lewis acids (e.g. thallium pentachloride on silica support [6]), acidic ion exchange resins [7], heteropolyacids [8], zeolites [9, 10] and clays [11].
In some cases this method is inapplicable, since the compound to be protected contains acid sensitive group(s). There are only few examples in the literature for neutral or base catalysed methods. A classical solution of this problem is the use of pyridinium p-toluenesulphonate [12], as homogeneous catalyst. Several other catalysts have also been published, thus CaCl2 [13], or the neutral N,N′-dibromo-N,N′-1,2-ethanediylbis(benzene-sulphonamide) [14]. An acid-free, organocatalytic tetrahydropyranylation method used N,N′-bis(3,5-bis(trifluoromethyl)-phenyl)thiourea and its polystyrene-bound analogue [15]. The use of a basic ion exchange resin (Amberlite IRA 400 iodide) has also been published for the tetrahydropyranylation of alcohols and phenols [16].
There are several disadvantages of these methods. The separation of the homogeneous catalysts is sometime tedious, the catalysts described are not available as commercial products [14–16], their preparation is a long process involving harmful or dangerous reagents and solvents such as elementary bromine and CCl4 [14]. Thus a simple, easily accessible heterogeneous catalyst may have synthetic importance.
Our research group works on the elaboration of new heterogeneous catalytic methods for the preparation of organic compounds using supported metal catalysts. During this work several metals, such as palladium [17–20], nickel [21] or copper [22–24] on different supports (Mg:La 3:1 mixed oxide, 4 Å molecular sieve) were used successfully in different organic syntheses. As 4 Å molecular sieve (4A) proved to be useful support in several cases, we examined the catalytic effect of different metals on this support. In this paper we report a method for tetrahydropyranylation of alcohols and phenols under mild basic conditions.
2 Experimental
Morphology of the catalyst samples was investigated by a JEOL 6380LVa (JEOL, Tokyo, Japan) type scanning electron microscope and elemental mapping was also accomplished using the energy-dispersive X-ray detector of the equipment. Each specimen was fixed by conductive double-sided carbon adhesive tape and sputtered by gold (using a JEOL 1200 instrument). Applied accelerating voltage and working distance were between 15 and 30 kV and 10 and 12 mm, respectively.
Nitrogen adsorption/desorption isotherms were measured at −196 °C with a computer controlled Nova 200e (Quantachrome) instrument. Transformation of the primary adsorption data and the (micro)pore analysis were performed with the Quantachrome software. The apparent surface area (S BET) was calculated using the Brunauer–Emmett–Teller (BET) model. The total pore volume (V t) was derived from the amount of vapour adsorbed at p/p 0 → 1, assuming that the pores are then filled with liquid adsorbate. The micropore volume (W 0) was derived from the Dubinin–Radushkevich (DR) plot.
Samples were evacuated for 24 h at 110 °C prior to the adsorption measurement.
GC–MS measurements were performed on an Agilent 6890 N-GC-5973 N-MSD chromatograph, using a 30 m × 0.25 mm Restek, Rtx-5SILMS column with a film layer of 0.25 μm. The initial temperature of column was 45 °C for 1 min, followed by programming at 10 °C/min up to 310 °C and a final period at 310 °C (isothermal) for 17 min. The temperature of the injector was 250 °C. The carrier gas was He and the operation mode was splitless.
1H NMR spectra were made on BRUKER Avance-300 instrument using TMS as internal standard in CDCl3.
2.1 Preparation of the Catalysts
Å molecular sieve (4A) was impregnated with the corresponding metal compound (TiCl4, ZrCl4, In(NO3)3xH2O) as follows: 1 mmol of the respective compound was dissolved in 100 ml of deionised water and stirred with 1 g 4A at room temperature for 24 h. The solid was filtered, washed with deionised water and with acetone, then dried in an oven at 150 °C for 1 h.
2.2 Determination of the pH of the Catalysts
The catalyst (1 g) was stirred in 30 mL deionised water under continuous measuring of the pH. The values were accepted after reaching a constant value at least during 10 min.
