Abstract
Enantioselective deacetylation of a set of benzylic acetates via alcoholysis catalyzed by Lipase B from Candida antarctica (CAL-B), under mild conditions is described. A systematic study allows to determine the appropriate combination nucleophile/organic solvent and also to explain the influence of these parameters on the enzymatic catalytic reaction. In all cases, (R)-alcohols are obtained with high ee (up to >99 %) at conversion 36 % < C < 48 %, the selectivity reaching E > 500. The enzymatic reactivity is influenced by the hydrophobicity of solvent and the structure/nature of the nucleophile. Furthermore, CAL-B allows enantio-complementary between transesterifications in non-aqueous media: alcoholysis and acetylation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The use of enzymes as effective catalysts tools in organic synthesis allows access to chiral molecules under mild and eco-compatible conditions [1–4]. The enzymatic kinetic resolution is commonly used to prepare a broad range of chiral building blocks, required in several domains, such as the manufacture of pharmaceuticals, cosmetics, flavors, agricultural and fine chemicals [5–7]. Especially benzylic alcohols are needed as key precursors for the generation of pharmaceuticals [8]. They can be obtained under enantiopure or enantiomeric enriched form by transesterification in organic solvents (acetylation or alcoholysis) [9, 10], or by enzymatic hydrolysis in aqueous or biphasic media, although deacetylation was scarcely investigated [11–15]. This may be an interesting way to be inserted into deracemization process, since efficient methods have been developed over the last decade and bring new pathways for the production of enantioenriched molecules with high yields and selectivities [16–18].
Previously, we have shown that the control of several parameters such as the amount of enzyme and the nature of the acetylating agent dramatically affects both reactivity and selectivity of the Candida antarctica lipase B (CAL-B) catalyzed kinetic resolution through transesterification [19–22]. We have demonstrated that the efficiency of the enzymatic acetylation is multifactorial, several parameters interact simultaneously on both enzymatic reactivity and selectivity. We supposed that the decrease of selectivity caused by reducing the amount of enzyme was probably due to competitive hydrolysis reactions in non aqueous media by the water brought by enzyme [20]. Similarly, we have recently revealed that the use of carbonate salts in the enzymatic hydrolysis of racemic acetates with CAL-B, in non aqueous media, allows a significant enhancement of the reactivity and selectivity of this lipase. Due to the high enantioselectivity of this hydrolysis with large series of substrates, deracemization via Mitsunobu inversion protocol was investigated [23, 24].
In the continuity of our investigations, with the aim to understand the mode of action of CAL-B and to clarify last observations related to hydrolysis competition, we have studied several possibilities to deacetylate acetates (Scheme 1).
In the present paper, we describe the enantioselective deacetylation of some benzylic acetates via alcoholysis catalyzed by CAL-B (Scheme 1, path C), a less studied path [27–36]. The influence of several parameters such as: the nature of the acetyl acceptor, the organic solvent and water on the progress of the enzymatic alcoholysis were examined in order to obtain optically pure alcohols.
2 Experimental Sections
2.1 Chemicals and Materials
All reagents and solvents were of analytical grade and were purchased from Sigma-Aldrich. The Candida antarctica lipase immobilized on acrylic resine CAL-B was purchased from Aldrich. Specific activity >10,000 U/g used without any pre-treatment. The monitoring of the reactions was conducted using TLC on Silica gel 60F254 plates type MERCK 5179, 250 mesh. The separation of the resulting alcohols and the remaining acetates was performed by column chromatography using Silica gel 60 Å, 70–230 mesh 63–200 µm.
2.2 Instrumentations
The spectroscopic characterisation was performed with Brüker spectrometers (300 MHz for 1H, 75 MHz for 13C). Chemical shifts were reported in δ ppm from tetramethylsilane with the solvent resonance as internal standard for 1H NMR and chloroform-d (δ 77.0 ppm) for 13C NMR. IR spectra were recorded on Shimadzu FTIR-8400S spectrometer. Melting points were measured using BÜCHI MELTING POINT B-545. The enantiomeric excesses were measured by gas chromatography on ThermoFinnigan Trace GC, equipped with an automatic autosampler and using a CHIRALSIL-DEX CB column (25 m; 0.25 mm; 0.25 µm). Retention times are reported in minutes.
