Abstract
Human periodontal ligament stem cells (hPDLSCs) are vital in cellular regeneration and tissue repair due to their multilineage differentiation potential. Low intensity pulsed ultrasound (LIPUS) has been applied for treating bone and cartilage defects. This study explored the role of LIPUS in the immunomodulation and osteogenesis of hPDLSCs. hPDLSCs were cultured in vitro, and the effect of different intensities of LIPUS (30, 60, and 90 mW/cm2) on hPDLSC viability was measured. hPDLSCs irradiated by LIPUS and stimulated by lipopolysaccharide (LPS) and LIPUS (90 mW/cm2) were co-cultured with peripheral blood mononuclear cells (PBMCs). Levels of immunomodulatory factors in hPDLSCs and inflammatory factors in PBMCs were estimated, along with determination of osteogenesis-related gene expression in LIPUS-irradiated hPDLSCs. The mineralized nodules and alkaline phosphatase (ALP) activity of hPDLSCs and levels of IκBα, p-IκBα, and p65 subunits of NF-κB were determined. hPDLSC viability was increased as LIPUS intensity increased. Immunomodulatory factors were elevated in LIPUS-irradiated hPDLSCs, and inflammatory factors were reduced in PBMCs. Osteogenesis-related genes, mineralized nodules, and ALP activity were promoted in LIPUS-irradiated hPDLSCs. The cytoplasm of hPDLSCs showed increased IκBα and p65 and decreased p-IκBα at increased LIPUS intensity. After LPS and LIPUS treatment, the inhibitory effect of LIPUS irradiation on the NF-κB pathway was partially reversed, and the immunoregulation and osteogenic differentiation of hPDLSCs were decreased. LIPUS irradiation enhanced immunomodulation and osteogenic differentiation abilities of hPDLSCs by inhibiting the NF-κB pathway, and the effect is dose-dependent. This study may offer novel insights relevant to periodontal tissue engineering.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is characterized by irreversible and progressive degradation of periodontal tissues, resulting in loss of tooth and alveolar bone (Slots 2017). Conventional treatments merely slow down the disease progression but are unable to restore the loss of alveolar bone; therefore, alveolar bone regeneration remains the ultimate therapeutic goal in the field of dentistry (Wen et al. 2019). The periodontal ligament (PDL) is a soft connective tissue between the cementum and inner wall of the alveolar bone, which is crucial for sustaining and supporting its function (Trubiani et al. 2019). Human PDL stem cells (hPDLSCs) are characterized by self-renewal and multi-differentiation capacities and contribute to physiological healing of the cementum-periodontal ligament complex and alveolar bone (Son et al. 2019). The osteogenic differentiation capacity endows hPDLSCs with immense potential in the promotion of functional restoration of periodontal tissues (Kato et al. 2011). In addition, a previous study has pointed out that host response to bacteria may lead to periodontal tissue changes, with the host immune responses influencing the progress of periodontitis (Bartold and Van Dyke 2013). Hence, mesenchymal stem cell (MSC)-based immunomodulation may play a vital role in dental tissue regeneration (Andrukhov et al. 2019). Intriguingly, hPDLSCs share several properties of MSCs, including immunomodulation, and are believed to be promising stem cells for periodontal regeneration therapy (Fawzy El-Sayed et al. 2019; Tomokiyo et al. 2019; Trubiani et al. 2019). The immunomodulatory ability of transplanted MSCs helps create a microenvironment that facilitates the activation of tissue repair mechanisms (Wang et al. 2014). Therefore, elucidating the potential mechanism of osteogenesis and immunomodulation of hPDLSCs has practical significance for periodontal regeneration therapy.
Low intensity pulsed ultrasound (LIPUS) is transmitted with low intensity, and its output is in the form of a pulse wave, which maintains sound energy transmission to target tissues with minimal energy loss and thermal side effects (Jiang et al. 2019). LIPUS can induce osteoblast differentiation and stimulate extracellular matrix production and calcium deposition, therefore facilitating cytokine secretion, improving microcirculation, and promoting tissue regeneration (Wang et al. 2018b). For example, LIPUS promotes the proliferation of human amnion‐derived MSCs and may emerge as a breakthrough in the application of stem cells in tissue engineering (Ling et al. 2017). LIPUS retards the progression of bisphosphonate-related osteonecrosis in rats by inducing a systemic regeneration response and accelerating local healing (Hidaka et al. 2019). Importantly, LIPUS has gradually been applied to periodontal regeneration and dental therapies as an innovative and noninvasive approach (Rego et al. 2012). LIPUS has been demonstrated to expedite periodontal wound healing and bone repair by enhancing the regeneration process of cementum and mandible (Ikai et al. 2008). LIPUS promotes hPDLSC migration, which may represent one of the mechanisms for LIPUS-mediated periodontal regeneration (Wang et al. 2018a). NF-κB has been demonstrated to participate in the osteogenesis of hPDLSCs (Chen et al. 2020; Mao et al. 2016; Xu et al. 2019a). Inhibition of the NF-κB pathway contributes to periodontal tissue repair in a rat model of periodontitis (Li et al. 2020b). The critical role of LIPUS in the formation of hPDLSC membrane has been unveiled (Kusuyama et al. 2019; Kusuyama et al. 2017; Li et al. 2021; Wang et al. 2018a), whether LIPUS can affect the osteogenic differentiation of hPDLSCs by regulating the NF-κB signaling pathway remains unknown. Therefore, this study focuses on the effect of LIPUS on the NF-κB signaling pathway, which includes some innovative contents. In this backdrop, we conducted a preliminary study on the role of LIPUS stimulation in osteogenesis and immunomodulation of hPDLSCs, which is expected to offer a novel direction for periodontal tissue engineering and its clinical application.
