Abstract
The use of processed nerve allografts as an alternative to autologous nerve grafts, the gold standard treatment for peripheral nerve defects, is increasing. However, it is not widely used in Korea due to cost and insurance issues. Moreover, the main detergent used in the conventional Hudson method is unavailable. Therefore, a new nerve allograft decellularization process is needed. We aimed to compare the traditional Hudson method with a novel decellularization process that may remove cellular content more efficiently while preserving the extracellular matrix (ECM) structure using low concentration sodium dodecyl sulfate (SDS) and nuclease. After each decellularization process, DNA content was measured in nerve tissue. Masson's trichrome staining and scanning electron microscopy were performed to determine the state of preservation of the ECM. A significantly greater amount of DNA content was removed in the novel method, and the ECM structure was preserved in both methods. For the in vivo study, a 15-mm long sciatic nerve defect was created in two groups of Sprague–Dawley rats, and processed nerve allografts decellularized using the Hudson or novel method were transplanted. Functional and histological recovery results were measured 12 weeks post-transplantation. Ankle contracture angle, maximal isometric tetanic force of the tibialis anterior (TA), and the TA mass were compared between the groups, as well as the percent neural tissue (100 × neural area/intrafascicular area). There was no significant difference in functional and histological nerve recovery between the methods. The novel method is appropriate for developing a processed nerve allograft.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral nerve injury can occur in situations, such as deep laceration, high-energy trauma, tumor resection surgery, chronic compression, or radiotherapy. In cases of minor nerve defects, tension-free end-to-end coaptation is a suitable choice of treatment. Conversely, if the nerve defect is too large to suture directly, an autologous nerve graft is the gold standard treatment. However, even with autografts, donor site morbidity, such as loss of sensation at the donor site, additional scar formation, and development of neuromas may occur. In addition, during the harvesting of the donor nerve tissue, the duration of surgery is inevitably increased, and the amount of available nerve tissue is limited. The shortcomings of autonomic nerve transplantation have become the driving force behind the development of new methods. As a result, synthetic conduits and processed nerve allografts were developed.
The processed nerve allograft achieves the most similar results to autologous nerve graft in terms of nerve recovery. This is based on the fact that the microstructure and molecules of the conserved extracellular matrix (ECM) promote axonal regeneration, proliferation, and migration of Schwann cells (Moore et al. 2011; Whitlock et al. 2009). In order to obtain a processed nerve allograft, it is necessary to preserve the microstructure and molecules of the extracellular matrix effectively removing the cellular components (Crapo et al. 2011). Although there are many nerve allograft decellularization methods, no consensus has been reached on the best method. Physical procedures and chemical, or biological, agents are used for the decellularization process, which are generally used in combination with each other (Crapo et al. 2011). In recent years, chemical decellularization using detergents has been most widely used and is still being investigated (Hudson et al. 2004b; Shin et al. 2019; Sondell et al. 1998; Zilic et al. 2016).
Hudson et al. published a report on a decellularization method developed by combining Triton X-200 and zwitterionic detergents in 2004 (Hudson et al. 2004a). In 2007, Neubauer et al. reported that axonal regeneration could be improved through chondroitinase ABC treatment in a 4-cm large nerve defect rat model (Neubauer et al. 2007). Based on the aforementioned studies, the Avance® processed nerve graft (AxoGen, Inc., Alachua, FL, USA) was developed and released using human nerve tissue. This is the only acellular nerve allograft currently available in the medical field that has been approved by the United States Food and Drug Administration (FDA). In 2009, Whitlock et al. compared a ready-made type I collagen conduit and AxoGen’s Avance® human decellularized allograft in a rat nerve defect model and reported that the processed nerve graft showed superior results (Whitlock et al. 2009). However, the Avance® processed nerve graft is not widely used in Korea due to cost and insurance-related issues. In addition, Triton X-200, which was used in the production of Avance® processed nerve grafts, cannot be used by other researchers due to a patent issue. Therefore, a new nerve allograft decellularization process is needed.