2.3 General Procedure for the Reduction of Ketones
The racemic alcohols were obtained after reduction of the corresponding ketones using an excess of LiAlH4 diluted in anhydrous ether or with NaBH4 in (THF/water; 4/1 v/v). The reaction mixture was stirred under at 0 °C. The evolution of the reactions was monitored by TLC. After total consumption of ketones, the resulting alcohols were obtained pure in good yields after standard work up. All spectroscopic analysis were detailed in the supplementary data.
2.4 General Procedure for the Chemical Acetylation of Racemic Alcohols (1–7)
The racemic acetates (1a–7a) were obtained by standard classical chemical acetylation of corresponding alcohols, according to the following procedure: to 1 equivalent of racemic alcohol (1–7), 1.2 equivalent of triethylamine and 0.1 equivalent of dimethylaminopyridine (DMAP) dissolved in 4 mL of ether, 1.5 equivalent acetic anhydride were added slowly. The evolution of the reactions was monitored by TLC. The acetates are obtained pure after standard work up, in good yields. All spectroscopic analysis were detailed in the supplementary data.
2.5 General Procedure for the Alcoholysis of Racemic Acetates (1a–7a) with Candida antarctica- B lipase
To 1 mmol of the racemic acetates (1a–7a) dissolved in 2 mL of organic solvent, 2 mmol of the appropriate alcohol and 12 mg of CAL-B, are added. The suspension was stirred at 40 °C for 24 h. The reaction mixture is filtered on Celite and concentrated in vacuo. The remaining acetate and the producing alcohol were separated by chromatography on silica gel (petroleum ether/ethyl acetate: 80/20) and analyzed by chiral GC. The same procedure was followed for the reactions in the presence of 60 mg of molecular sieves 4 Å.
3 Results and Discussion
The effect of both nucleophile and the organic solvent on the deacetylation via alcoholysis of phenylethyl acetate (1a), have been studied. These parameters should have strong influences on the selectivity of the CAL-B in catalyzed kinetic resolution. Thus seven alcohols with different structures were selected, methanol, ethanol, n-propanol, n-butanol, 2-propanol, 2-butanol, t-butanol and two organic solvents with different hydrophobicities: di-isopropylether and toluene.
3.1 Influence of the Nature of Nucleophile on the Enzymatic Alcoholysis of 1-Phenylethyl Acetate (1a)
Alcoholysis of racemic phenylethyl acetate (1a) by the selected alcohols was performed in the presence of catalytic amount of CAL-B, in the appropriate organic solvent (Scheme 2).
The conversion and selectivity of the kinetic resolution were quantified by chiral chromatography, and the results are collected in Table 1.
The data from Table 1 showed high selectivity of CAL-B catalyzed deacylation of (1a) with selectivity factor values reaching E > 500 in favor of the (R)-alcohol enantiomer in all cases. It is to be reported that neither alcoholysis or hydrolysis was observed without enzyme, in both used solvents. On the other hand, a significant conversion rates (respectively 14.7 and 11.2 %, entries 1 and 9) of (1a) deacetylation was recorded without use of alcohols, which strengthens our hypothesis concerning the existence of competitive hydrolysis reactions in non aqueous media [23]. The reactivity was modulated by the nature of the nucleophile and the length of the carbon chain, whether in DIPE or in toluene, conversion vary between 14 % < C < 44 %. Using primary alcohols in DIPE, the reaction rate increases with the length of the chain of the nucleophile. With n-propanol 40.9 % conversion is observed compared to 22.1 % in methanol (entry 4 vs. 2), but conversion decreases (15 %) in presence of n-butanol (entry 5). The best conversion was noted with 2-butanol in DIPE (C = 43.5 %) and (R)-alcohol was obtained with enantiomeric excess eep >99 % (entry 7).