Materials and methods
Culture and identification of hPDLSCs
hPDLSCs were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. hPDLSCs at passage 3 were labeled with antibodies specific to CD34, CD45, CD44, CD90, CD105, and CD29, which were then detected on a flow cytometer (BD Biosciences, San Jose, CA, USA). hPDLSCs were induced by using osteogenic induction medium (Alpha modified Eagle’s minimal essential medium containing 10 mol/L sodium β-glycerophosphate, 50 g/mL ascorbic acid, 10 mol/L dexamethasone, and 10% FBS) and adipogenic induction medium (containing 10% FBS, 1 μmol/L dexamethasone, 0.5 mmol/L IBMX, 10 mg/L insulin, and 100 mmol/L indomethacin) for 7 and 14 days, respectively. Afterwards, cells in the osteogenic and adipogenic induction groups were stained with Alizarin red and Oil Red O, respectively. Cells were observed and imaged using an optical microscope.
LIPUS irradiation of hPDLSCs
Peripheral blood mononuclear cells (PBMCs) of healthy human were purchased from Shanghai MTBio Technology Co., Ltd. (SER-PBMC-F, Shanghai, China). Cells were resuspended in Roswell Park Memorial Institute-1640 medium (10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin) (FY-PLS1368, FUYUBIO, Fuyu Biotechnology Co., Ltd., Shanghai, China), seeded into independent Transwell chambers in 6-well plates (2 mL cells/well), and cultured at 37 °C with 5% CO2 for 2–3 days. After cells were seeded for 24 h, hPDLSCs were stimulated with LIPUS at intensities of 30, 60, and 90 mW/cm2 in a water bath maintained at 37 °C for 7 days (30 min/day). The frequency of applied LIPUS was 1.5 MHz, pulse duty cycle was 1:4, and pulse repetition rate was 1.0 kHz, as described previously (Li et al. 2020a; Wang et al. 2018a). hPDLSCs stimulated by LIPUS were co-cultured with PBMCs at a ratio of 1:1 in Transwell chambers (3 × 105 cells/well) (Demircan et al. 2011), and PBMCs were seeded on top of hPDLSCs. Some hPDLSCs were treated with the NF-κB pathway activator, lipopolysaccharide (LPS) (1 μg/mL; Hengdu Biotech Co., Ltd, Shanghai, China), during LIPUS stimulation (90 mW/cm2). LIPUS stimulation was conducted for 7 days (15 min/day). Cells were collected on the 8th day for further experiments (Duan et al. 2019; Wang et al. 2020).
Grouping was as follows: (1) control group: hPDLSCs without any treatment were co-cultured with PBMCs; (2) blank group, LIPUSa (30 mW/cm2) group, LIPUSa (60 mW/cm2) group, LIPUSa (90 mW/cm2): hPDLSCs were stimulated with LIPUS at 0 mW/cm2, 30 mW/cm2, 60 mW/cm2 and 90 mW/cm2, respectively; (3) LIPUS (30 mW/cm2) group: hPDLSCs irradiated with LIPUS at 30 mW/cm2were co-cultured with PBMCs; (4) LIPUS (60 mW/cm2) group: hPDLSCs irradiated with LIPUS at 60 mW/cm2were co-cultured with PBMCs; (5) LIPUS (90 mW/cm2) group: hPDLSCs irradiated with LIPUS at 90 mW/cm2were co-cultured with PBMCs; (6) LPS group: hPDLSCs treated with LPS (1 μg/mL) but not irradiated by LIPUS were co-cultured with PBMCs; (7) LPS + LIPUS (90 mW/cm2) group: hPDLSCs irradiated with LIPUS at 90 mW/cm2 and treated with LPS (1 μg/mL) were co-cultured with PBMCs.
Cell counting kit-8 (CCK-8) assay
hPDLSCs were seeded into 96-well plates (103 cells/well) and cultured at 37 °C for 24 h. Then, cells in each well were supplemented with 10 mL of CCK-8 solution and cultured at 37 °C for 1 h. The absorbance value of cells at 450 nm on the 1st, 3rd, 5th, 7th, and 9th days was evaluated using a Synergy™ 2 Multi-Mode Microplate Reader (BioTek Instruments Inc., Winooski, VT, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells and tissues using TRIzol reagent (Invitrogen Inc., Carlsbad, CA, USA) and reverse transcribed into cDNA using the SYBR® Premix Ex Taq™ II (Takara, Dalian, China). qPCR was performed on an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The reaction conditions were as follows: 96 °C for 30 s and 40 cycles at 96 °C for 30 s, 57 °C for 30 s, 72 °C for 30 s, and 72 °C for 10 min. Primer sequences are listed in Table 1. The relative gene expression was examined by the 2-ΔΔCt method, with β-actin as the internal reference.
Enzyme-linked immunosorbent assay (ELISA)
The levels of multiple immunomodulatory cytokines such as transforming growth factor-β (TGF-β), indoleamine 2, 3-dioxygenase (IDO), and interleukin (IL)-10 and that of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and IL-lα were estimated using ELISA kits (eBioscience, San Diego, CA, USA). The optical density was measured at 450 nm and plotted with the corresponding standard curve to determine the appropriate protein concentration.
Preparation of cytoplasmic extracts
The cytoplasmic and nuclear extracts were prepared using a Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Pierce, Rockford, IL, USA). Briefly, cells were collected and resuspended in precooled cytoplasmic extraction reagent I, and cytoplasmic extraction reagent II was added to the mixture and centrifuged at 16,700 × g for 20 min. The upper solution was transferred into a sterile tube and used as a cytoplasmic extract.