2.3 Typical reaction conditions
A typical reaction was carried out in a 10 mL flask. Alcohol (5 mmol), 3,4-dihydro-2H-pyran (DHP, 5.5 mmol), Ti4+/4A (0.5 g) and dichloromethane (3 mL) were stirred at 40 °C for 7 h. The solid was filtered, and washed with dichloromethane, then the filtrate was evaporated. The residue was subjected to GC–MS analysis and NMR spectroscopy. The crude product could be purified by column chromatography (Kieselgel, hexane:acetone 4:1).
All products have satisfactory spectral data (1H NMR, MS). The spectral data of the known compounds were identical with those reported in the literature. Representative physical and spectroscopic data of the products:
2.4 2-(3′-Methylbut-2′-enyloxy)tetrahydro-2H-pyran (Table 3, entry 1)
1H NMR, CDCl3: 1.54–1.86 (m, 14H); 3.51 (m, 1H); 3.88 (m, 1H); 3.98 (m, 1H); 4.22 (m, 1H); 5.36 (m 1H).
2.5 Decyloxy-tetrahydro-2H-pyran (Table 3, entry 5)
1H NMR, CDCl3: 0.88 (t, 3H); 1.27 (m, 16H); 1.54-1.59 (m, 6H); 3.34–3.41 (m, 1H); 3.48–3.52 (m, 1H); 3.69–3.77 (m, 1H); 3.84–3.89 (m, 1H), 4.58 (t, 1H).
2.6 Benzyloxy-tetrahydro-2H-pyran (Table 3, entry 7)
1H NMR, CDCl3: 1.50–1.84 (m, 6H); 3.54 (m, 1H); 3.9 (m, 1H); 4.49 (d, 1H); 4.71 (t, 1H); 4.78 (d, 1H); 7.26–7.35 (m, 5H).
2.7 (2-Phenyl-propan-1-yloxy)tetrahydro-2H-pyran (Table 3, entry 10)
1H NMR, CDCl3: 1.32 (m, 3H); 1.4–1.85 (m, 6H); 3.03 (qua, 1H); 3.38–3.52 (m, 1H); 3.73–3.82 (m, 2H); 4.54 (d, 1H); 7.18–7.31 (m, 5H).
3 Results and Discussion
First several catalysts were tested in the reaction of 3-methyl-2-butene-1-ol with DHP. All of them have slightly basic properties (see Table 1). The results are summarised in Table 2.
The pure support 4A proved to be inefficient. The best result was obtained with Ti4+/4A, in boiling dichloromethane (DCM). The desired product was obtained with complete conversion (94 % isolated yield, see Table 2). Zirconium on 4A showed less activity, while indium on 4A was practically inefficient. Thus, as the bulk basicity of the catalyst increases, the yield decreases.
The structure of the Ti4+/4A catalyst was investigated by scanning electron microscopy. Titanium is evenly distributed on the surface of the support. EDS showed 13.67 w/w % titanium on the surface. Comparing this data with the titanium content by ICP-OES (3.99 w/w %), one can state, that titanium incorporated mostly on the surface of the support.
Nitrogen adsorption measurements also verified a remarkable change of the surface of the support. The specific surface of 4A changed from 800 to 122 m2/g. The total pore volume of the molecular sieve decreased from 0.3 to 0.201 cm3/g, the micropore volume was 0.038 cm3/g.
Based on the reaction conditions elaborated a variety of alcohols were reacted with DHP. The results are collected in Table 3.
Primary alcohols gave generally good to excellent isolated yield, except allyl alcohol (entry 3), where the low yield might be explained with the quite high volatility of the product. Long-chain aliphatic alcohols gave good yields (entries 4–6). The difference between the yield obtained with benzyl alcohol (entry 7) and 2-nitrobenzyl alcohol (entry 8) can be explained either by a steric factor of a bulky group in o-position, or by the intramolecular H-bond between the alcoholic hydrogen and the oxygen of the nitro group, which decreases the reactivity of the hydroxyl function. Menthol as secondary alcohol gave the desired product with moderate yield (entry 12). Satisfactory yields were obtained with tertiary alcohols (entries 13–14). In these cases steric effects may play the most important role as well, as dehydration of the alcohols—which is a common side reaction of the tertiary alcohols in acidic media—could not be observed. The low preparative yield obtained for tert-butoxy-tetrahydro-2H-pyran (entry 13) might also be explained with its high volatility.
Substituted phenols (entries 15–16) had poorer reactivity even after a longer reaction time.
