Abstract
Platelet Rich Plasma (PRP) contains high concentrations of growth factors, therefore, PRP activation results in their release, stimulating the process of healing and regeneration. The study was conducted to check whether activated platelet-rich plasma (aPRP) treatment can improve regeneration of the endometrium in an experimental model of ethanol-induced disturbed endometrium. Seventy-two female Wistar rats were randomly assigned into the control group, disturbed endometrium (DE) group and aPRP treated group. Activation of PRP was performed by adding thrombin. All the animals were sacrificed on day 1, day 3, day 6 and day 9 and samples were taken from the miduterine horn. Quantification of Cytokine and chemokine profiles of activated and non-activated PRP for CCL2, TNF- α, IL-1β, CXCL8, CXCL10, IL2, IL4, IL-6 IL-10, IL-12, IL-17A, TGF- β, IFN-γ was carried out. Functional and structural recovery of the endometrium was analyzed by hematoxylin–eosin (HE) and immunohistochemical (IHC) analyses. HE confirmed proliferated epithelial lining and stromal reconstruction with decreased fibrosis in PRP treated group compared to the DE group. Epithelial thickness in aPRP treated on day 1, day 3, day 6 and day 9 revealed an significant increase (p ≤ 0.05). Significantly stronger IHC expression of alpha smooth muscle actin, Cytokeratin 18, Cytokeratin 19, Connexin-40, E-Cadherin, Claudin-1, Zona Occludin-1was found in the aPRP treated group compared to the DE group. Furthermore, aPRP treatment was associated with birth of live pups. Our results suggest that intrauterine administration of aPRP stimulated and accelerated the regeneration of endometrium in the murine model of disturbed endometrium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regenerative Medicine is considered an advanced technology that utilizes stem cells for the healing and regeneration of diseased organs. However, the prevailing concern for autologous stem cells is the requirement of additional procedures related to the isolation of cells and allogenic stem cells have chance of immunological reaction. In such a scenario, autologous PRP offers promising direction as it is non-immunogenic, able to induce activation of stem cells, remodelling of extracellular matrix (ECM), and new blood vessel formation.
The endometrium is a dynamic organ, which undergoes cyclic rejuvenation and breakdown during the reproductive phase. Endometrial receptivity is a significant factor in implantation. Dysregulated endometrium is a major cause of female infertility. It is usually exemplified by deprived glandular epithelial growth, scattered stroma, reduced vascular development and altered expression of cell adhesion molecules. In vitro fertilization (IVF) treatments require the need of proper endometrial thickness to improve pregnancy rates. The correlation between implantation and junction proteins has been mentioned in various studies (Article 2014; Alleyassin et al. 2016; Warembourg et al. 2014; Report 2018). Several treatments have been attempted to restore endometrial function including estrogen, aspirin, vitamin E, and pentoxifylline, however, outcomes are still poor. Recent studies have reported that intra-uterine infusion of granulocyte colony-stimulating factor (GCSF), and bone marrow-derived stem cells (BM-SCs) are used for the regeneration of damaged endometrium in murine models. However, there are some limitations and some concerning issues that are still unexplained with respect to usability including immune reactions (Zhao et al. 2014). In recent years some studies have stated that intrauterine infusion of platelet-rich plasma (PRP) may be effective in patients with thin and unresponsive endometrium to traditional treatments. However, the scientific basis for the PRP effect on endometrium has not been elucidated, which impedes the acceptance of PRP therapy. A study has validated molecular biological trials to quantify the endometrial regeneration by PRP treatment in a rat model but did not elucidate functional analysis of PRP (Jang et al. 2017). PRP is a rich source of platelet concentration including growth factors (GFs). The GFs regulate normal tissue structure and promote rapid healing of tissue damage. PRP contains platelet-derived growth factor (PDGF), Interleukin (IL), transforming growth factor-beta(TGF-β), epidermal