Western blot analysis
Total protein was extracted in a radioimmunoprecipitation assay buffer (Solarbio, Beijing, China) containing 1% phenylmethylsulfonyl fluoride (Solarbio) for 30 min and then centrifuged at 12,000 × g at 4 °C for 20 min. The supernatant was collected, and the protein concentration was estimated using a Bicinchoninic Acid Protein Assay Kit (Beyotime, Shanghai, China). Next, the protein was separated with 10% SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Invitrogen). These membranes were incubated with primary antibodies against IκBα (#4814, Cell Signaling Technology, Beverly, MA, USA), p-IκBα (#2859, Cell Signaling Technology), p65 (#8242, Cell Signaling Technology), p-p65 (ab76302, Abcam Inc., Cambridge, MA, USA), osteocalcin (OCN) (ab133612, Abcam), osteopontin (OPN) (ab214050, Abcam), osterix (OSX) (ab227820, Abcam), and RUNX2 (ab236639, Abcam) at 4 °C overnight. Afterwards, the membranes were washed with tris-buffered saline-tween (TBST) buffer three times (10 min/step). The membranes were then treated with a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary antibody (1/5000, ComWin Biotech, Beijing, China) for 1 h, followed by three washes with TBST (10 min/time). The membranes were developed and visualized using an enhanced chemiluminescence reagent. Protein blotting was analyzed, with GAPDH as the internal reference (ab181602, 1/10000, Abcam).
Immunofluorescence
For localization of the p65 subunit of NF-κB, cells were fixed with 4% paraformaldehyde and treated with 0.1% Triton X-100. NF-κB p65 antibody and FITC-labeled secondary antibody (Santa Cruz, CA, USA) were used to stain the cells. For observation of the nucleus, cells were treated with 4ʹ, 6-diamidino-2-phenylindole for 30 min and then washed with phosphate-buffered saline (PBS). The slides were fixed with fixative and then examined under an Eclipse Ti fluorescent microscope (Nikon, Tokyo, Japan) (Han, et al. 2020).
Alizarin red staining
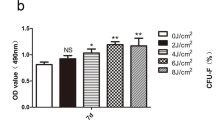
hPDLSCs were washed with PBS three times and fixed in 10% neutral formalin for 30 min. After removal of the fixative solution, cells were washed thrice with PBS and incubated with alizarin red (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 5 min. Then, the staining solution was removed, and cells were again washed thrice with PBS. Osteogenic nodules were observed and imaged using an inverted microscope (Olympus Optical Co. Ltd, Tokyo, Japan). The absorbance value at 450 nm was evaluated using a microplate reader.
Estimation of alkaline phosphatase (ALP) activity
Cells from a one-well plate were randomly selected from each group for checking ALP activity. The culture medium was collected and stored at −80 °C. Cells were isolated using trypsin and incubated in 0.2% Triton X-100 at 4 °C for 24 h, followed by ultrasonic treatment. ALP activity was determined using an ALP detection kit (Beyotime). Absorbance was measured at 520 nm for evaluation of the relative activity.
Statistical analysis
Data analysis was performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0.1 (GraphPad Software Inc., San Diego, CA, USA). The Kolmogorov–Smirnov test was used to check whether the data followed a normal distribution. Data are expressed as the mean ± standard deviation. The t-test was adopted for analysis of comparisons between two groups. One-way analysis of variance (ANOVA) was employed for comparisons among multiple groups, and Tukey’s multiple comparison test was used for the post-hoc test. P value was obtained from the bilateral tests. A value of p < 0.05 was considered to denote a statistically significant difference.
Results
LIPUS increased hPDLSC viability
hPDLSCs were found to be positive for MSC surface positive markers (CD90, CD105, CD29, and CD44) and negative for the negative markers of hematopoietic stem cells (CD34 and CD45), suggesting the stem cell characteristics of hPDLSCs (Fig. 1A). After osteogenic and adipogenic induction, the presence of alizarin red-positive calcium deposition (Fig. 1B) and oil red O-positive lipid droplets (Fig. 1C), respectively, confirmed the adipogenic ability of hPDLSCs. To explore the effect of LIPUS irradiation at different intensities on hPDLSCs, we first evaluated the effect of different intensities of LIPUS irradiation on the viability of hPDLSCs, and found that the viability of LIPUS-irradiated hPDLSCs was significantly enhanced compared with that of unirradiated cells; the viability of hPDLSCs was promoted at increased intensities of LIPUS irradiation (Fig. 1D, E, p < 0.01). In brief, LIPUS could work as a safe irradiation method to enhance the viability of hPDLSCs in a dose-dependent manner.