The reaction of meparfynol (entry 14) was tested under the classical conditions using p-toluenesulphonic acid catalyst. The reaction mixture darkened immediately after the addition of the catalyst, and a complex mixture of products was formed. 1H NMR investigation of the reaction mixture showed that the yield did not exceed 50 %. As titanium is located on the surface of the support, forming potentially acidic sites, the reaction may happen on these parts of the catalysts. Meanwhile the bulk basic phase may help to avoid the disadvantageous, acid-catalysed side reactions. This may prove the effectiveness of our method.
The reusability of the catalysts was examined also in the reaction of benzyl alcohol and DHP. After the reaction the solid was filtered and washed with dichloromethane, then the solid was treated in boiling dichloromethane for an hour, filtered, then heated at ca. 150 °C for 1 h. The yields in the 1st, 2nd and 3rd runs were 98, 96 and 92 %, respectively. There was no significant difference in the titanium content of the freshly prepared catalyst (3.99 w/w %) and a sample taken after the 1st use (3.85 w/w %).
4 Conclusions
In conclusion, titanium on 4 Å molecular sieve support proved to be useful catalyst for the tetrahydropyranylation of alcohols under mild, slightly basic conditions, which is almost unprecedented in the literature. The catalyst could be simply prepared and it was reused several times with good result after a short treatment with boiling dichloromethane.
References
Sartoni G, Ballini R, Bigi F, Bosica G, Maggi R, Righi P (2004) Chem Rev 104:199–250
Forrest Woods G, Kramer DN (1947) J Am Chem Soc 69:2246
Bernady KF, Floyd MB, Poletto JE, Weiss MJ (1979) J Org Chem 44:1438–1447
Alper H, Dinkes L (1972) Synthesis 81–82
Rezai N, Meybodi FA, Salehi P (2000) Synth Commun 30:1799–1805
Chandrasekhar S, Takhi M, Reddy YR, Mohapatra S, Rao CR, Reddy KV (1997) Tetrahedron 53:14997
Haynes LJ, Plimmer JR (1956) J Chem Soc 4665
Molnár Á, Beregszászi T (1996) Tetrahedron Lett 37:8597–8600
Kumar P, Dinesh CU, Reddy RS, Pandey B (1993) Synthesis 1069–1070
Hegedüs A, Vígh I, Hell Z (2004) Synth Comm 34(22):4145–4152
Hoyer S, Laszlo P, Orlovri M, Polla E (1986) Synthesis 655–657
Miyashita M, Yoshikoshi A, Grieco PA (1977) J Org Chem 42:3772–3774
Bandgar BP, Sadavarte VS, Uppalla LS, Patil SV (2003) Monatshefte 134:425–428
Khazaei A, Rostami A, Mahboubifar M (2007) Catalysis Comm 8:383–388
Kotke M, Schreiner PR (2007) Synthesis 779–790
Chaturverdi D, Kumar A, Ray S (2004) Indian J Chem SecB 43:437–438
Cwik A, Hell Z, Figueras F (2006) Adv Synth Catal 348:523–530
Cwik A, Hell Z, Figueras F (2006) Tetrahedron Lett 47:3023–3026
Cwik A, Hell Z, Figueras F (2005) Org Biomol Chem 3:4307–4309
Németh J, Hell Z (2013) React Kinet Mech Catal 111:115–121
Kiss A, Hell Z, Bálint M (2010) Org Biomol Chem 8:331–335
Fodor A, Kiss A, Debreczeni N, Hell Z, Gresits I (2010) Org Biomol Chem 8:4575–4581
Kiss A, Hell Z (2013) Synth Comm 43:1778–1786
Kiss A, Hell Z (2011) Tetrahedron Lett 52:6021–6023
Acknowledgments
Á. M. is grateful to Chinoin Pharmaceuticals Ltd. for the financial support. The authors express their gratitude to Prof. K. László for the gas adsorption measurements. This work was financially supported by the New Széchenyi Development Plan (TÁMOP-4.2.1/B-09/1/KMR-2010-0002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magyar, Á., Nagy, B. & Hell, Z. Tetrahydropyranylation of Alcohols in the Presence of a Slightly Basic, Heterogeneous Titanium Catalyst. Catal Lett 145, 1876–1879 (2015). https://doi.org/10.1007/s10562-015-1590-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1590-8