growth factor (EGF), fibroblast growth factor (FGF), Chemokine C–C motif ligand (CCL2), tumor necrosis factor-alpha (TNF- α), chemokine C-X-c motif ligand (CXCL) 8, CXCL10, interferon-GAMMA (IFN-γ). These growth factors play a role in endometrial cell proliferation and differentiation. PRP may have beneficial regenerative effects on damaged endometrium (El-sharkawy et al. 2007). Activation of platelet might influence the release of bioactive molecules exuded by platelet α-granules influencing stem cell trafficking, proliferation, and differentiation with a complex effect on pro-inflammatory processes. In the present study, we explored whether activated PRP (aPRP) treatment can have a beneficial effect on ethanol-disturbed endometrium rat model. The aPRP infusion in disturbed endometrium (DE) can promote endometrial regeneration. To evaluate the potential use of PRP in the endometrial regeneration, we developed a rat model of DE and conducted histological, biological, and functional analysis to investigate whether PRP administration could restore endometrial function and improve pregnancy outcomes. The main finding of our study is that the activation modality of PRP influences PRP clot formation, leading to release of platelet-derived GFs. This activated PRP forms gel layer within intrauterine cavity which will be fruitful for intrauterine cell delivery for endometrial regeneration. These released GFs from activated PRP will boost the adhesion, regeneration and proliferation for delivered cells along with it.
Materials and methods
Preparation of PRP
Two rats were anaesthetized with a subcutaneous injection of Thiosol 1 g/vial, (Neon laboratories Ltd.) using an insulin syringe at a dose of 12 units/gm of body weight. 5 ml of blood was drawn into a sodium citrate tube via cardiac puncture and postcava to collect a good amount of blood from the experimental animals. A needle of 25G was inserted into the ventricle and posterior vena cava slowly. The same procedure was repeated three to four times to collect more blood samples. The blood samples from the tail vein and jugular vein puncture were collected to extract a sufficient amount of PRP but we failed to collect the required amount. The centrifugation of blood was carried out at 1200 rpm for 10 min in a cooling centrifuge. After the centrifugation process, blood gets separated into three layers viz. plasma, buffy-coat, and erythrocytes. The top-most layer, plasma, was then aspirated and stored at -4 °C.Then the middle buffy coat layer was extracted and transferred into a new sterile tube. This layer underwent another 10 min of centrifugation. The platelets were allowed to sediment at the bottom of the tube. While the upper part of the platelet-poor plasma layer was discarded, the lower half of the PRP was carefully collected using a pipette. The entire procedure was performed in highly sterile conditions.
Preparation of thrombin
The plasma of 1000 μl concentration which was stored at -4 °C was diluted 10 times with distilled water, to make a 10 ml solution. The pH was adjusted to 5.3 by adding 100 μl of 1% acetic acid. As thrombin precipitates at pH ≤ 5, it resulted in precipitate formation. It was then centrifuged at 2000 rpm for 5 min and later kept still for 30 min. The precipitate was then re-suspended in normal saline to make a volume of 10 ml solution. The pH was adjusted to approximately 7.This solution was later placed in a 37 °C water bath followed by an addition of 0.1 ml of 0.1 M Calcium Chloride (C5670, Sigma-Aldrich) to it. A clot was formed which was removed in 5 min. Then a water-clear form of thrombin was obtained and stored at -4 °C.
PRP activation
Activation of PRP is done by commercially available thrombin, which is derived from bovine plasma (Plains 1990). The platelets in the PRP contain platelet growth factor (PGF). Whereas the α-granules of the nonactivated PRP contain nonfunctional PGF because they are in contact with the tissue or not released yet. To stimulate the release of these growth factors, platelets must be activated. Thrombin being a potent platelet activator facilitates immediate PGF release from the PRP (Lacoste et al. 2003; Schoenecker et al. 2000). The 0.5 ml of thrombin was added into 0.5 ml of PRP, to activate it, which changed into a semi-solid, jelly-like structure in 3 min.
