Abstract
The corneal endothelium is a monolayer, which mediates solute and water flux across the posterior corneal surface. Alcaine’s main component proparacaine is paramount in human corneal endothelium (HCE) cell regulation. This study explored the mechanism of alcaine in regulating HCE cells. HCE cell morphology under gradient concentrations was observed by an optical microscope. Cell proliferation and viability were detected by MTT assay to determine the half inhibitory concentration (IC 50). Cell apoptosis rate, HIF-1α mRNA expression, and HIF-1α, p/t-JNK and Caspase-3 protein levels were detected by flow cytometry, RT-qPCR, and Western blot. After treatment with alcaine at 0.625–5 g/L concentration range for 24 h, HCE cells showed cytoplasmic vacuolation, cell shrinkage, separation from culture matrix, and eventual death. Alcaine treated-HCE cell proliferation was decreased in a dose-dependent manner. The IC 50 of alcaine was 1.26 g/L. After alcaine treatment, HCE cell apoptosis rate was promoted and HIF-1α levels in HCE cells were stimulated. Knockdown of HIF-1α partially annulled the effects of alcaine on inhibiting HCE cell proliferation and facilitating apoptosis. Alcaine might activate the JNK/caspase-3 pathway by increasing HIF-1α. The inhibition of the JNK/caspase-3 pathway partially abrogated the effects of alcaine on inhibiting HCE cell proliferation and promoting apoptosis. Alcaine might affect HCE cell proliferation and apoptosis by upregulating HIF-1α and activating the JNK/caspase-3 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human corneal endothelium (HCE) is the most essential monolayer of the cornea physiologically (Tokuda et al. 2020). The unique hexagonal cells work as a barrier for the fluid moving into the stroma because glycoaminoglycan composition can adsorb huge amounts of fluid creating an edematous cornea, which lead to vision loss, and the cell density and monolayer integrity are the key to maintaining the normal thickness, transparency, and visual acuity of the cornea (Peh et al. 2011). In adulthood, HCE cells that have formed a confluent monolayer not only lose their proliferation ability but also die at a rate of 0.3–0.6% per year, which can only rely on the expansion and migration of adjacent cells to repair the integrity of the monolayer (Sabater et al. 2013; Yu et al. 2014). In addition to aging, accidental injury, surgical trauma, and diseases may also lead to excessive loss of HCE cells, thus damaging the physiological function of the cornea (Ishino et al. 2004; Schmedt et al. 2012; Zhu et al. 2008). The decrease of cell density beyond a threshold will damage the integrity of corneal endothelium, which can cause mild corneal edema, reduced transparency and decreased visual acuity, and more seriously, lead to HCE decompensation, and eventually cause corneal endothelium blindness (Tran et al. 2020). Increasing evidence has shown that HCE injury is related to the abuse of local anesthetics (Borazan et al. 2009; Schellini et al. 2007; Wen et al. 2015).