LIPUS treatment increased hPDLSC viability. A expression of surface markers of hPDLSCs were detected using flow cytometry; B hPDLSCs were stained with alizarin red; C hPDLSCs were stained with oil red O; D viability of hPDLSCs measured on the 1st, 3rd, 5th, 7th, and 9th day after LIPUS irradiation using the CCK-8 assay; E viability of hPDLSCs irradiated by different intensities of LIPUS (30, 60, and 90 mW/cm2) on the 9th day measured using the CCK-8 assay. Cellular experiments were repeated three times. Data are presented as the mean ± standard deviation and were analyzed using one-way ANOVA, followed by Tukey's multiple comparison test, **p < 0.01 vs. control group
LIPUS promoted immunomodulation of hPDLSCs
The effect of different LIPUS intensities (0, 30, 60, and 90 mW/cm2) on the mRNA expression and protein levels of immunomodulatory factors (TGF-β, IDO, and IL-10) in hPDLSCs and inflammatory factors (TNF-α, IFN-γ, and IL-lα) in the supernatant of PBMCs was measured. RT-qPCR results showed gradual increases in the mRNA level of immunomodulatory factors (TGF-β, IDO, and IL-10) in hPDLSCs after LIPUS irradiation, while the mRNA level of inflammatory factors (TNF-α, IFN-γ, and IL-lα) in PBMCs decreased gradually, and the effect was dose-dependent (Fig. 2A/B, p < 0.01). Results obtained from ELISA similarly demonstrated that the levels of immunomodulatory factors (TGF-β, IDO, and IL-10) in hPDLSCs increased, while that of inflammatory factors (TNF-α, IFN-γ, and IL-lα) in PBMCs decreased (p < 0.05) and the change was most obvious when the irradiation intensity was 90 mW/cm2 (Fig. 2C, D, p < 0.01). These results suggest that LIPUS can promote the immunomodulation of hPDLSCs in a dose-dependent manner.
LIPUS promoted immunoregulation of hPDLSCs. A mRNA expression of immunoregulatory factors (TGF-β, IDO, and IL-10) in hPDLSCs detected using RT-qPCR; B mRNA expression of inflammatory factors (TNF-α, IFN-γ, and IL-lα) in PBMCs measured using RT-qPCR; C, D proteins level of immunoregulatory factors (TGF-β, IDO, and IL-10) in the supernatant of hPDLSCs and inflammatory cytokines (TNF-A, IFN-y, IL-1α) in the supernatant of PBMCs was detected using ELISA; Cell experiments were repeated three times. Data are presented as mean ± standard deviation and were analyzed using one-way ANOVA, followed by Tukey's multiple comparison test, ** p < 0.01, * p < 0.05
LIPUS facilitated osteogenic differentiation of hPDLSCs
Subsequently, the hPDLSCs were irradiated with different intensities (0, 30, 60, and 90 mW/cm2) of LIPUS. qRT-PCR displayed that the levels of Runx2, OPN, OSX, and OCN were apparently elevated after LIPUS irradiation. With an increase in irradiation intensity, the levels of these genes in hPDLSCs were notably increased (Fig. 3A, p < 0.05). Compared with the control cells, LIPUS-irradiated hPDLSCs showed larger and darker mineralized nodules in the matrix, as well as enhanced ALP activity in a dose-dependent manner (Fig. 3B, C, p < 0.05 for all). Taken together, LIPUS promoted osteogenic differentiation of hPDLSCs.
LIPUS facilitated osteogenic differentiation of hPDLSCs. A mRNA expression of osteogenic differentiation-related genes (Runx2, OPN, OSX, and OCN) detected using RT-qPCR; B number of mineralized nodules in hPDLSCs measured using alizarin red staining; C ALP activity of hPDLSCs measured using ALP staining. Cell experiments were repeated three times. Data are presented as mean ± standard deviation and were analyzed using one-way ANOVA, followed by Tukey's multiple comparison test, **p < 0.01, * p < 0.05
LIPUS enhanced immunomodulation and osteogenic differentiation of hPDLSCs by inhibiting the NF-κB pathway
A previous study has revealed that activation of the NF-κB pathway could inhibit hPDLSC osteogenesis (Xu et al. 2019a). Meanwhile, activation of the NF-κB pathway has been reported to inhibit osteogenic differentiation of hPDLSC (Xu et al. 2019b; Zhang et al. 2021). WB demonstrated that the ratios of p-IκBα/IκBα and p-p65/p65 in hPDLSCs were significantly decreased after LIPUS irradiation, along with decreased entry of p65 into the nuclei, and the effect was dose-dependent. Then, we treated LIPUS-irradiated (90 mW/cm2) hPDLSCs and non-irradiated hPDLSCs with the NF-κB activator LPS and co-cultured them with PBMCs, respectively. WB uncovered that compared with the control group, the ratios of p-IκBα/IκBα and p-p65/p65 were increased in the LPS group, along with enhanced entry of p65 into the nuclei. The ratio of p-IκBα/IκBα and p-p65/p65, and the level of p65 entry into the nuclei in the LPS + LIPUS (90 m W/cm2) group were lower than those in the LPS group, and higher than those in the LIPUS (90 m W/cm2) group (Fig. 4A, B, p < 0.01). Next, the immunomodulation factors and osteogenic differentiation ability of hPDLSCs were examined. The results manifested that compared with the control group, the levels of immunomodulatory factors (TGF-β, IDO, and IL-10), osteogenic genes (Runx-2, OPN, OSX, and OCN), the degree of mineralized nodules and ALP activity of hPDLSCs in the LPS group were lowered, whereas the levels of inflammatory factors (TNF-a, IFN-y, and IL-1α) in PBMCs were increased. Compared with the LIPUS (90 m W/cm2) group, the LPS + LIPUS (90 m W/cm2) group had lower levels of immunomodulatory factors, osteogenic genes, mineralized nodules degree and ALP activity in hPDLSCs, and higher inflammatory factors in PBMCs; Compared with the LPS group, the levels of immunomodulatory factors, osteogenic genes, the degree of mineralized nodules and the activity of ALP were raised in the LPS + LIPUS (90 m W/cm2) group, while the levels of inflammatory factors in PBMCs were decreased (Fig. 4C–H, p < 0.01 for all). Therefore, LIPUS was observed to suppress the NF-κB pathway, thus enhancing the immunomodulation and osteogenic differentiation ability of hPDLSCs.
