Abstract
Reconstruction of large skeletal defects is a significant and challenging issue. Bone allografts are often used for such reconstructions. However, sterilizing bone allografts by using γ-irradiation, damages collagen and causes the bone to become weak, brittle and less fatigue resistant. In a previous study, we successfully protected the mechanical properties of human cortical bone by conducting a pre-treatment with ribose, a natural and biocompatible agent. This study focuses on examining possible mechanisms by which ribose might protect the bone. We examined the mechanical properties, crosslinking, connectivity and free radical scavenging potentials of the ribose treatment. Human cortical bone beams were treated with varying concentration of ribose (0.06–1.2 M) and γ-irradiation before testing them in 3-point bending. The connectivity and amounts of crosslinking were determined with Hydrothermal-Isometric-Tension testing and High-Performance-Liquid-Chromatography, respectively. The free radical content was measured using Electron Paramagnetic Resonance. Ribose pre-treatment improved the mechanical properties of irradiation sterilized human bone in a pre-treatment concentration-dependent manner. The 1.2 M pre-treatment provided >100% of ultimate strength of normal controls and protected 76% of the work-to-fracture (toughness) lost in the irradiated controls. Similarly, the ribose pre-treatment improved the thermo-mechanical properties of irradiation-sterilized human bone collagen in a concentration-dependent manner. Greater free radical content and pentosidine content were modified in the ribose treated bone. This study shows that the mechanical properties of irradiation-sterilized cortical bone allografts can be protected by incubating the bone in a ribose solution prior to irradiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Critically sized defects in bone are gaps that are too large for the body’s physiology to heal on its own. Human bone transplants (allografts) are used in the orthopaedic reconstruction of skeletal defects resulting from traumatic injuries, diseases such as bone cancer and revision arthroplasty. In these situations, some form of reconstruction is necessary involving a graft material to bridge the gap and restore structure and function. Reconstruction of a critically sized segmental defect in a long bone commonly involves the use of a large cortical bone allograft or a megaprosthesis. Over 1.5 million structural bone-grafting procedures are performed each year in the USA (Kawaguchi and Hart 2015).
Under normal physiological loading conditions, micro-cracks will accumulate in bone tissue (Norman and Wang 1997; Ritchie et al. 2005; Zimmermann et al. 2014; Zioupos et al. 2008). This micro-cracking is normal and the cells present in bone (osteoclasts and osteoblasts) will remodel the damage accumulated by laying new bone in its place via osteonal remodelling. Since allograft tissue is dead bone, the normal mechanisms of remodelling are limited to the region close to the host-graft junction, or do not take place at all (Enneking and Campanacci 2001). The micro-cracks that accumulate constitute flaws in the material and become stress concentrations when the bone is loaded. High local stress can cause the cracks to grow. If the stresses are high enough and the cracks are large enough, the allograft will fail. Fracture toughness, or resistance to crack growth, is essential to limit the propagation of micro-cracks. Graft fracture is a clinically recognized failure mode and structural allograft reconstructions fail in this manner an estimated 20–40% of the time (Goldberg 2008; Thompson et al. 2000).
In order to ensure the safety of the recipient, bone transplants are often sterilized to reduce the biological risk of pathogens. γ-irradiation is the gold standard, as it is an effective method to destroy pathogens. This method, however, causes the bone to have insufficient mechanical properties (Akkus and Belaney 2005; Burton et al. 2014; Currey et al. 1997; Greenwald et al. 2001; Komender 1976) and graft fracture is a documented clinical failure mode (Lietman et al. 2000). The irradiation is known to cause damage to the bone collagen, which decreases the mechanical toughness, ultimate strength, strain-to-failure, fracture toughness and fatigue resistance particularly under tension (Akkus and Rimnac 2001; Mitchell et al. 2004) and one study found that this almost doubled the clinically observed graft fracture rate (~40%) (Lietman et al. 2000). For bone tissue, sterilization is often done with a relatively large dose of γ-irradiation. Tissue banks often use doses in the range of ~20 to 30 kGy (Salehpour et al. 1995). The formation of macromolecular free radicals in the organic phase of bone is a known result of scission sites in the collagen and defects in the mineral generated by γ-irradiation. This seems to be the major contributor to the loss of collagen connectivity and bone toughness previously reported (Akkus et al. 2005; Burton et al. 2014; Seto et al. 2008).