Topical ocular anesthesia plays a vital role in the practice of ophthalmology, both for procedures in the operating room and in the office, and although generally well tolerated, it can be toxic, especially when abused, which has toxicities to the ocular surface including deep corneal ulceration, infiltrates and even perforation (Shah et al. 2010). The most commonly adopted drugs at present are tetracaine, proparacaine (0.5% alcaine), lidocaine, and benoxinate (oxybuprocaine) cocaine (McGee and Fraunfelder 2007). Proparacaine, the main component of alcaine, one of the commonly used surface anesthetics, is an active anesthetic agent, which is a commonly used anesthetic for ophthalmology surgery (Dang et al. 2022). Proparacaine has cytotoxicity to both cat corneal endothelial cells in vivo and HCE cells in vitro, and its time- and dose-dependent cytotoxicity to HCE cells is realized by inducing apoptosis through the mitochondrion-mediated caspase-dependent pathway (Wen et al. 2015). Hypoxia inducible factor-1α (HIF-1α) is a transcription factor in response to the hypoxic conditions, and its involvement in apoptosis regulation is thought to be achieved indirectly through transcriptional activation of genes encoding pro- or anti-apoptotic factors (Mylonis et al. 2017). Silenced HIF-1α reduces the area of corneal neovascularization, represses neovascularization, and improves pathological changes (Fu and Xin 2019). c-Jun N-terminal kinase (JNK) participates in oxidative stress injury and also has a critical role in mediating mitochondria-related apoptosis (Xia et al. 2020). Additionally, both extrinsic and intrinsic pathways of apoptosis eventually merge in the caspase cascade, with activated caspase-3 as the most abundant executioner caspases (Beroske et al. 2021). Importantly, HIF-1α is identified to be involved in cell apoptosis by regulating the JNK/caspase-3 signaling pathway (Song et al. 2020). However, the role of alcaine in HCE cell proliferation remains elusive. This study aimed to identify the effect of alcaine on the proliferation of the HCE cells, so as to provide some reference values for the ophthalmic diseases.
Materials and methods
Ethics statement
The experiments were authorized by the academic ethics committee of Tianjin Eye Hospital. All procedures strictly followed the code of Declaration of Helsinki.
Cell culture and transfection
HCE cells (CP-H134) acquired from Procell (Wuhan, Hubei, China) were cultured in the Dulbecco’s modified Eagle medium/Nutrient mixture F12 (DMEM/F12) containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) at 37 °C with 5% CO2.
Cells were allocated to the following 4 groups: (1) control group (without treatment); (2) alcaine group (treated with 1.25 g/L alcaine for 24 h); (3) alcaine + si-NC group (transfected with si-NC and treated with 1.25 g/L alcaine for 24 h); (4) alcaine + si-HIF-1α group (transfected with si-HIF-1α and treated with 1.25 g/L alcaine for 24 h); (5) Alcaine + DMSO group [added with the same dose of dimethyl sulfoxide (DMSO), and treated with 1.25 g/L alcaine for 24 h]; (6) Alcaine + SP600125 group (added with 10 μM SP600125, and treated with 1.25 g/L alcaine for 24 h). si-NC and si-HIF-1α were synthesized by GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine®2000. The transfection concentration was 50 nM. After 24 h, the following experiments were performed. JNK inhibition was induced with 10 μM SP600125 inhibitor (Calbiochem, La Jolla, CA, USA) with a 10 μM stock solution in DMSO (Lo et al. 2022).
Morphological observation of HCE cells
HCE cells were seeded into 24-well plates and cultured in the DMEM/F12 medium containing 10% FBS at 37 °C with 5% CO2 for 24 h. Cells in the logarithmic phase were cultured with 0.625 g/L, 1.25 g/L, 2.5 g/L, and 5 g/L alcaine (Sigma-Aldrich, St. Louis, MO, USA). HCE cells without alcaine treatment were used as the control. After 24 h, the cell morphology and growth state were observed under an Eclipse TS100 inverted light microscope (Nikon, Tokyo, Japan).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
HCE cells were seeded at 1 × 104 into the 96-well plates and cultured under the same conditions. After cells were treated with 0.625–5 g/L alcaine for 0, 6, 12, 24, and 48 h, the medium was refreshed with the serum-free DMEM/F12 (200 μL) supplemented with 1.1 mM MTT (all from Sigma-Aldrich). After 4-h incubation in the dark at 37 °C, the produced formazan was dissolved with 150 μL DMSO (Sigma-Aldrich), and the optical density at 490 nm was detected using a microplate reader (Thermo Scientific, Waltham, MA, USA).