Sodium dodecyl sulfate (SDS) is one of the most commonly used chemical agents for decellularization. SDS is known to effectively remove nuclei from dense tissues or organs, such as the kidneys (Lumpkins et al. 2008; Nakayama et al. 2010); however, favorable decellularization results have also been reported in the cornea, blood vessels, and nerves (Du et al. 2011; Wilshaw et al. 2012; Zilic et al. 2016). In 2016, Zilic et al. published an effective decellularization method for making xenogeneic nerve grafts from porcine peripheral nerves using low-concentration SDS, hypotonic buffers, and nuclease enzymes based on the method developed by Wilshaw et al. (Wilshaw et al. 2012; Zilic et al. 2016). These authors reported that the acellular nerve retained its native 3D endoneurial microstructure and mechanical properties while eliminating over 95% of the cellular components. We hypothesized that the results obtained using the novel Zilic method would be comparable to the traditional Hudson method in terms of decellularization and nerve recovery. Therefore, we performed an in vitro and in vivo comparative study using rat sciatic nerves to test this hypothesis. We also attempted to determine whether the novel Zilic method was suitable as a new allograft processing method.
Material and methods
Experimental design
All animal care and experimental procedures were approved by the Catholic University of Korea College of Medicine, St. Vincent’s Hospital Institutional Animal Care and Use Committee (IRB17-7). Thirty-six adult Sprague–Dawley (SD) (250–300 g) outbred male rats were used in the study: 12 as sciatic nerve donors, and 24 as recipients. Since SD rats are outbred rats and do not have homogenous genotypes, nerve transplantation between SD rats was considered an allograft. Twenty-four sciatic nerves were collected from both hind limbs of 12 donor SD rats with a length of about 20-mm. Nerves of 12 were decellularized using the Hudson method and the nerves of the remaining 12 using the novel Zilic method. After treatment, about 5-mm of the nerve tissue was excised, and DNA content, histologic analysis, and reagent residue measurement were performed. Twenty-four recipient SD rats were randomly assigned to groups I and II. Group I rats were implanted with the sciatic nerve treated with the Hudson method and group II rats were implanted with the sciatic nerve treated with the Zilic method. Decellularized nerves were transplanted 4 weeks after collection, and functional and histological evaluation of nerve recovery was performed 12 weeks after transplantation.
Preperation of the processed nerve allograft
The twelve sciatic nerves out of a total of 24 were decellularized using the Hudson method as previously described (Hudson et al. 2004a, b). However, the Triton X-200 was unavailable, so it was replaced with the Triton X-100. As previously mentioned, the remaining 12 nerves were decellularized using the Zilic method and the allograft processing method was as follows. On the first day, the nerves were washed three times in phosphate buffered saline (PBS; Oxoid, Basingstoke, UK) containing 0.1% (w/v) ethylene diamine tetra acetic acid (EDTA; VWRi) and hypotonic buffer (10 mM TRIS–HCl; pH 8.0) with agitation in the presence of aprotinin (10 kIU/mL; Nordic Pharma) and EDTA (0.1%; w/v; VWRi) at 4 °C for 24 h. On day 2, the nerves were subjected to hypotonic buffer (10 mM Tris–HCl, pH 8.0) containing 0.1% (w/v) SDS (Sigma) with agitation in the presence of aprotinin (10 kIU/mL; Nordic Pharma) and EDTA (0.1%; w/v; VWRi) at room temperature for 24 h. On day 3, the nerves were washed in PBS three times for 30 min with agitation and treated using nuclease buffer (50 mM TRIS, 10 mM MgCl2, 50 μg/L human serum albumin, 10 kIU aprotinin, 50 U/mL DNase, 1 U/mL RNase; pH 7.5) for 3 h at 37 °C. Then, each nerve was washed in hypertonic buffer (1.5 M NaCl in 50 mM TRIS–HCl, pH 7.6) for 24 h with agitation. On day 4, the nerves were washed three times in PBS at 4 °C for 30 min with agitation (Table 1). Both the Hudson method and the Zilic method took approximately 4 days to complete.
Surgical procedure
Rats were anesthetized by isoflurane (Bkpharm, Korea) gas inhalation. Ibuprofen (10–30 mg/kg) was injected intraperitoneally for pain control before procedure. Twenty-four sciatic nerves that were approximately 20-mm-long were harvested from both hindlimbs of twelve donor SD rats for decellularization; subsequently, the animals were euthanized with CO gas inhalation. In the recipient groups, sciatic nerves were fully exposed from the inferior margin of the piriformis muscle to the bifurcation of the peroneal nerve and tibial nerve. A 10-mm segment of the intact sciatic nerve was excised, and the processed nerve graft was implanted in the defect. Nerve grafts were sutured to the proximal and distal nerve stumps using two 9–0 nylon sutures at each side under the microscope. After implantation, the incision was irrigated, and the fascia and skin were closed using 4–0 vicryl and 4–0 nylon, respectively.