LIPUS promoted immunoregulation and osteogenic differentiation of hPDLSCs by inhibiting the NF-κB signaling pathway. A: protein levels of IκBα, p-IκBα, p65, and p-p65 examined using western blotting; B entry of p65 into nuclei detected using immunofluorescence; C proteins level of immunoregulatory factors (TGF-β, IDO, and IL-10) in hPDLSCs and inflammatory cytokines (TNF-α, IFN-γ, IL-1α) in PBMCs was detected using ELISA; D mRNA levels of immunoregulatory factors (TGF-β, IDO, and IL-10) in hPDLSCs and inflammatory factors (TNF-α, IFN-γ, and IL-lα) in PBMCs in co-culture system were measured using RT-qPCR; E mRNA levels of osteogenic differentiation-related genes (Runx2, OPN, OSX, and OCN) in hPDLSCs in co-culture system were detected using RT-qPCR; F protein levels of osteogenic differentiation-related genes (Runx2, OPN, OSX, and OCN) in hPDLSCs in co-culture system were detected using WB. G number of mineralized nodules in hPDLSCs in co-culture system counted using alizarin red staining; H ALP activity of hPDLSCs in co-culture system was measured using ALP staining. Cellular experiments were repeated three times. Data are presented as mean ± standard deviation and were analyzed using one-way ANOVA, followed by Tukey's multiple comparison test, **p < 0.01, * p < 0.05
Discussion
hPDLSCs represent ideal candidates for periodontal tissue regeneration due to their strong proliferation and differentiation potentials (Wu et al. 2017; Xue et al. 2018). The effect of LIPUS on tissue regeneration has attracted extensive attention, and a considerable number of studies have evaluated the potential application of LIPUS in tissue engineering (Tanaka et al. 2015). Against this backdrop, the present study investigated the potential role of LIPUS in periodontal tissue regeneration and revealed the positive influence of LIPUS on immunomodulation and osteogenesis of hPDLSCs, and the effect was dose-dependent.
Previous literature determines 120 mW/cm2 as high intensity pulsed ultrasound, and the range of LIPUS is less than 100 mW/cm2 (Saito et al. 2004). Most studies have clarified the effects of LIPUS on hPDLSCs (Li et al. 2020a; Li et al. 2021). LIPUS can stimulate the viability and differentiation of hematopoietic stem/progenitor cells in peripheral blood leukocytes (Xu et al. 2012). LIPUS promotes the osteogenic differentiation of periosteal cells in vitro (Maung et al. 2021). LIPUS is also known to rescue the viability of H2O2-treated cells, thereby contributing to alveolar bone homeostasis in experimental periodontitis (Ying et al. 2020). We focused on the effect of LIPUS on hPDLSCs, and designed the gradient LIPUS starting from 90 mW/cm2 according to the literature (Hu et al. 2014). We showed here that the viability of hPDLSCs was elevated significantly after LIPUS treatment.
The immunomodulatory function of somatic stem cells has been highlighted in different dental tissues, including PDL (Ding et al. 2010; Su et al. 2011). The host immune response has been identified to stimulate the progression of periodontitis (Shi et al. 2020). Activated immune cells mainly generate inflammatory cytokines, and immune cells and somatic cells control each other reciprocally; this interaction is acknowledged to be critical for tissue homeostasis and repair (Tomokiyo et al. 2019). Beccaria et al. have suggested that opening of the blood–brain barrier on LIPUS treatment contributes to immune modulation and could lead to direct immunotherapy of brain tumors (Beccaria et al. 2020). To the best of our knowledge, the role of LIPUS in the immunomodulation of stem cells is rarely studied. In the current study, the effects of different intensities of LIPUS on the levels of immunomodulatory factors in hPDLSCs and inflammatory factors in PBMCs were measured. With enhancement in LIPUS intensity, the levels of immunomodulatory factors in hPDLSCs increased gradually and were the highest when the intensity of LIPUS was 90 mW/cm2. However, the levels of inflammatory factors in PBMCs decreased gradually with increasing LIPUS intensity and reached the lowest level when the intensity of LIPUS was 90 mW/cm2. Consistent with our observations, Li et al. showed that LIPUS activates autophagy in hPDLSCs and contributes to the anti-inflammatory mechanism (Li et al. 2020c). Additionally, stimulating autophagy could partially restore the osteogenic potential of hPDLSCs, possibly conferring a reference for periodontal tissue regeneration (Kuang et al. 2020). Elevated expression of inflammatory factors hinders the osteogenesis of hPDLSCs, and LIPUS could reduce LPS-induced inflammation in hPDLSCs (Li et al. 2020a). LIPUS enhances BMP9-induced osteogenesis and represses inflammatory responses of hPDLSCs (Kusuyama et al. 2017).