Flow cytometry
Apoptosis was detected using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Biosciences, San Jose, CA, USA). In short, HCE cells in 24-well plates were treated with alcaine, and the cells were collected at 24 h according to the previous method. The cell particles were resuspended with 0.1 mL serum-free DMEM/F12, washed twice with phosphate buffer saline, added with 500 μL buffer solution and 10 μL annexin V-FITC, and reacted at 37 °C in the dark for 30 min after shaking. The apoptosis was detected by flow cytometry.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HCE cells using the TRIzol reagent (Invitrogen). The total RNA was reverse transcribed into cDNA using the Prime Script RT kit (Perfect Real Time) (Takara, Tokyo, Japan). Then, qPCR was performed using SYBR®Premix Ex Taq™II (Takara) on ABI 7900HT rapid PCR real-time system (ABI, Foster City, CA, USA). The reaction conditions were as follows: pre-denaturation at 95 °C for 10 min, and 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s and extension at 72 °C for 34 s. With β-actin as the internal reference, the expression of HIF-1α was measured. The relative expression was calculated by the 2−△△ct method. The experiment was conducted in triplicate. Primer sequences are shown in Table 1.
Western blot
The cells were treated with radio-immunoprecipitation assay lysis (Acmec Biochemical Co., Ltd, Shanghai, China) for 20 min. The cell lysate was centrifuged at 16,000 rpm for 10 min and the supernatant was collected. The protein was separated by 10–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to the nitrocellulose membranes, and incubated with primary antibodies HIF-1α (ab179483, 1:1000, Abcam, Cambridge, MA, USA), JNK (ab179461, 1:1000, Abcam), p-JNK (ab124956, 1:1000, Abcam), Caspase-3 (ab32351, 1:5000, Abcam), and β-Actin (ab8227, 1:1000, Abcam) at 4 °C overnight. Subsequently, the membranes were washed 3 times with Tris-buffered saline with Tween-20 and incubated with horseradish peroxidase-labeled secondary antibody IgG (ab6721, 1:5000, Abcam) at room temperature for 1 h. The band density was measured using ImageJ analysis software (Wright Cell Imaging Facility, Toronto, ON, Canada). β-actin was used as an internal parameter.
Statistical analysis
SPSS 21.0 statistical software (IBM Corp. Armonk, NY, USA) and GraphPad Prism 8.01 (GraphPad Software Inc., San Diego, CA, USA) were employed for statistical analysis and mapping. Shapiro Wilk test revealed that the data were normally distributed. The data were expressed as mean ± standard deviation. Independent t test was employed for comparisons between groups and one-way analysis of variance (ANOVA) was applied for comparisons among groups, followed by Tukey’s multiple comparisons test. P < 0.05 was indicative of statistical significance.
Results
Alcaine inhibited the proliferation of HCE cells in a concentration dependent manner
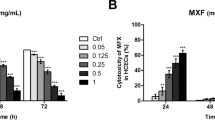
To evaluate the cytotoxicity of alcaine in vitro, the morphology, growth state, and viability of alcaine-treated HCE cells were studied. The morphology of HCE cells was observed using an optical microscope. HCE cells treated with alcaine at the concentration range of 0.625–5 g/L for 24 h showed growth delay and abnormal morphological changes similar to apoptotic cells in a dose-dependent manner, including cytoplasmic vacuolation, cell shrinkage, detachment from the culture matrix, and eventual death (Fig. 1A). In addition, MTT results revealed that with the increase of alcaine concentration, cell proliferation rate showed a gradual downward trend, and when the concentration of alcaine was 0.625 g/L, 1.25 g/L, 2.5 g/L and 5 g/L, and the action time increased from 6 to 24 h, cell survival rate was decreased (P < 0.05). But interestingly, when the action time increased from 24 to 48 h, cell proliferation rate did not decrease significantly (P > 0.05) (Fig. 1B). In addition, through calculation, the IC50 values of alcaine at 6 h, 12 h, 24 h and 48 h were 7.27 g/L, 3.36 g/L, 1.26 g/L and 1.06 g/L, respectively, and there was no significant difference between the IC50 values at 24 h and 48 h (Fig. 1B). Therefore, alcaine 1.25 g/L was selected for the following experiments.