Likewise, with toluene as organic solvent, we observed a direct relationship between the deacetylation efficiency of the 1-phenylethyl acetate (1a) through CAL-B alcoholysis, and the length of the alcohol alkyl radical, without any exception (entries 10–13). Similar observations when using DIPE as organic medium were recorded during the alcoholysis of (1a) by secondary and tertiary alcohols (entries 6, 7 and 8 vs. 14, 15 and 16), and like with DIPE, the deacetylation using 2-butanol gave the highest reaction rate C = 42.6 % with (R)-alcohol enantiopreference (entry15). Furthermore, DIPE and toluene used as solvent gave similar results with the tertiary alcohol: t-BuOH (32.3 and 33.3 % for C, entries 8 and 16). This result should be appealing, since this nucleophile is usually employed as inert solvent in enzymatic catalysis [33, 37, 38] or as co-solvent in enzymatic hydrolysis of 1-phenylethyl acetate (1a) in biphasic systems [39]. These results suggest that this alcohol could perform secondary reactions in enzymatic reactions processes.
3.2 Effect of Molecular Sieves on the Enzymatic Alcoholysis of Benzylic Acetates (1a–3a)
In order to reduce the competition between the nucleophiles present in reaction media (Water/ROH), and regulate the enzymatic catalysis, we have added molecular sieves 4 Å, which have indeed shown an effect on both reactivity and selectivity of Candida Rugosa Lipase (CRL) in transesterification [21]. Thus the addition of the molecular sieves was investigated for the alcoholysis of the phenylethyl acetate [40, 41], the substrate model of our study, as well as for two other acetates [8, 42] (1a–3a).
Each enzymatic alcoholysis reaction was carried out according to Sect. 2.5 (Scheme 3). The progress and the selectivity of the kinetic resolution were analyzed by chiral chromatography, and the results are summarized in Table 2.
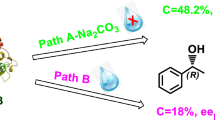
As shown in Table 2, the addition of molecular sieves with both solvents, strongly improves the alcoholysis rate of the deacylation of acetate (1a) using the CAL-B. The deacetylation was extremely enantioselective (E > 500) in all cases. Indeed, either the nature of the nucleophile or the hydrophobicity of the solvent modulate the reactivity of CAL-B catalyzed deacetylation of (1a) (18 % < C < 47.8 %). This is probably due to the addition of molecular sieves which reduces the amount of water introduced by the solvent, the reagents or the immobilized enzyme. This limits the competition between nucleophiles present in the reaction suspension: alcohol and water. The highest conversion was achieved using ethanol in DIPE: C = 47.8 % (entry 3). With the primary acetyl acceptor, CAL-B reactivity depends on the length of the alkyl radical. The same effect, was recently reported in the course of the enzymatic esterification of mandelic acid [43]. This effect is more important in toluene (entries 2–5 vs. 10–13). Using the tertiary alcohol, the reaction rate was markedly decreased (entries 8, 16), it must be underlined, that the best results were recorded with the primary and secondary alcohols.
Furthermore, as shown in the results depicted in Table 2 the alcoholysis of acetates (2a–3a) presents similarities with those obtained with (1a). With acetates (2a) and (3a), the optimal conversions were obtained using 2-BuOH in DIPE and with MeOH in toluene [entries 22, 24 for (2a) and entries 36, 38 for (3a)]. These results indicate a significant dependence 〈〈acetyl acceptor/hydrophobicity of solvent 〉〉 for the catalytic deacetylation catalyzed by CAL-B (Fig. 1).
3.3 Deacetylation via CAL-B Alcoholysis of Some Acetate Precursors of Drugs (1a–7a)
In order to examine if the promising results obtained with acetates (1a–3a) could be extended to a wider scope of substrate, we have decided to test the same experimental conditions with benzylic acetates of well-known pharmacotherapeutic interest. With the suitable deacetylation conditions above described, we have performed a series of reactions on racemic acetates (1a–7a), with 2-BuOH in DIPE and methanol in toluene. The enantiomeric excesses of the recovered acetates and the produced alcohols were evaluated by chiral chromatography. The isolated chemical yields of products were measured after separation by chromatography on silica gel. The results are collected in Table 3.