The regeneration and repair of periodontal tissues mainly depend on the osteogenic differentiation ability of hPDLSCs (Li et al. 2018). LIPUS stimulation has been demonstrated to enhance the osteogenesis of hPDLSCs (Hu et al. 2014; Kusuyama et al. 2019; Li et al. 2020a). Runx2 is a major marker of osteoblast differentiation and chondrocyte maturation (Komori 2018). OSX is another osteoblast-specific transcription factor expressed in tooth germ mesenchymal cells (Chen et al. 2009). OCN is implicated in the progression of bone formation and mineralization (Neve et al. 2013), and OPN is regarded as a specific marker of osteoblast matrix formation and maturation, with the increase in OPN expression correlated to osteoblast maturity (Singh et al. 2018). Liu et al. have revealed that enhancement of osteogenesis-related genes (Runx2, OSX, OPN, and OCN) can be observed in LIPUS-irradiated hPDLSCs (Liu et al. 2020), which is consistent with the results of our present study. LIPUS stimulates osteogenic differentiation of periosteum-derived cells and further enhances BMP-2 and OSX expression in an osteogenic medium, leading to mineral apposition (Maung et al. 2021). Moreover, the ability to generate calcium nodules and mineralized matrix is a vital factor for osteogenesis of hPDLSCs (Vitale-Brovarone et al. 2007). ALP activity is an early marker of osteogenesis and is involved in the mineralization process (Jiang and Hua 2016). We found that LIPUS-irradiated hPDLSCs showed large mineralized nodules in the matrix and enhanced ALP activity. Briefly, LIPUS facilitated osteogenesis of hPDLSCs.
LIPUS can directly regulate the transient expression of key osteogenic genes or interact with signaling pathways to promote the formation of mineralized matrix (Chen et al. 2019). Emerging evidence has indicated that inhibition of the NF-κB pathway contributes to periodontal tissue repair in a rat model of periodontitis (Li et al. 2020b). Intriguingly, LIPUS represses activation of the NF-κB pathway in response to inflammatory stimulation (Sato et al. 2015). Therefore, we conducted relevant studies on the NF-κB pathway. The results manifested that with enhancement in the LIPUS intensity, the cytoplasm of hPDLSCs showed increased IκBα and p65 and decreased p-IκBα levels, indicating that LIPUS inhibited the NF-κB pathway. Generally, the NF-κB pathway is activated to modulate the downstream target gene transcription through phosphorylation of IκBα and translocation of p65 from the cytoplasm to the nucleus (Manna 2012). Therefore, LIPUS-irradiated hPDLSCs were treated with LPS (which functions as a NF-κB activator). Our results showed that the levels of immunomodulatory factors in hPDLSCs were decreased and that of inflammatory factors in PBMCs were increased with increasing radiation intensity. LPS also reduced the expression of osteogenesis-related genes, number of mineralized nodules, and ALP activity of LIPUS-irradiated hPDLSCs. The NF-κB pathway is a crucial inflammation-related pathway, which is implicated in the inflammatory secretion and osteogenesis of hPDLSCs (Liu et al. 2020).
Conclusion
LIPUS promoted immunomodulation and osteogenesis of hPDLSCs by suppressing the NF-κB pathway. Emerging lines of evidence have unveiled the critical role of LIPUS in the formation of hPDLSC membrane, although existing studies do not confirm whether LIPUS could affect the osteogenic differentiation of hPDLSCs by regulation of the NF-κB signaling pathway. Therefore, this study was focused on the effect of LIPUS on the NF-κB signaling pathway and provided some novel insights into the underlying mechanism. In particular, observation from this study highlights the therapeutic implications of LIPUS in periodontal tissue repair. This study also has some limitations. We detected the cell viability at different time points using CCK-8 assay, and the subsequent experiments were conducted after LIPUS stimulation for 7 days. However, due to the COVID-19 and the limitation of experimental conditions, we failed to show the data of two or three time points in time-course under LIPUS stimulation in RT-qPCR and Western blot analysis. In the future, we will carry out the relevant research in the appropriate experimental conditions. Further research is necessary to clarify the mechanism by which LIPUS facilitates immunomodulation and osteogenesis of hPDLSCs via the NF-κB pathway. Additionally, the role of LIPUS in other osteogenesis-related pathways (such as the Wnt and JNK pathways) remains elusive and needs further exploration along similar lines.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Andrukhov O, Behm C, Blufstein A, Rausch-Fan X (2019) Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells 11:604–617
Bartold PM, Van Dyke TE (2013) Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearn Learn Concepts Periodontol 62:203–217
Beccaria K, Sabbagh A, de Groot J, Canney M, Carpentier A, Heimberger AB (2020) Blood-brain barrier opening with low intensity pulsed ultrasound for immune modulation and immune therapeutic delivery to CNS tumors. J Neurooncol 151:65
Chen S, Gluhak-Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L, Yuan GH, Dong J, Gay I, MacDougall M (2009) Runx2, osx, and dspp in tooth development. J Dent Res 88:904–909