Alcaine inhibited the proliferation of HCE cells in a concentration dependent manner. The HCE cells were treated with alcaine (0.625–5 g/L) or without alcaine for 24 h, and A The morphology of HCE cells at gradient concentration was observed using an optical microscope; B Cell proliferation rate was detected by MTT assay. The cell experiment was performed 3 times independently, and the data were expressed as mean ± standard deviation. One-way ANOVA was applied for comparisons among groups, followed by Tukey’s multiple comparisons test. *P < 0.05, **P <0.01
Alcaine might affect HCE cell apoptosis through HIF-1α
To determine whether the cytotoxicity of alcaine was achieved by inducing apoptosis, the apoptosis was detected by flow cytometry. Compared with the control group, the apoptosis rate of alcaine-treated HCE cells was promoted (P < 0.01) (Fig. 2A), indicating that alcaine increased the apoptosis rate of HCE cells. HIF-1α is involved in apoptosis (Song et al. 2020). Therefore, the expression of HIF-1α in HCE cells was measured by RT-qPCR and Western blot. Compared with the control group, the expression of HIF-1α in alcaine-treated HCE cells was facilitated (P < 0.01) (Fig. 2B, C). These suggested that alcaine might affect HCE cell apoptosis through HIF-1α.
Alcaine may affect HCE cell apoptosis through HIF-1α. A The apoptosis rate of HCE cells was detected by flow cytometry; B mRNA expression of HIF-1α in HCE cells was detected by RT-qPCR; C Protein level of HIF-1α in HCE cells was detected by Western blot. The cell experiment was performed 3 times independently, and the data were expressed as mean ± standard deviation. Independent t test was applied for comparisons between groups. **P < 0.01
Knockdown of HIF-1α partially annulled the effects of alcaine on HCE cells
To further explore the mechanism of HIF-1α in HCE cell apoptosis, the expression of HIF-1α was silenced by introducing si-HIF-1α into alcaine-treated HCE cells. RT-qPCR and Western blot demonstrated that compared with the alcaine + si-NC group, HIF-1α levels in the alcaine + si-HIF-1α group were diminished (P < 0.01) (Fig. 3A, B). Cell proliferation was detected by MTT assay. Inhibition of HIF-1α expression partially abrogated the inhibiting effect of alcaine on HCE cell proliferation (P < 0.01) (Fig. 3C). Flow cytometry manifested that silencing of HIF-1α expression partially averted the promoting effect of alcaine on HCE cell apoptosis (P < 0.01) (Fig. 3D). These results suggested that silencing HIF-1α partially reversed the effects of alcaine on HCE cells.
Inhibition of HIF-1α partially averted the effects of alcaine on HCE cells. The expression of HIF-1α was knocked down by transfection of si-HIF-1α into alcaine-treated HCE cells. A The mRNA expression of HIF-1α in HCE cells was measured by RT-qPCR; B Protein level of HIF-1α in HCE cells was measured by Western blot; C Cell proliferation rate was detected by MTT assay; D The apoptosis rate of HCE cells was detected by flow cytometry. The cell experiment was performed 3 times independently, and the data were expressed as mean ± standard deviation. Independent t test was applied for comparisons between groups. **P < 0.01
Alcaine might activate the JNK/caspase-3 pathway by stimulating HIF-1α
HIF-1α can act as a pro-apoptotic factor by activating the JNK/caspase-3 pathway (Song et al. 2020). To further study whether alcaine activated the JNK/caspase-3 pathway by increasing HIF-1α, the protein levels of p/t-JNK and Caspase-3 in HCE cells of each treatment group were analyzed by Western blot. As shown in Fig. 4, compared with the control group, the protein levels of p/t-JNK and Caspase-3 in HCE cells treated with alcaine was elevated (P < 0.01). Compared with the alcaine + si-NC group, the levels of p/t-JNK and Caspase-3 in the alcaine + si-HIF-1α group were lowered (P < 0.01). The results suggested that alcaine might affect the proliferation and apoptosis of HCE cells by increasing HIF-1α and activating the JNK/caspase-3 pathway.