Cytokine and chemokine profiling of PRP
It is known that thrombin-activated PRP (aPRP) produces high levels of various cytokines and Chemokines. We have analyzed the supernatants from aPRP for these factors. Relative quantification of cytokines and chemokines was carried out by using LEGEND plex Rat Th Cytokine Panel (13-plex) (Biolegend, San Diego, CA) Kit. Cytokine levels of CCL2, TNF- α, IL-1β, CXCL8, CXCL10, IL2, IL4, IL-6 IL-10, IL-12, IL-17A, TGF-β, IFN-γ were measured with flow cytometry, by following the manufacturer’s protocol.
Experimental animals
The study was approved by the Institutional Animal Ethical Committee (1800/PO/ReBi/S/15 /CPCSEA). Male (n = 5) adult Wistar rats and female adult Wistar rats (n = 72), weighing approximately 220 g (range, 190-250 g) each, were used. Two rats were housed together in a standard animal cage of size 420 × 270 × 180 mm. Rats were housed in a light-controlled room with free access to tap water and chow food/pellets, with 12 h of light and 12 h of darkness, and were maintained at a temperature of approximately 24 ºC.
Animal study groups and treatments
The 72 female Wistar rats were allocated into three groups: (I) control group (II) Disrupted endometrium (DE) group (III) activated PRP (aPRP) treated group. The animals were anaesthetized for the surgical procedures with a subcutaneous, extra-peritoneal injection of Thiosol 1 g/vial, (Neon laboratories Ltd.) at a dose of 12 units/250 gm of body weight.. The aPRP treated group was mated with 5 healthy male rats at 15 days after experiment initiation. In the Control group (n = 24), the uterine horns were exteriorized and clamped just above the cervix with small curved hemostatic forceps. The 0.25 ml intrauterine infusion of normal physiological saline was subjected to bilateral intrauterine horns. Similarly DE group (n = 24) was injected with 95% ethanol into the uterine horns to disrupt the endometrial lining. After one day of endometrial disruption, each rat in the DE group was administered with aPRP. The aPRP-treated group (n = 24) subjected to the administration of 0.5mlthrombin activated PRP into both uterine cavities in the same manner initially. The six animals from each group were sacrificed at Day 1 (D1), Day 3 (D3), Day 6 (D6) and Day 9 (D9) intervals from each group. Mid uterine horns were excised immediately after the animals were sacrificed and placed into 10% neutral buffer formalin for histological analysis.
Hematoxylin–eosin staining analysis
The uterine specimens were fixed in 10% formalin for 24 h, embedded in paraffin, sectioned into 4-μm thick sections and allowed to stain with hematoxylin–eosin (H&E). Endometrial morphology was analyzed by H&E staining, and images were captured using the bright-field microscope (Nikon). Images were captured at magnifications of 20 × . To determine the area of the endometrium (μm), each slide was analyzed in a double-blinded manner by two experts using Image J image analysis software.
IHC analysis
The intensification of epithelial, stromal, and vascular cells was assessed by immunohistochemical (IHC) for alpha smooth muscle actin (α-SMA), Cytokeratin (CK)18, CK 19, Connexin-40 (Cx-40), E-Cadherin (E-Cad), Claudin-1(Cla-1), Zona Occludin-1 (ZO-1).After deparaffinization and rehydration, antigen retrieval was carried out with 0.01 M sodium citrate buffer (pH 6.0)in a pressure cooker at 100 °C for 20 min followed by a 20 min cooling time. The tissue sections were incubated and nonspecific binding was blocked with respective serum for 45 min at room temperature. Sections were then incubated withα-SMA(mouse monoclonal;14–9760-82; Invitrogen; USA), CK 18 (mouse monoclonal; ab7753; Abcam, UK), CK 19 (mouse monoclonal; ab7753, ABD serotech; UK), E-Cad (Mab anti human: Invitrogen, USA), Cla-1 (Rabbit anti human: Invitrogen, USA), ZO-1 (Mab anti human: Invitrogen, USA) at a dilution of 1:200, in BSA (Hi Media) in humid chamber followed by washing with DW containing 0.05% Tween 20. This was followed by incubation of sections for 60 min at room temperature with a secondary antibody labeled with Alexa 488 (Molecular Probe) in dark according to the manufacturer’s instructions.