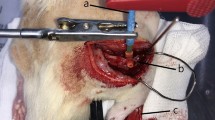
At 12 weeks postoperatively, both the maximal isometric tetanic force (MITF) and muscle mass of the tibialis anterior (TA) were assessed using a method described by Shin et al. (Shin et al. 2008) as functional parameters. Recipient sciatic nerves and bifurcation of peroneal and tibial nerves were re-exposed under inhalation anesthesia (Fig. 1). An electrode was attached to the peroneal branch of the sciatic nerve, and another skin incision was made on the dorsal aspect of the hind limb to expose the entire TA musculature. The distal tendinous portion of the TA was held with a hemostat, which was attached to a force transducer (MDB-2.5, Transducer Techniques, Temecula, CA). After measuring the MIFT, the entire TA muscle was extracted from the hind limb and weighed. The same procedure was performed on the contralateral normal hind limb. For histomorphometric analysis, the implanted sciatic nerve was extracted from the experimental side. After all measurements were completed, the recipient rats were euthanized using CO inhalation.
Histologic analysis
After the decellularization treatment, the tissue was observed with Masson's trichrome (MT) staining and scanning electron microscopy (SEM) to confirm the elimination of cellular contents and preservation of ECM. For MT staining, we used the Masson’s Trichrome Stain Kit (#25,088, Polysciences, Warrington, PA, USA) according to the manufacturer’s instruction. The SEM specimens were fixed for 24 h in Karnovsky’s fixative (2% glutaraldehyde, 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) and were washed two times for 30 min in 0.1 M PB. They were postfixed with 1% OsO4 for 2 h and dehydrated in ascending gradual series (50–100%) of ethanol using a critical point dryer (LEICA EM CPD300). They were coated with platinum by ion sputter (LEICA EM ACE600) and observed with a field emission scanning electron microscope (MERLIN, ZEISS).
Hematoxylin & eosin (H&E; Sigma Aldrich, MO, USA) staining and toluidine blue staining were performed to confirm axonal regeneration in the explanted tissue after transplantation. H&E staining was performed to confirm the tissue histoarchitecture, and toluidine blue staining was performed to calculate the population of regenerated axons. A blinded observer measured the percent neural tissue (100× neural area/intrafascicular area) in the cross section in the middle of the repaired graft using light microscopy and an image analysis program (CellSense; Olympus, Tokyo, Japan).
DNA content
The decellularized nerves (n = 6) in each group were washed in water and lyophilized for 72 h. Samples were then weighed, and DNA was isolated using a DNeasy Blood and Tissue Kit (Qiagen, Germany). The total DNA content was measured by absorption at 260 nm on a spectrophotometer (NanoDrop 1000, Thermo Fisher Scientific, Waltham, MA). All samples were normalized to the sample dry weight in each case.
Reagent residue
After decellularization treatment, residual amounts of the major drugs were tested to indirectly assess cytotoxicity. The residual amounts of Triton X-100 in the neural tissue used in group I and SDS in group II were investigated.
To accurately measure the residual amounts of detergents in the tissue, the supernatant was analyzed using high-performance liquid chromatography (HPLC). Pretreatment of the calibration standard sample was performed by modifying and supplementing the analysis method described by Karlsson et al. (Karlsson et al. 2002). The decellularized neural tissue sample was mixed with tertiary distilled water (ratio 1:4, W/V) before being ground and centrifuged at 4000 rpm for 14 min. The supernatant was collected and stored at –80 °C. To analyze the tissue sample, the sample stored at –80 °C was allowed to stand at room temperature to dissolve, before being pretreated in the same manner as that for preparing a calibration standard sample and was injected into the HPLC column. The ratio of the peak area of the detergents to the peak area of the internal standard was calculated from the obtained chromatogram, and the concentration of residual detergents in the sample was obtained from the calibration curve prepared in advance.
Functional assessment
Ankle contracture angle
The angle between the anterior border of the tibia and the dorsal aspect of the foot with maximal ankle plantar flexion was measured under anesthesia (Fig. 2). The rats developed ankle contracture, which limited plantar flexion at the resting position that kept their ankles in complete dorsiflexion. A larger angle indicated less ankle contracture, and a smaller ankle angle indicated greater nerve regeneration (Lee et al. 2013; Lin et al. 1996).