Alcaine might activate the JNK/caspase-3 pathway by stimulating HIF-1α. The protein levels of p/t-JNK and Caspase-3 in HCE cells were measured by Western blot. The cell experiment was performed 3 times independently, and the data were expressed as mean ± standard deviation. One-way ANOVA was applied for comparisons among groups, followed by Tukey’s multiple comparisons test. **P < 0.01
Inhibition of the JNK/caspase-3 pathway partially reversed the effects of alcaine on HCE cells
To further confirm that alcaine activated the JNK/caspase-3 pathway, we added 10 μM SP600125 (JNK inhibitor) to inhibit the JNK/caspase-3 pathway in alcaine-treated HCE cells. Western blot revealed that the expression of JNK in the Alcaine + SP600125 group was lower than that in the Alcaine + DMSO group (P < 0.01) (Fig. 5A). MTT assay showed that inhibition of JNK expression partially averted the effect of alcaine on HCE cell proliferation (P < 0.01) (Fig. 5B). Flow cytometry manifested that inhibition of JNK expression partially annulled the effect of alcaine on HCE cell apoptosis (P < 0.01) (Fig. 5C). The results indicated that inhibition of the JNK/caspase-3 pathway partially averted the effects of alcaine on HCE cells.
Inhibition of the JNK/caspase-3 pathway partially averted the effects of alcaine on HCE cells. The JNK/caspase-3 pathway was inhibited by adding 10 μM SP600125 (JNK inhibitor) to alcaine-treated HCE cells. A The expression levels of JNK and caspase-3 in HCE cells were assessed by Western blot; B MTT assay was used to detect cell proliferation rate; C HCE cell apoptosis rate was detected by flow cytometry. Cell experiment was repeated 3 times independently. Data were expressed as mean ± standard deviation. The comparison between 2 groups was performed by independent sample t-test. **P < 0.01
Discussion
A transparent cornea is principal for vision because it mediates the light entering into the eyes, and the HCE cells that form a monolayer of hexagonal, polarized cells lying on the Descemet’s membrane, influence the entire cornea transparency because its main property is to maintain the adequate thickness and hydration of the corneal stroma (Smeringaiova et al. 2021). The damage to HCE cells by a number of pathological conditions results in severe vision loss (Tokuda et al. 2020). Topical anesthetic agents including proparacaine have a role in ophthalmic operations, and the abuse of local anesthetics is associated with HCE injury (Borazan et al. 2009; Moreira et al. 1999; Schellini et al. 2007). This study revealed that alcaine might affect HCE cell proliferation and apoptosis by upregulating HIF-1α and activating the JNK/caspase-3 pathway.
Topical ocular anesthesia, including proparacaine, is essential for ophthalmology practice but can be toxic to the ocular surface including deep corneal ulceration, infiltrates and even perforation (Shah et al. 2010). To evaluate the cytotoxicity of alcaine, we treated HCE cells with alcaine at a concentration range of 0.625–5 g/L for 24 h and observed that the cells manifested growth delay, abnormal morphological changes, and decreased proliferation ability in a dose-dependent manner, and the IC 50 of alcaine was 1.26 g/L. Consistently, HCE cells have a time- and dose-dependent toxic response to proparacaine (Wen et al. 2015). Therefore, we chose alcaine with 1.26 g/L concentration for study.