After washing with D/W containing 0.05% tween 20, sections were counterstained with DAPI (Invitrogen). Sections were mounted in fluorescent mounting medium (Dako, Agilent Technologies, California, USA). Negative controls were stained without primary antibodies. Stained sections were examined under the fluorescence microscope (NIKON, Japan). This assessment was successively analyzed in a double-blind manner by two investigators with ImageJ software as previously described. For quantitative assessment of protein expressions of IHC staining, five randomly selected section fields of endometrial tissue slides were scanned into a TIFF image file at a magnification of × 20. Immunostaining is assessed using H score. H score = Ʃ Pi(i + 1), where i is the intensity of staining (Greene et al. 2000; Poncelet et al. 2010). The α-SMA, CK-18, CK 19, E-Cad, Cla-1, ZO-1,Connexin-40 positive samples were defined as having low positive intensity (1), medium positive intensity (2) or high positive intensity (3) signal and Pi is the percentage of stained epithelial cells ranging from 0 to 100%. The H-score ranges from 0 to 4.
Treatment of aPRP improves the birth rate of live pups
Female rats in aPRP treated group were mated with healthy males fifteen days after initiation of the experimental study. Pregnancy outcomes included the time to conceive and live-birth rate.
Results
Activated PRP released cytokines and chemokines
The cytokines and chemokines release profile is summarized in Table 1 and Fig. 1.
Cytokine levels in PRP and aPRP (highly significant***; significant**) Detection of cytokines and chemokines in inactivated and activated PRP. The levels of common cytokines and chemokinesCCL2, TNF- α, IL-1 BETA, CXCL8, CXCL10, IL2, IL4, IL-6 IL-10, IL-12, IL-17A, TGF-BETA, IFN-γ in rat PRP were assessed using LEGENDplex Rat ThCytokine Panel (13-plex) (Biolegend, San Diego, CA) Kit. It showed high levels of IL-6 and IL-8, while low levels of IL-1α, IL-1β and GM-CSF were detected
No detectable levels of IL-β, IL 4 and IL 12 were found in activated samples. Significantly lower amounts of TNF-α, IL 6, IL 10 and IL 17 were detected in activated PRP as compared to the non-activated PRP (p < 0.05). Activated PRP produced significantly higher amounts of CCl2, CXCL 10, IL-2, TGF-β with respect to that of TNF-α, IL 6, IL 10 and IL 17 (p < 0.05). CXCL-8 and IFNγ induced a significantly high Cytokine and Chemokine release with respect to that of IL-β, IL 4 and IL 12 (p < 0.05)(Fig. 1).
Intrauterine Infusion of aPRP restores endometrial epithelium in a DE rat Model
The effect of aPRP was assessed on DE at 1,3,6,9daysafteraPRP treatment. For this purpose, we conducted hematoxylin and eosin staining (HE). In tissue sections from DE that did not undergo PRP treatment (DE group),HE revealed a thin endometrial atrophic columnar epithelium lining, with degenerative changes and discrete stroma (Fig. 2, middle panel).However, well-organized proliferated glandular and endometrial stromal cells were observed in the aPRP treated group (Fig. 2,right panel) as compared to the control (Fig. 2, Left panel). aPRP treatment group revealed an significant increase in epithelial thickness at Day 1, day 3, day 6 and day 9. As compared to the control and aPRP treated group, the DE group showed much decline in epithelial thickness. These results specified that aPRP treatment on DE promoted the recovery of endometrial structure in disturbed endometrium (Fig. 3).The evaluation of epithelial thickness was calculated by using image J software Tables 2 and 3.