Chen D, Xiang M, Gong Y, Xu L, Zhang T, He Y, Zhou M, Xin L, Li J, Song J (2019) LIPUS promotes FOXO1 accumulation by downregulating miR-182 to enhance osteogenic differentiation in hPDLCs. Biochimie 165:219–228
Chen J, Yu M, Li X, Sun QF, Yang CZ, Yang PS (2020) Progranulin promotes osteogenic differentiation of human periodontal ligament stem cells via tumor necrosis factor receptors to inhibit TNF-alpha sensitized NF-kB and activate ERK/JNK signaling. J Periodontal Res 55:363–373
Demircan PC, Sariboyaci AE, Unal ZS, Gacar G, Subasi C, Karaoz E (2011) Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 13:1205–1220
Ding G, Wang W, Liu Y, An Y, Zhang C, Shi S, Wang S (2010) Effect of cryopreservation on biological and immunological properties of stem cells from apical papilla. J Cell Physiol 223:415–422
Duan Y, An W, Wu H, Wu Y (2019) Salvianolic acid C attenuates LPS-induced inflammation and apoptosis in human periodontal ligament stem cells via toll-like receptors 4 (TLR4)/nuclear factor kappa B (NF-kappaB) pathway. Med Sci Monit 25:9499–9508
Fawzy El-Sayed KM, Elahmady M, Adawi Z, Aboushadi N, Elnaggar A, Eid M, Hamdy N, Sanaa D, Dorfer CE (2019) The periodontal stem/progenitor cell inflammatory-regenerative cross talk: a new perspective. J Periodontal Res 54:81–94
Han BH, Song CH, Yoon JJ, Kim HY, Seo CS, Kang DG, Lee YJ, Lee HS (2020) Anti-vascular inflammatory effect of ethanol extract from securinega suffruticosa in human umbilical vein endothelial cells. Nutrients 12:3448
Hidaka K, Mikuni-Takagaki Y, Wada-Takahashi S, Saita M, Kawamata R, Sato T, Kawata A, Miyamoto C, Maehata Y, Watabe H, Tani-Ishii N, Hamada N, Takahashi SS, Deguchi S, Takeuchi R (2019) Low-intensity pulsed ultrasound prevents development of bisphosphonate-related osteonecrosis of the jaw-like pathophysiology in a Rat model. Ultrasound Med Biol 45:1721–1732
Hu B, Zhang Y, Zhou J, Li J, Deng F, Wang Z, Song J (2014) Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS ONE 9:e95168
Ikai H, Tamura T, Watanabe T, Itou M, Sugaya A, Iwabuchi S, Mikuni-Takagaki Y, Deguchi S (2008) Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J Periodontal Res 43:212–216
Jiang Z, Hua Y (2016) Hydrogen sulfide promotes osteogenic differentiation of human periodontal ligament cells via p38-MAPK signaling pathway under proper tension stimulation. Arch Oral Biol 72:8–13
Jiang X, Savchenko O, Li Y, Qi S, Yang T, Zhang W, Chen J (2019) A review of low-intensity pulsed ultrasound for therapeutic applications. IEEE Trans Biomed Eng 66:2704–2718
Kato T, Hattori K, Deguchi T, Katsube Y, Matsumoto T, Ohgushi H, Numabe Y (2011) Osteogenic potential of rat stromal cells derived from periodontal ligament. J Tissue Eng Regen Med 5:798–805
Komori T (2018) Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol 149:313–323
Kuang Y, Hu B, Feng G, Xiang M, Deng Y, Tan M, Li J, Song J (2020) Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 21:13–27
Kusuyama J, Nakamura T, Ohnishi T, Eiraku N, Noguchi K, Matsuguchi T (2017) Low-intensity pulsed ultrasound (lipus) promotes BMP9-induced osteogenesis and suppresses inflammatory responses in human periodontal ligament-derived stem cells. J Orthop Trauma 31:S4
Kusuyama J, Nakamura T, Ohnishi T, Albertson BG, Ebe Y, Eiraku N, Noguchi K, Matsuguchi T (2019) Low-intensity pulsed ultrasound promotes bone morphogenic protein 9-induced osteogenesis and suppresses inhibitory effects of inflammatory cytokines on cellular responses via Rho-associated kinase 1 in human periodontal ligament fibroblasts. J Cell Biochem 120:14657–14669
Li X, Yang H, Zhang Z, Yan Z, Lv H, Zhang Y, Wu B (2018) Plateletrich fibrin exudate promotes the proliferation and osteogenic differentiation of human periodontal ligament cells in vitro. Mol Med Rep 18:4477–4485
Li Q, Liu F, Dang R, Feng C, Xiao R, Hua Y, Wang W, Jia Z, Liu D (2020b) Epigenetic modifier trichostatin a enhanced osteogenic differentiation of mesenchymal stem cells by inhibiting NF-kappaB (p65) DNA binding and promoted periodontal repair in rats. J Cell Physiol 235:9691–9701
Li Y, Sun C, Feng G, He Y, Li J, Song J (2020c) Low-intensity pulsed ultrasound activates autophagy in periodontal ligament cells in the presence or absence of lipopolysaccharide. Arch Oral Biol 117:104769
Li H, Zhou J, Zhu M, Ying S, Li L, Chen D, Li J, Song J (2020a) Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J Biomed Mater Res A 109:1101
Ling L, Wei T, He L, Wang Y, Wang Y, Feng X, Zhang W, and Xiong Z(2017) Low-intensity pulsed ultrasound activates ERK1/2 and PI3K-Akt signalling pathways and promotes the proliferation of human amnion-derived mesenchymal stem cells. Cell Prolif 50
Liu S, Zhou M, Li J, Hu B, Jiang D, Huang H, Song J (2020) LIPUS inhibited the expression of inflammatory factors and promoted the osteogenic differentiation capacity of hPDLCs by inhibiting the NF-kappaB signaling pathway. J Periodontal Res 55:125–140
Li H, Zhou J, Zhu M, Ying S, Li L, Chen D, Li J, Song J (2021) Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J Biomed Mater Res A 109:1101–1112
Manna SK (2012) Double-edged sword effect of biochanin to inhibit nuclear factor kappaB: suppression of serine/threonine and tyrosine kinases. Biochem Pharmacol 83:1383–1392
Mao CY, Wang YG, Zhang X, Zheng XY, Tang TT, Lu EY (2016) Double-edged-sword effect of IL-1beta on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-kappaB, MAPK and BMP/Smad signaling pathways. Cell Death Dis 7:e2296