HIF-1α is involved in the regulation of cell apoptosis (Song et al. 2020). To determine whether the cytotoxicity of alcaine was achieved by inducing apoptosis, our results manifested that alcaine stimulated the apoptosis rate of HCE cells. HIF-1α expression in alcaine-treated HCE cells was augmented. It is consistent that proparacaine induces mitochondria-dependent apoptosis and cytotoxicity in corneal stromal cells both in vivo and in vitro (Fan et al. 2016). However, at present, there is little foreign and abroad report on the relationship between alcaine or proparacaine and HIF-1α. This study demonstrated that alcaine might affect HCE cell apoptosis through HIF-1α for the first time. To further study the effects of HIF-1α on HCE cell apoptosis, we knocked down HIF-1α expression in HCE cells and discovered that knockdown of HIF-1α partially annulled the effects of alcaine on blocking HCE cell proliferation and inducing HCE cell apoptosis. Similarly, downregulation of HIF-1α represses the apoptosis of aortic endothelial cells in chronic intermittent hypoxia mice (Ding et al. 2020). Knockdown of HIF-1α prevented hypoxia-induced increase of C/EBP homologous protein and apoptosis in alveolar epithelial cells (Delbrel et al. 2018). In conclusion, HIF-1α silencing partially abrogated the effects of alcaine on HCE cells.
Shenshuaikang Enema inhibits hypoxia and reoxygenation-mediated apoptosis in renal tubular epithelial cells by repressing the oxidative damage-dependent JNK/Caspase-3 pathways (Lu et al. 2020). The attenuation of the JNK/caspase-3 pathway activation reduces apoptosis in Neuro2a cells (Wang et al. 2021). To further determine whether alcaine activated the JNK/caspase-3 pathway by stimulating HIF-1α expression, we analyzed p/t-JNK and Caspase-3 protein levels in HCE cells and found that the p/t-JNK and Caspase-3 protein levels were raised in alcaine-treated HCE cells, while the levels were diminished after knockdown of HIF-1α. Consistently, proparacaine has cytotoxicity to HCE cells, and its dose-dependent cytotoxicity to HCE cells is realized by promoting apoptosis through a caspase-dependent pathway (Wen et al. 2015). HIF-1α induces hypoxic apoptosis of osteocytes through the JNK/caspase-3 pathway and the apoptotic-osteocyte-mediated osteoclastogenesis in vitro (Song et al. 2020). In short, alcaine might influence HCE cell apoptosis and proliferation by promoting HIF-1α expression and activating the JNK/caspase-3 pathway. Subsequently, we inhibited the JNK/caspase-3 pathway using 10 μM SP600125 (JNK inhibitor) and elaborated that the inhibition of the JNK/caspase-3 pathway partially averted the effects of alcaine on inhibiting HCE cell proliferation and promoting apoptosis.
In summary, this study supported that alcaine might affect HCE cell proliferation and apoptosis by upregulating HIF-1α and activating the JNK/caspase-3 pathway. However, the results of this study lack further animal experiment in vivo validation and clinical data. The specific mechanism of alcaine in activating the JNK/caspase-3 pathway by regulating HIF-1α expression remains elusive. In addition, other target genes and downstream pathways that may be regulated by alcaine need to be further studied. In the further, we will carry out experimental validation and clinical research in animals and study the mechanism of alcaine regulating other downstream target genes. Moreover, we will study the mechanism of other topical ocular anesthesia on the proliferation and apoptosis of HCE cells.