The data from Table 3 show that CAL-B catalyzed alcoholysis of racemic acetate (1a–7a) in organic medium is highly enantioselective. The conversion rates vary between 40 % < C < 48 % and E > 500 under the tested reaction conditions. The CAL-B reactivity is modulated by the combination of two factors: the nature of alcohol (ROH) and the hydrophobicity of the solvent used for the enzymatic catalytic deacetylation. The lipase produced the (R)-alcohols enantiomerically pure in satisfying isolated chemical yields. These results validate the efficiency of our deacetylation conditions for enzymatic alcoholysis of 1-phenylethyl acetate (1a).
A comparison of the results using CAL-B as an efficient biocatalyst in both alcoholysis of phenylthyl acetate (1a) and transesterification of the corresponding alcohol (1) with isopropenyl acetate reveals a perfect enantiocomplementary of those reactions in favor of the (R)-enantiomer (Scheme 4). Moreover, the alcoholysis reaction appears as an interesting alternative path, complementary to the hydrolysis for resolving acetates. This study contributes to the understanding of factors that significantly affect play a role in the CAL-B catalyzed alcoholysis.
4 Conclusions
We have examined the effect of two important parameters on the enzymatic alcoholysis of a set of benzylic acetates using candida antarctica lipase (CAL-B): the nature of the nucleophile and the hydrophobicity of the solvent. We have optimized these parameters and achieved a highly selective enzymatic deacetylation reaction. In all cases, high selectivity factor values E > 500, with (R)-enantiopreference were recorded. CAL-B reactivity is dependent upon the nature of nucleophile (ROH) whatever the solvent employed. As expected, the primary and secondary alcohols exhibit the best conversion rates C > 40 %, and selectivities E > 500 in kinetic resolution via alcoholysis by the CAL-B lipase in both explored solvents. The addition of molecular sieves 4 Å decreasing the amount of water, enhances the enzymatic reactivity. The best results were recorded using 2-BuOH in DIPE and MeOH in toluene, indicating dependence 〈〈acetyl acceptor/hydrophobicity of solvent〉〉. The novelty of this study is represented by the results obtained by t-butanol used as nucleophile in the alcoholysis of acetates, which shows the influence of alcohol structure on conversion. The results of the alcoholysis catalyzed by CAL-B of a series of benzylic acetates show thus a perfect enantiocomplementary with the enzymatic transesterification studied by our team.
Finally, the improved alcoholysis conditions described appear to be an efficient pathway for enzymatic deacetylation of acetates which offers many opportunities for development, particularly interesting to insert in deracemization processes.
References
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, New York
Anastas PT, Li PT (2010) In: Yukawa H, Blaschek H (eds) Water as a green solvent. Wiley, New York
Anastas PT, Eghbali N (2010) Chem Soc Rev 39:301
Trost BM (1995) Angew Chem Int Ed 107:285
Carrea G, Riva S (2000) Angew Chem Int Ed 39:2226
Faber K (2011) Biotransformations in organic chemistry, 6th edn. Springer, Berlin
Patel RN (2003) Curr Opin Drug Discov Dev 6:902
Patel RN (2008) Coord Chem Rev 252:659
Ahmed M, Kelly T, Ghanem A (2012) Tetrahedron 68:6781