Hematoxylin and eosin analysis The aPRP infusion improves the regeneration of the DE and diminishes fibrotic changes. Hematoxylin and eosin staining of the endometrial tissues to evaluate morphologic structures. Control (left panel) showed normal morphology of epithelial cells. DE (middle panel) evaluated discrete epithelial morphology with fibrosis. The aPRP-treated (right panel) revealed normal epithelial morphology with no fibrosis similar to the control group
Immunohistochemical evaluation
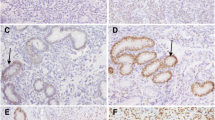
Immunohistochemical expressions were evaluated for the surface epithelial cells, glandular epithelial cells and stromal cells. Positive nuclei to α-SMA, CK18, CK 19, Cx-40, E-Cad, Cla-1, ZO-1 were fluorescent green stained. Treated animals showed the highest expressions in surface epithelium, stromal region and glandular epithelium at D1, D3, D6 and D9 compared to DE group (P < 0.05). The control animals attained high expression score in surface epithelium and stromal cells that is not statistically different compared to treated D1group (P > 0.05).
CK was mainly localized in the endometrial epithelial cells and endometrial glands; whereas, the expressions of Cx-40, E-Cad, Cla-1 and ZO-1 were observed in the endometrial stromal cells and endometrial epithelial cells. IHC nuclear staining for α-SMA was observed nearly similar to control group in the endometrial cells. Compared to the control group, quantitative comparison of IHC staining in the DE group showed significantly decreased expressions of α-SMA, CK18, CK 19, Cx-40, E-Cad, Cla-1 and ZO-1 in all days group. The expression of these factors was significantly higher in the activated PRP treated group, compared to the DE group. In addition, there was no significant difference in the expression of these factors between the PRP-treated group and the control group (Fig. 4).
Treatment of Activated PRP in DE Improves Live pups in Wistar rat
Wistar rats took an average of three days to conceive, as confirmed by vaginal plug smears.
The female rat was mated with a fertile male. All rats from the control group gave birth to live pups. DE group did not give birth to any live pups. All rats from aPRP treated groups delivered healthy pups. The Control group delivered average 8 live pups while aPRP treated group gave birth to 13 pups. Though the average days required to conceive for aPRP treated group was a little longer, the number of live pups indicates that aPRP treatment improves better receptivity. These results indicate that endometrial disruption caused by endometrial injury could be restored by activated PRP treatment. Activated PRP treatment improved the rate of livebirths since DE group failed to deliver.
Discussion
This study proposes that intrauterine administration of autologous PRP exerts morphological proliferative effects on damaged endometrium. PRP is known to restrain growth factors and cytokines that accelerate cell proliferation, vascular angiogenesis and cell migration resulting in speedy healing and regeneration (Gonzalez et al. 2021). To date, there have been no specific studies evaluating the effects of thrombin-activated PRP on endometrial regeneration in murine models. This study revealed that CCL2, TNF- α, IL-1 β, CXCL8, CXCL10, IL2, IL4, IL-6 IL-10, IL-12, IL-17A, TGF- β, IFN-γ play critical roles in cellular proliferation within the endometrium. These cytokines and chemokines produced by aPRP potentially exhibited an anti-inflammatory potential. Since PRP was activated and lysed by the action of thrombin, PRP was capable to release cytokines. Thereby, making it an easier and more effective strategy to deliver platelet bioactive molecules. In the present study, using cytokine and chemokine profiling, we detected the expression of CCL2, TNF- α, IL-1β, CXCL8, CXCL10, IL2, IL4, IL-6 IL-10, IL-12, IL-17A, TGF-β, IFN-γ. Significantly increased expression of CCl2, CXCL 10, IL-2, and TGF-β is noted in the aPRP when compared to non-activated PRP, but no significant change in the expressions of IL- β, IL 4 and IL 12. Significantly minor amounts of TNF-α, IL 6, IL 10 and IL 17 were detected in activated PRP as compared to the non-activated PRP (ρ < 0.05). aPRP secrete elevated levels of IL 6, IL 10 and IL 17 as an anti-inflammatory cytokine, which is mediated through inhibitory effects on TNF-α. Thus, the high levels of IL 6, IL 10 and IL 17 produced by aPRP may counterbalance the disturbed endometrium-induced inflammation caused by pro-inflammatory cytokines. CCL2 regulates cellular adhesion through the activation of β integrin. TGF-β, CXCL 8 and CXCL10 are involved in the wound healing cascade. CCl2 and TGF-β are engaged in the normal progression of the healing processes. These cytokines and chemokines are involved in the modulation of endometrial cell growth, proliferation and implantation.