Availability of data and materials
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Beroske L, Van den Wyngaert T, Stroobants S, Van der Veken P, Elvas F (2021) Molecular imaging of apoptosis: the case of caspase-3 radiotracers. Int J Mol Sci. https://doi.org/10.3390/ijms22083948
Borazan M, Karalezli A, Oto S, Akova YA, Karabay G, Kocbiyik A, Celasun B, Demirhan B (2009) Induction of apoptosis of rabbit corneal endothelial cells by preservative-free lidocaine hydrochloride 2%, ropivacaine 1%, or levobupivacaine 0.75%. J Cataract Refract Surg 35:753–758. https://doi.org/10.1016/j.jcrs.2008.12.016
Dang A, Reddy AJ, Pokala V, Rabara J, Brahmbhatt H (2022) An analysis of the use of proparacaine in cataract surgery. Cureus 14:e22175. https://doi.org/10.7759/cureus.22175
Delbrel E, Soumare A, Naguez A, Label R, Bernard O, Bruhat A, Fafournoux P, Tremblais G, Marchant D, Gille T, Bernaudin JF, Callard P, Kambouchner M, Martinod E, Valeyre D, Uzunhan Y, Planes C, Boncoeur E (2018) HIF-1alpha triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci Rep 8:17939. https://doi.org/10.1038/s41598-018-36063-2
Ding H, Huang J, Wu D, Zhao J, Huang J, Lin Q (2020) Silencing of the long non-coding RNA MEG3 suppresses the apoptosis of aortic endothelial cells in mice with chronic intermittent hypoxia via downregulation of HIF-1alpha by competitively binding to microRNA-135a. J Thorac Dis 12:1903–1916. https://doi.org/10.21037/jtd-19-2472
Fan WY, Sui YL, Fan TJ (2016) Proparacaine induces cytotoxicity and mitochondria-dependent apoptosis in corneal stromal cells both in vitro and in vivo. Toxicol Res (camb) 5:1434–1444. https://doi.org/10.1039/c6tx00286b
Fu YC, Xin ZM (2019) Inhibited corneal neovascularization in rabbits following corneal alkali burn by double-target interference for VEGF and HIF-1alpha. Biosci Rep. https://doi.org/10.1042/BSR20180552
Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, Kinoshita S (2004) Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol vis Sci 45:800–806. https://doi.org/10.1167/iovs.03-0016
Lo CH, Li LC, Yang SF, Tsai CF, Chuang YT, Chu HJ, Ueng KC (2022) MicroRNA Let-7a, -7e and -133a attenuate hypoxia-induced atrial fibrosis via targeting collagen expression and the JNK pathway in HL1 cardiomyocytes. Int J Mol Sci. https://doi.org/10.3390/ijms23179636
Lu H, Luo X, He Y, Qu B, Zhao L, Li M (2020) Shenshuaikang enema, a Chinese herbal remedy, inhibited hypoxia and reoxygenation-induced apoptosis in renal tubular epithelial cells by inhibiting oxidative damage-dependent JNK/Caspase-3 signaling pathways using network pharmacology. Evid Based Complement Alternat Med 2020:9457101. https://doi.org/10.1155/2020/9457101
McGee HT, Fraunfelder FW (2007) Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf 6:637–640. https://doi.org/10.1517/14740338.6.6.637
Moreira LB, Kasetsuwan N, Sanchez D, Shah SS, LaBree L, McDonnell PJ (1999) Toxicity of topical anesthetic agents to human keratocytes in vivo. J Cataract Refract Surg 25:975–980. https://doi.org/10.1016/s0886-3350(99)00075-9
Mylonis I, Kourti M, Samiotaki M, Panayotou G, Simos G (2017) Mortalin-mediated and ERK-controlled targeting of HIF-1alpha to mitochondria confers resistance to apoptosis under hypoxia. J Cell Sci 130:466–479. https://doi.org/10.1242/jcs.195339
Peh GS, Beuerman RW, Colman A, Tan DT, Mehta JS (2011) Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation 91:811–819. https://doi.org/10.1097/TP.0b013e3182111f01
Sabater AL, Guarnieri A, Espana EM, Li W, Prosper F, Moreno-Montanes J (2013) Strategies of human corneal endothelial tissue regeneration. Regen Med 8:183–195. https://doi.org/10.2217/rme.13.11