Maung WM, Nakata H, Miura M, Miyasaka M, Kim YK, Kasugai S, Kuroda S (2021) Low-intensity pulsed ultrasound stimulates osteogenic differentiation of periosteal cells in vitro. Tissue Eng Part A 27:63–73
Neve A, Corrado A, Cantatore FP (2013) Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol 228:1149–1153
Rego EB, Takata T, Tanne K, Tanaka E (2012) Current status of low intensity pulsed ultrasound for dental purposes. Open Dent J 6:220–225
Saito M, Soshi S, Tanaka T, Fujii K (2004) Intensity-related differences in collagen post-translational modification in MC3T3-E1 osteoblasts after exposure to low- and high-intensity pulsed ultrasound. Bone 35:644–655
Sato M, Kuroda S, Mansjur KQ, Khaliunaa G, Nagata K, Horiuchi S, Inubushi T, Yamamura Y, Azuma M, Tanaka E (2015) Low-intensity pulsed ultrasound rescues insufficient salivary secretion in autoimmune sialadenitis. Arthritis Res Ther 17:278
Shi T, Jin Y, Miao Y, Wang Y, Zhou Y, Lin X (2020) IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology 108:350–357
Singh A, Gill G, Kaur H, Amhmed M, Jakhu H (2018) Role of osteopontin in bone remodeling and orthodontic tooth movement: a review. Prog Orthod 19:18
Slots J (2017) Periodontitis: facts, fallacies and the future. Periodontol 75:7–23
Son H, Jeon M, Choi HJ, Lee HS, Kim IH, Kang CM, Song JS (2019) Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 14:e0221236
Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD (2011) Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells 29:1849–1860
Tanaka E, Kuroda S, Horiuchi S, Tabata A, El-Bialy T (2015) Low-intensity pulsed ultrasound in dentofacial tissue engineering. Ann Biomed Eng 43:871–886
Tomokiyo A, Wada N, Maeda H (2019) Periodontal ligament stem cells: regenerative potency in periodontium. Stem Cells Dev 28:974–985
Trubiani O, Pizzicannella J, Caputi S, Marchisio M, Mazzon E, Paganelli R, Paganelli A, Diomede F (2019) Periodontal ligament stem cells: current knowledge and future perspectives. Stem Cells Dev 28:995–1003
Vitale-Brovarone C, Verne E, Robiglio L, Appendino P, Bassi F, Martinasso G, Muzio G, Canuto R (2007) Development of glass-ceramic scaffolds for bone tissue engineering: characterisation, proliferation of human osteoblasts and nodule formation. Acta Biomater 3:199–208
Wang Y, Chen X, Cao W, Shi Y (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15:1009–1016
Wang Y, Li J, Qiu Y, Hu B, Chen J, Fu T, Zhou P, Song J (2018a) Lowintensity pulsed ultrasound promotes periodontal ligament stem cell migration through TWIST1mediated SDF1 expression. Int J Mol Med 42:322–330
Wang Y, Qiu Y, Li J, Zhao C, Song J (2018b) Low-intensity pulsed ultrasound promotes alveolar bone regeneration in a periodontal injury model. Ultrasonics 90:166–172
Wang W, Luo D, Chen J, Chen J, Xia Y, Chen W, and Wang Y(2020) Amelioration of cyclophosphamide-induced myelosuppression during treatment to rats with breast cancer through low-intensity pulsed ultrasound. Biosci Rep 40
Wen Y, Yang H, Wu J, Wang A, Chen X, Hu S, Zhang Y, Bai D, Jin Z (2019) COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of periodontal ligament stem cells. Theranostics 9:4265–4286
Wu Y, Wang Y, Ji Y, Ou Y, Xia H, Zhang B, Zhao Y (2017) C4orf7 modulates osteogenesis and adipogenesis of human periodontal ligament cells. Am J Transl Res 9:5708–5718
Xu P, Gul-Uludag H, Ang WT, Yang X, Huang M, Marquez-Curtis L, McGann L, Janowska-Wieczorek A, Xing J, Swanson E, Chen J (2012) Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol Lett 34:1965–1973
Xu Y, Ren C, Zhao X, Wang W, Zhang N (2019a) microRNA-132 inhibits osteogenic differentiation of periodontal ligament stem cells via GDF5 and the NF-kappaB signaling pathway. Pathol Res Pract 215:152722
Xu Y, Wang Y, Pang X, Li Z, Wu J, Zhou Z, Xu T, Gobin Beharee R, Jin L, Yu J (2019b) Potassium dihydrogen phosphate promotes the proliferation and differentiation of human periodontal ligament stem cells via nuclear factor kappa B pathway. Exp Cell Res 384:111593
Xue N, Qi L, Zhang G, Zhang Y (2018) miRNA-125b regulates osteogenic differentiation of periodontal ligament cells through NKIRAS2/NF-kappaB pathway. Cell Physiol Biochem 48:1771–1781
Ying S, Tan M, Feng G, Kuang Y, Chen D, Li J, Song J (2020) Low-intensity pulsed ultrasound regulates alveolar bone homeostasis in experimental periodontitis by diminishing oxidative stress. Theranostics 10:9789–9807
Zhang L, Cheng L, Cui Y, Wu Z, Cai L, Yang L, Duan M, Zhang D, Zhou C, Xie J (2021) The virulence factor GroEL directs the osteogenic and adipogenic differentiation of human periodontal ligament stem cells through the involvement of JNK/MAPK and NF-kappaB signaling. J Periodontol 92:103–115
Acknowledgements
This study was supported by Natural Science Foundation of Guangdong Province (No: 2015A030313861).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethic approval
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, H., Wang, Q., Quan, C. et al. Low-intensity pulsed ultrasound enhances immunomodulation and facilitates osteogenesis of human periodontal ligament stem cells by inhibiting the NF-κB pathway. Cell Tissue Bank 24, 45–58 (2023). https://doi.org/10.1007/s10561-022-10010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-022-10010-y