Endometrial epithelial lining and junction proteins are studied by HE and IHC analysis respectively. Treatment of thrombin-activated PRP on the DE rat model illustrated the enhancement of epithelial proliferation in endometrium. Moreover, treatment with PRP may suggest a decrease in fibrotic lesions in the disturbed endometrium. These results provided correlative evidence for the possible use of activated PRP in achieving implantation with improvement in uterine vascularization and endometrial receptivity. Immunohistochemical analysis on the expression of α-SMA, CK18, CK 19, Cx-40, E-Cad, Cla-1 and ZO-1 showed significant differences between the DE group and aPRP-treated groups. In the present study, an increased expression of above-mentioned proteins was noted in the aPRP-treated group, compared to the DE group which indicates cellular proliferation in the endometrium. Additionally, α-SMA is known to play a central role in the remodelling of cellular functions and the healing process. The intensity of CK 18 and CK 19 was significantly strong in the aPRP-treated group, compared to the DE group located in the epithelial lining and columnar epithelium. CK is a cellular marker for the epithelium. Thus, aPRP may accelerate the induction of endometrial epithelial differentiation. Our analysis revealed the expressions of Cx 40 and E-Cad at stromal endometrium and epithelial lining regulate cell adhesion and proper exchange of molecules within cells. Cla-1, ZO-1 along with Cx 40 and E-Cad are involved in regulating endometrial function and development, along with endometrial receptivity for establishing the necessary conditions for implantation in rats. The expressions of junction proteins identified as cell surface-specific markers of stem cells in the human endometrium. These results assisted us to consider that endometrial stem cells may be involved in the proliferative effects of aPRP on disturbed endometrium. The aPRP may stimulate the endometrial stem cells and promote regeneration. Hence the combined use of aPRP and stem cells may improve the outcome of treatment for disturbed endometrium.
On the other hand, the functional analysis of PRP infusion effectiveness is clarified. The intrauterine infusion of aPRP can promote endometrial regeneration after endometrial damage and improve endometrial receptivity in a DE rat model by restoring the histological structure. Furthermore, aPRP treatment augmented receptivity outcomes. The rats carried full-term pregnancies and delivered healthy pups, thereby, ascertaining the clinical use of aPRP in disrupted endometrium. The results suggest that aPRP infusion may be a productive approach for treating impaired endometrium in the clinical setting.
Another significant task is to determine which PRP elements improve which pathways and to investigate the underlying mechanisms. The PRP infusion affects the pro-inflammatory factors and endometrial proliferation, emphasizing the valuable effect of aPRP treatment in the murine model. We demonstrated that aPRP helps down-regulate fibrosis, restores endometrial function and improves implantation outcomes following endometrial disturbance in rats, enabling full term delivery and live births. These promoting findings set up the theoretical basis for the capacity of aPRP to promote endometrial regeneration and improve implantation outcomes. It will support the clinical application of aPRP treatment with compromised endometrial pathologies.
Conclusion
This study showed the positive effects of aPRP as a potential treatment for disturbed unresponsive endometrium. The clinical application of aPRP and its effects on compromised endometrium is still at a preliminary stage. Further explorations of study and clinical trials are necessitated for the optimization of aPRP application along with a larger randomized study to determine the effect of PRP in implantation failure in humans.