Schellini SA, Creppe MC, Gregorio EA, Padovani CR (2007) Lidocaine effects on corneal endothelial cell ultrastructure. Vet Ophthalmol 10:239–244. https://doi.org/10.1111/j.1463-5224.2007.00545.x
Schmedt T, Chen Y, Nguyen TT, Li S, Bonanno JA, Jurkunas UV (2012) Telomerase immortalization of human corneal endothelial cells yields functional hexagonal monolayers. PLoS ONE 7:e51427. https://doi.org/10.1371/journal.pone.0051427
Shah H, Reichel E, Busbee B (2010) A novel lidocaine hydrochloride ophthalmic gel for topical ocular anesthesia. Local Reg Anesth 3:57–63. https://doi.org/10.2147/lra.s6453
Smeringaiova I, Utheim TP, Jirsova K (2021) Ex vivo expansion and characterization of human corneal endothelium for transplantation: a review. Stem Cell Res Ther 12:554. https://doi.org/10.1186/s13287-021-02611-3
Song X, Tang Y, Zhu J, Tian Y, Song Z, Hu X, Hong C, Cai Y, Kang F (2020) HIF-1alpha induces hypoxic apoptosis of MLO-Y4 osteocytes via JNK/caspase-3 pathway and the apoptotic-osteocyte-mediated osteoclastogenesis in vitro. Tissue Cell 67:101402. https://doi.org/10.1016/j.tice.2020.101402
Tokuda Y, Okumura N, Komori Y, Hanada N, Tashiro K, Koizumi N, Nakano M (2020) Transcriptome dataset of human corneal endothelium based on ribosomal RNA-depleted RNA-Seq data. Sci Data 7:407. https://doi.org/10.1038/s41597-020-00754-1
Tran TM, Duong H, Bonnet C, Kashanchi A, Buckshey A, Aldave AJ (2020) Corneal blindness in Asia: a systematic review and meta-analysis to identify challenges and opportunities. Cornea 39:1196–1205. https://doi.org/10.1097/ICO.0000000000002374
Wang M, Hayashi H, Horinokita I, Asada M, Iwatani Y, Liu JX, Takagi N (2021) Neuroprotective effects of Senkyunolide I against glutamate-induced cells death by attenuating JNK/caspase-3 activation and apoptosis. Biomed Pharmacother 140:111696. https://doi.org/10.1016/j.biopha.2021.111696
Wen Q, Fan T, Bai S, Sui Y (2015) Cytotoxicity of proparacaine to human corneal endothelial cells in vitro. J Toxicol Sci 40:427–436. https://doi.org/10.2131/jts.40.427
Xia P, Zhang F, Yuan Y, Chen C, Huang Y, Li L, Wang E, Guo Q, Ye Z (2020) ALDH 2 conferred neuroprotection on cerebral ischemic injury by alleviating mitochondria-related apoptosis through JNK/caspase-3 signing pathway. Int J Biol Sci 16:1303–1323. https://doi.org/10.7150/ijbs.38962
Yu HZ, Li YH, Wang RX, Zhou X, Yu MM, Ge Y, Zhao J, Fan TJ (2014) Cytotoxicity of lidocaine to human corneal endothelial cells in vitro. Basic Clin Pharmacol Toxicol 114:352–359. https://doi.org/10.1111/bcpt.12186
Zhu C, Rawe I, Joyce NC (2008) Differential protein expression in human corneal endothelial cells cultured from young and older donors. Mol Vis 14:1805–1814
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
QW and ZZ contributed to the study concepts, study design; QW, XG contributed to the experimental studies and data acquisition; ZZ and XG contributed to the data analysis and statistical analysis; QW, ZZ contributed to the manuscript preparation and XG contributed to the manuscript editing and review; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The experiments were authorized by the academic ethics committee of Tianjin Eye Hospital. All procedures strictly followed the code of Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Zhang, Z. & Gao, X. Effects of ophthalmic surface anesthetic alcaine on the proliferation and apoptosis of human corneal endothelial cells through HIF-1α regulation. Cell Tissue Bank 24, 561–570 (2023). https://doi.org/10.1007/s10561-022-10057-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-022-10057-x