Maximum isometric tetanic force
The MITF was measured based on the method described by Shin et al. (Shin et al. 2008). The recipient rats were placed prone with the knee and ankle attached to the testing block. A bipolar electrode was attached to the peroneal branch of the sciatic nerve. The TA muscle was dissected from the surrounding tissue and released from the insertion without damaging the neurovascular pedicle and muscle fibers. The TA muscle was kept moist with a saline drip. The distal TA tendon was secured with a modified surgical hemostat, which was attached to a force transducer (MDB-2.5; Transducer Techniques, Temecula, CA, USA) (Fig. 3). The force transducer signals were displayed using a smart sensor indicator (Transducer Techniques, Temecula, CA, USA). To deliver supra-maximal stimuli, we set a frequency of 100 Hz, an intensity of 3.6 V, a duration of 0.3 ms, and a 5-min interval between stimuli to prevent muscle fatigue. After performing the same work on the side of the inoperable hind limb, the result was expressed as the percentage (%) of the experimental side to the normal side.
Wet muscle weight
Following the measurement of the MITF, the whole TA muscle was carefully removed and weighed in grams. After the weight was measured on the contralateral normal side in a similar manner, it was expressed as a percentage (%) of the experimental side to the normal side.
Statistical analysis
Data were analyzed using IBM SPSS Statistics software, version 21 (Systat Software, Inc., San Jose, CA, USA). Measurements of the two groups were compared using Student’s t-test when they had normality (assessed by Kolmogorov–Smirnov normality test) and compared using the Mann–Whitney test when they did not. Significance was set at 0.05 (P < 0.05), and all results are reported as the mean ± standard deviation.
Result
Histologic analysis
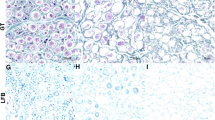
First, we report the results of the histologic analysis of decellularized nerves. On MT staining, the ECM structure was well preserved in both groups I and II, and some cell nuclei were seen in greater number in group I (Fig. 4). SEM also showed maintained ECM structure in both groups; however, some cellular materials were observed in groups I (Fig. 5).
Next, we report the histological results of explanted tissues 12 weeks after transplantation. H&E staining was performed to observe the overall histoarchitecture, and confirmed that the nerve fascicles, consisting of the epineurium, perineurium, and endoneurium connective tissue, were well formed in both group I- and II-transplanted nerves (Fig. 6). The regenerated population of axons was identified through toluidine blue staining, and the percent neural tissues (100 × neural area/intrafascicular area) in groups I and group II were 18.21% ± 6.45% and 23.45% ± 10.15%, respectively. There appeared to be an increased recovery rate in group II; however, the difference was not statistically significant (P = 0.164) (Fig. 7).
Cross section of harvested sciatic nerve 12 weeks after transplantation under toluidine blue staining. a Group I: Triton X-100 b Group II: SDS c Percent neural tissues (100 × neural area/intrafascicular area) of group I and group II were 18.21 ± 6.45% and 23.45 ± 10.15%, respectively. There appeared to be an increased recovery rate in Group II; however, there was no statistically significant difference (P = 0.164). Scale bar: 50 μm
DNA content
In groups I and II, dsDNA level was measured as 36.31 ± 2.31 and 7.50 ± 5.71 ng dsDNA/mg dry weight, respectively, and it was significantly lower in group II (P < 0.05). However, both are compatible with the current decellularization guidelines of 50 ng dsDNA/mg ECM proposed by Crapo et al. (Crapo et al. 2011).
Reagent residue
Based on the decellularization method, the concentration of each of the three specimens in the tissue was measured. Triton X-100 was measured at 0.03, 0.45, and 0.04 µg/mg, and SDS was measured at 4.2, 7.6, and 6.5 µg/mg, respectively. In the decellularized tissues, when the tissue concentration of the detergent is 10 µg/mg, or less than 50 mg/L in the last wash solution, it has been shown to be non-cytotoxic and have no effect on the growth of new cells (Cebotari et al. 2010; Keane et al. 2015).