Pan J, Yu HL, Xu JH, Lin GQ (2011) Advances in biocatalysis: enzymatic reactions and their applications. In: Ma S (ed) Asymmetric catalysis from a Chinese perspective. Springer, Berlin, pp 67–103
Aribi-Zouioueche L, Fiaud JC (2000) Tetrahedron Lett 41:4085
Bidjou C, Aribi-Zouioueche L, Fiaud JC (2002) Tetrahedron Lett 43:3025
Bora PP, Bez G, Anal JMH (2011) J Mol Catal B 72:270
Kumaragura T, Fadnavis NW (2012) Tetrahedron 23:775
Bouzemi N, Grib I, Houiene Z, Aribi-Zouioueche L (2014) Catalysts 4:215
Turner NJ (2003) Curr Opin Biotechnol 14:401
Kamal A, Azhar MA, Krishnaji T, Malik MH, Azeeza S (2008) Coord Chem Rev 252:569
Merabet-Khelassi M, Vriamont N, Riant O, Aribi-Zouioueche L (2011) Tetrahedron 22:1790
Bouzemi N, Debbeche H, Aribi-Zouioueche L, Fiand JC (2004) Tetrahedron Lett 45:627
Merabet-Khelassi M, Bouzemi N, Fiaud JC, Riant O, Aribi-Zouioueche L (2011) C R Chim 14:978
Merabet-Khelassi M, Aribi-Zouioueche L, Riant O (2008) Tetrahedron 19:2378
Merabet-Khelassi M, Aribi-Zouioueche L, Riant O (2009) Tetrahedron 20:1371
Merabet-Khelassi M, Houiene Z, Aribi-Zouioueche L, Riant O (2012) Tetrahedron 23:823
Houiene Z, Merabet-Khelassi M, Bouzemi N, Aribi-Zouioueche L, Riant O (2013) Tetrahedron 24:290
Chen CS, Fujimoto Y, Sih CJ (1982) J Am Chem Soc 104:7294
Kagan HB, Fiaud JC (1998) Kinetic resolution. In: Eliel EL, Wilen SH (eds) Topics in stereochemistry, vol 18. Wiley & Sons, New York, pp 249–330
Baldessari A, Iglesias LE (2012) Methods Mol Biol 861:457
Zhang J, Wu J, Yang L (2004) J Mol Catal B 31:67
Tosa M, Pilbak S, Moldovan P, Paizs C, Szatzker G, Szakacs G, Novak L, Irimie FD, Poppe L (2008) Tetrahedron 19:1844
Kawasaki M, Nakamura K, Kawabata S (1999) J Mol Catal B 6:447
Zhou R, Xu JH (2005) Biochem Eng J 23:11
Kato K, Gong Y, Irimescu R, Saito T, Yokogawa Y (2002) Biotechnol Lett 24:1623
Schieweck F, Altenbach HJ (1998) Tetrahedron 9:403
Bencze LC, Paizs C, Tos MI, Trif M, Irimie FD (2010) Tetrahedron 21:1999
Brem J, Pilbak S, Paizs C, Banoczi G, Irimie FD, Tos MI, Poppe L (2011) Tetrahedron 22:916
Faigl F, Kovacs E, Balogh D, Holczbauer T, Czugler M, Simandi B (2014) Central Eur J Chem 12(1):25
Kitaguchi H, Fitzpatrick PA, Huber JE, Klibanov AM (1989) J Am Chem Soc 111:3094
Woudenberg-van Oosterom M, van Rantwijk F, Sheldon RA (1996) Biotechnol Bioeng 49:328
Fan Y, Xie Z, Zhang H, Qian J (2011) Kinet Catal 52(5):686
Suan CL, Sarmidi MR (2004) J Mol Catal B 28:111
Zilbeyaz K, Taskin M, Kurbanoglu EB, Kurbanoglu NI, Kilic H (2010) Chirality 22:543
Sheldon RA (1996) J Chem Tech Biotechnol 67:1
Pan Y, Tang K-W, He C-Q, Yi W, Zhu W, Liu Y-N (2014) Biotechnol Appl Biochem 61:274
Acknowledgments
Algerian Ministry of Higher Education and Scientific Research (MESRS, FNR 2000) and ANDRU (PNR) are gratefully acknowledged for financial support of this work. Prof. Olivier RIANT (IMCN/Louvain Catholic University -UCL- Louvain-La-Neuve, Belgium) is acknowledged for his help and the welcome of Amna ZAÏDI and Mounia MERABET to perform specific analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zaïdi, A., Merabet-Khelassi, M. & Aribi-Zouioueche, L. CAL-B-Catalyzed Enantioselective Deacetylation of Some Benzylic Acetate Derivatives Via Alcoholysis in Non-aqueous Media. Catal Lett 145, 1054–1061 (2015). https://doi.org/10.1007/s10562-014-1470-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1470-7