References
Alleyassin A, Abiri A, Fatemeh MA. (2016) The value of routine hysteroscopy before the first intracytoplasmic sperm injection treatment cycle.
Article O. (2014) In vitro fertilization outcome following embryo transfer with or without preinstillation of human chorionic gonadotropin into the uterine cavity : a randomized controlled trial.
El-sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M et al (2007) Platelet-Rich Plasma : growth factors and pro-and anti-inflammatory properties. J Periodontol 78(4):661–669
Gonzalez R, Silva G, Alkass N, Liana T, Ohana G, Furtado L et al (2021) Cytokine The regenerative mechanisms of platelet-rich plasma : a review. Cytokine 144:155560
Greene W, Paulson M, Meyer WR, Fritz MA (2000) Reproductive Endocrinology use of integrins to date the endometrium. Fertil Steril 73(4):779–787
Jang H, Myoung SM, Choe JM, Kim T, Cheon Y, Kim YM et al (2017) Effects of autologous platelet-rich plasma on regeneration of damaged endometrium in female rats. Yonsei Med J 58(6):1195–1203
Lacoste E, Martineau I, Gagnon G (2003) Platelet concentrates: effects of calcium and thrombin on endothelial cell proliferation and growth factor release. J Periodontol 74(10):1498–1507
Plains M. (1990) Antibodies to thrombin and factor v with recurrent bleeding in a patient exposed to topical bovine thrombin.
Poncelet C, Tepper M, Sauce E, Magan N, Wolf JP, Ziol M et al (2010) Expression of E- and N-cadherin and CD44 in endometrium and hydrosalpinges from infertile women. Fertil Steril 94(7):2909–2912
Report C (2018) Unexplained primary infertility is associated with lack of tight and adherence junction between endometrial cells. Hum Reprod 10:1–4
Schoenecker JG, Hauck RK, Mercer MEGC, Parker W, Lawson JH (2000) Exposure to topical bovine thrombin during surgery elicits a response against the xenogeneic carbohydrate galactose ␣ 1–3galactose. J Clin Immunol 20(6):434–444
Warembourg S, Huberlant S, Garric X, Leprince S, De TR, Letouzey V (2014) Prévention et traitement des synéchies endo-utérines : revue de la littérature Prevention and treatment of intra-uterine synechiae : review. J Gynecol Obstet Biol La Reprod. https://doi.org/10.1016/j.jgyn.2014.10.014
Zhao J, Zhang Q, Wang Y, Li Y (2014) Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reproductive Sci 22(2):181–188
Acknowledgements
Dr. Meghnad G. Joshi acknowledges the research funding support from the D.Y. Patil Education Society (Institution Deemed to be University) (DYPES/DU/R&D/2023/261). Authors like to acknowledge Mr. Nimish Deshpande for help with Prism and Image J software.
Author information
Authors and Affiliations
Contributions
The experiments were carried out by JK, MJ, and AK, who also wrote the manuscript. The data was interpreted by MJ, KT, MD, ND, AK, and LC. Data was examined by RK and MJ. The final text was reviewed and approved by all authors. All of the authors, whose names are on the article, approved of its substance and gave their explicit approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The Institutional Animal Ethics Committee (1800/PO/ReBi/S/15/CPCSEA) granted us permission. The work was authorized by the Institutional Research Committee of the D.Y. Patil Education Society (Institution Considered to Be a University), Kolhapur (DYPES/DU/2017/03).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kshersagar, J., Kawale, A.A., Tardalkar, K. et al. Activated platelet-rich plasma accelerate endometrial regeneration and improve pregnancy outcomes in murine model of disturbed endometrium. Cell Tissue Bank 25, 453–461 (2024). https://doi.org/10.1007/s10561-023-10101-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-023-10101-4