Functional assessment
The mean ankle contracture angles for groups I and II were 141.18° ± 4.96° and 138.73° ± 5.62°, respectively. A smaller ankle angle indicated greater nerve regeneration; however, there was no statistically significant difference between the two groups (P = 0.29). The mean MITF was measured as 46.05% ± 5.89% in group I and 58.89% ± 4.87% in group II. Although group II had an increased MITF compared to group I, the difference was not statistically significant (P = 0.12). The mean TA muscle mass was 58.03% ± 7.01% in group I and 57.99% ± 4.85% in group II, and there was no statistically significant difference between groups I and II (P = 0.99) (Fig. 8).
Result of functional assessments. a Mean ankle contracture angle for group I and group II was 141.18 ± 4.96° and 138.73 ± 5.62°, respectively, and there was no statistically significant difference between the two groups (P = 0.29). b Mean MITF was measured as 46.05 ± 5.89% in group I and 58.89 ± 4.87% in group II, and the MITF was greater in group 2; however, there was no statistically significant difference (P = 0.12). c Mean TA muscle mass was 58.03 ± 7.01% in group I and 57.99 ± 4.85% in group II, and there was no statistically significant difference between group I and group II (P = 0.99)
Discussion
Processed nerve allografts prepared by two different decellularization methods were compared through in vitro and in vivo studies. Based on MT staining and SEM image, ECM structure was well maintained in both groups, but cell nucleus and cellular component was more clearly observed in group I. This difference was verified as having statistical significance in measuring DNA content, however both methods satisfied the DNA content criteria suggested by Crapo et al. On the other hand, in the in vivo study, the results demonstrated that there were no significant differences in the functional and histological results between the two groups. Moreover, there were no complications, such as host rejection, surgical site infection, or neuroma formation, after surgery. The time required for each decellularization process was similar; however, the cost of the Zilic method was lower. Specifically, under the same controlled laboratory conditions, the Hudson method cost approximately $61 for decellularization of the 10-cm-long SD rat sciatic nerve, while the Zilic method cost approximately $19, which was 1/3 cheaper. In conclusion, the Zilic method is more advantageous for the removal of the cellular component, and it is a more cost effective than the Hudson method, showing equal or superior results in terms of nerve recovery. Therefore, the novel method can be considered appropriate as an allograft processing method.
The Avance® processed nerve graft (AxoGen, Inc., Alachua, Florida) is the only acellular nerve allograft currently available in the medical field with FDA approval. It is made from human peripheral nerve tissue using a combination of detergent decellularization, chondroitinase-mediated chondroitin sulfate proteoglycans degradation, and gamma-irradiation sterilization based on the Hudson method of decellularization (Hudson et al. 2004b; Whitlock et al. 2009). To date, there are several reports on the surgical results obtained using the Avance® product. Means et al. conducted a prospective randomized pilot study of the treatment results using a hollow conduit and processed nerve allografts for 31 digital nerve injuries in 23 patients at four centers. In the static two-point discrimination and moving two-point discrimination tests, the processed allograft showed significantly better outcomes than the hollow conduit (Means et al. 2016). Moreover, Rbia et al. reported a better than good recovery in ≥ 94% of patients at the 12-month postoperative evaluation of nerve defects less than 2.5 cm (Rbia et al. 2019). Rinker et al. reported an S3 or better postoperative recovery in 86% of nerves with an average large nerve gap of 35 ± 8 mm, which was a positive result compared to using autografts or a conduit (Rinker et al. 2017). As mentioned above, to date, Axogen’s Avance®, which has been widely used since receiving FDA approval in 2008, is believed to have produced positive results. However, it is not widely used in Korea due to cost and insurance-related issues.
The Zilic method was developed based on the method of Whilshaw et al. and is characterized by a combination of low concentrations of SDS, hypotonic buffer, and nuclease enzyme (Wilshaw et al. 2012). SDS can cause destruction of the ECM ultrastructure and loss of collagen and glycosaminoglycans when used at high concentrations (Wilshaw et al. 2012). However, the low-concentration SDS method has already been used for decellularization of soft tissues such as arteries, veins, and heart valves (Korossis et al. 2002; Schaner et al. 2004; Wilshaw et al. 2012). Hypotonic buffer was used to lyse neuronal and Schwann cells. The nuclease enzyme catalyzes the hydrolysis of DNA and ribonucleated (RNA) chains. Zilic et al. decellularized porcine peroneal and tibial nerves and compared the results before and after the procedure. In their study, important ECM components, such as collagen, laminin, and fibronectin, were well maintained in the nerve after decellularization. In addition, the microstructure and mechanical properties of the nerve were well preserved, whereas more than 95% of cellular components, such as DNA, were removed, demonstrating its use as an effective decellularization method. However, the study by Zilic et al. was performed in vitro and did not include nerve transplantation; thus, it was not possible to confirm nerve recovery after transplantation.
In our study, it was found that the Zilic method significantly better removed DNA content. The cause may be due to the use of nuclease, and it is conventionally known that SDS is more effective in removing cellular content (Crapo et al. 2011). In addition, the Triton X-200 is currently unavailable and has been replaced with the Triton X-100, but it is known that the Triton X-200 is effective in removing cellular content (Hudson et al. 2004a). In the present study, there was no statistically significant difference in the results of recovery after nerve transplantation between the two groups, and no complications related to transplantation were observed. Previous studies have also reported successful decellularization results with SDS or nucleases. Han et al. reported that the method using SDS was the most efficient in their study comparing four different methods, including SDS and Triton X-100, separately (Han et al. 2020). Shin et al. also reported that the combination of amphoteric detergent and nuclease was the most effective in a study comparing six different decellularization methods, including Triton X-100, sodium deoxycholate, and amphoteric detergents (Shin et al. 2019). Although no laboratory analysis was performed regarding the mechanical properties of the processed nerve allografts in our study, the nerves decellularized using the Zilic method were stiffer than those decellularized by the Hudson method; the stiffness made manipulation of the graft easier. In general, it is known that decellularized tissue becomes stiffer than the native tissue (Freed and Doehring 2005; Williams et al. 2009), and a study has shown that the tissue decellularized with Triton X-100 is softer than when using SDS (Lumpkins et al. 2008).
A limitation of this study is that it is difficult to generalize the study results due to the small number of animals in each experimental group. Second, the sciatic nerve of SD rats was used; however, the nerve defect created was a short gap of 15-mm, and the diameter was thin. In clinical situations, large gaps > 15 mm and thick nerve defects may occur. Third, this study was conducted using SD rats, and there is a possibility that different results would be observed in the human peripheral nerve. Finally, it is necessary to verify the safety of the decellularized tissue by conducting a direct cytotoxic assay, rather than using the amount of residual reagent in the tissue. Further research and experiments are required to develop a new human allograft processing method.
Conclusion
The Zilic method is more advantageous for the removal of the cellular component, and it is a more cost effective than the Hudson method, showing equal or superior results in terms of nerve recovery. Therefore, the novel method can be considered appropriate as an allograft processing method.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz EE, Wisotsky SM, Cho MS, Wilson C, Cooper EO, Ingari JV, Safa B, Parrett BM, Buncke GM (2012) Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 32:1–14. https://doi.org/10.1002/micr.20975
Cebotari S, Tudorache I, Jaekel T, Hilfiker A, Dorfman S, Ternes W, Haverich A, Lichtenberg A (2010) Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs 34:206–210. https://doi.org/10.1111/j.1525-1594.2009.00796.x
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243. https://doi.org/10.1016/j.biomaterials.2011.01.057
Du L, Wu X, Pang K, Yang Y (2011) Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br J Ophthalmol 95:410–414. https://doi.org/10.1136/bjo.2008.142539
Freed AD, Doehring TC (2005) Elastic model for crimped collagen fibrils. J Biomech Eng 127:587–593. https://doi.org/10.1115/1.1934145
Han LW, Xu G, Guo MY, Chang YA, Zhang Y, Zhao YT, Li ZH (2020) Comparison of SB-SDS and other decellularization methods for the acellular nerve graft: biological evaluation and nerve repair in vitro and in vivo. Synapse 74:e22143. https://doi.org/10.1002/syn.22143
Hudson TW, Liu SY, Schmidt CE (2004a) Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng 10:1346–1358. https://doi.org/10.1089/ten.2004.10.1641
Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE (2004b) Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 10:1641–1651. https://doi.org/10.1089/ten.2004.10.1641
Karlsson G, Hinz AC, Henriksson E, Winge S (2002) Determination of triton X-100 in plasma-derived coagulation factor VIII and factor IX products by reversed-phase high-performance liquid chromatography. J Chromatogr A 946:163–168. https://doi.org/10.1016/s0021-9673(01)01565-5
Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84:25–34. https://doi.org/10.1016/j.ymeth.2015.03.005
Korossis SA, Booth C, Wilcox HE, Watterson KG, Kearney JN, Fisher J, Ingham E (2002) Tissue engineering of cardiac valve prostheses II: biomechanical characterization of decellularized porcine aortic heart valves. J Heart Valve Dis 11:463–471
Lee JY, Giusti G, Wang H, Friedrich PF, Bishop AT, Shin AY (2013) Functional evaluation in the rat sciatic nerve defect model: a comparison of the sciatic functional index, ankle angles, and isometric tetanic force. Plast Reconstr Surg 132:1173–1180. https://doi.org/10.1097/PRS.0b013e3182a3bfeb
Lin FM, Pan YC, Hom C, Sabbahi M, Shenaq S (1996) Ankle stance angle: a functional index for the evaluation of sciatic nerve recovery after complete transection. J Reconstr Microsurg 12:173–177. https://doi.org/10.1055/s-2007-1006472
Lumpkins SB, Pierre N, McFetridge PS (2008) A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater 4:808–816. https://doi.org/10.1016/j.actbio.2008.01.016
Means KR Jr, Rinker BD, Higgins JP, Payne SH Jr, Merrell GA, Wilgis EFS (2016) A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand (N Y) 11:144–151. https://doi.org/10.1177/1558944715627233
Moore AM, MacEwan M, Santosa KB, Chenard KE, Ray WZ, Hunter DA, Mackinnon SE, Johnson PJ (2011) Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve 44:221–234. https://doi.org/10.1002/mus.22033
Nakayama KH, Batchelder CA, Lee CI, Tarantal AF (2010) Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A 16:2207–2216. https://doi.org/10.1089/ten.tea.2009.0602
Neubauer D, Graham JB, Muir D (2007) Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol 207:163–170. https://doi.org/10.1016/j.expneurol.2007.06.006
Rbia N, Bulstra LF, Saffari TM, Hovius SER, Shin AY (2019) Collagen nerve conduits and processed nerve allografts for the reconstruction of digital nerve gaps: a single-institution case series and review of the literature. World Neurosurg 127:e1176–e1184. https://doi.org/10.1016/j.wneu.2019.04.087
Rinker B, Zoldos J, Weber RV, Ko J, Thayer W, Greenberg J, Leversedge FJ, Safa B, Buncke G (2017) Use of processed nerve allografts to repair nerve injuries greater than 25 mm in the hand. Ann Plast Surg 78:S292–S295. https://doi.org/10.1097/SAP.0000000000001037
Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, Dimuzio PJ (2004) Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg 40:146–153. https://doi.org/10.1016/j.jvs.2004.03.033
Shin RH, Vathana T, Giessler GA, Friedrich PF, Bishop AT, Shin AY (2008) Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery 28:452–457. https://doi.org/10.1002/micr.20520
Shin YH, Park SY, Kim JK (2019) Comparison of systematically combined detergent and nuclease-based decellularization methods for acellular nerve graft: an ex vivo characterization and in vivo evaluation. J Tissue Eng Regen Med 13:1241–1252. https://doi.org/10.1002/term.2874
Sondell M, Lundborg G, Kanje M (1998) Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 795:44–54. https://doi.org/10.1016/s0006-8993(98)00251-0
Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH (2009) Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 39:787–799. https://doi.org/10.1002/mus.21220
Williams C, Liao J, Joyce EM, Wang B, Leach JB, Sacks MS, Wong JY (2009) Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomater 5:993–1005. https://doi.org/10.1016/j.actbio.2008.11.028
Wilshaw SP, Rooney P, Berry H, Kearney JN, Homer-Vanniasinkam S, Fisher J, Ingham E (2012) Development and characterization of acellular allogeneic arterial matrices. Tissue Eng Part A 18:471–483. https://doi.org/10.1089/ten.tea.2011.0287
Zilic L, Wilshaw SP, Haycock JW (2016) Decellularisation and histological characterisation of porcine peripheral nerves. Biotechnol Bioeng 113:2041–2053. https://doi.org/10.1002/bit.25964
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Ethical approval
All of animal care and experimental procedures were approved by the Catholic University of Korea College of Medicine, St. Vincent’s Hospital Institutional Animal Care and Use Committee (IRB17-7).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang, HV., Im, JH., Chung, YG. et al. Comparison of two different decellularization methods for processed nerve allograft. Cell Tissue Bank 22, 575–585 (2021). https://doi.org/10.1007/s10561-021-09965-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-021-09965-1