In a previous study by the Musculoskeletal Research Laboratory at Mount Sinai Hospital, it has been shown that the pre-treatment with ribose protected collagen connectivity and thermal stability of bovine cortical bone (Burton 2013). This protection is most likely due to the fact that γ-irradiation weakens bone collagen through cleavage of peptide bonds, and high temperature ribose treatment prior to irradiation induces non-enzymatic glycation crosslinking (Pentosidine) that stabilizes the organic network against formation of these cleavage sites. We have developed a novel method for improving quasi-static mechanical properties of human cortical bone by using ribose to increase the stability and connectivity of the bone collagen network prior to the irradiation (Willett et al. 2015). This results in the connectivity and thermal stability of the bone collagen being protected. About 70% of the toughness that was lost during irradiation without pre-treatment was protected in the ribose treated specimens. In addition to the toughness improvement, excellent protection of the ultimate strength, fracture toughness and failure strain was detected (Burton 2013; Willett et al. 2015).
This study focuses on examining two possible mechanisms by which ribose might protect the bone: (1) the first part of this study examines the correlation of the mechanical properties and the concentration-dependent effects of pre-treatment of human bone with ribose. To further characterize the correlation, the Pentosidine crosslink content and the collagen connectivity were examined. (2) The second part of the study is to characterize the free radical content, using electron paramagnetic resonance (EPR). Our hypothesis was that limiting the free radical damage pathway to the collagen phase by using ribose will minimize the mechanical impairment of γ-radiation sterilized bone tissue. In addition, we attempted to separate the effects of ribose bound to the collagen and free ribose to better understand their respective roles in the protective effect.
Materials and methods
Experiment 1: dose dependent treatment of ribose in human bone
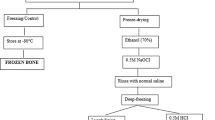
Cortical bone blocks were cut from the diaphysis of five human femurs (males aged 59–67 years) with an Isomet 1000 diamond wafer saw (Buehler Canada, Whitby, ON, Canada). 15 sets of 2 mm (t) × 4 mm (w) × 60 mm (l) rectangular beams were cut from these blocks using a diamond wire saw (Delaware Diamond Knives, Wilmington, DE, USA). The length was oriented along the longitudinal direction and the thickness in the radial direction. The endosteal side of the beam was marked to track orientation. Each set consisted of seven different treatment groups: non-irradiated control (Normal), irradiated control (Irradiated), and different concentrations of ribose pre-treated ± irradiated groups: 0.06, 0.3, 0.6, and 1.2 M in phosphate buffered solution (PBS) with calcium supplementation. The ribose treated specimens were incubated in a solution containing D-ribose with different concentrations at 60 °C for 24 h (pH 7.4) and then irradiated to 30 kGy using a Cobalt-60 source (Isomedix Steris, Whitby, ON, Canada) on dry ice. Irradiated controls received incubation in PBS only and were then irradiated. Non-Irradiated controls (Normal) were kept frozen until preparation for mechanical testing. The R1.2x group received ribose pre-treatment but no irradiation.
Three-point bending test
15 sets were prepared for 3-point bend testing. After irradiation, bone beams were thawed and polished to a 1 μm finish by hand to remove surface flaws and microcracks that could change the mechanical properties of the samples. Following polishing, each bone beam was soaked in 15 ml PBS for 4 h at room temperature to rehydrate the sample. The thickness and width were measured for each sample using a digital micrometer (Mitutoyo Canada Inc., Mississauga ON, Canada) to normalize the data to the geometry of each sample and calculate the stress/strain data. An Instron ElectroPuls E1000 mechanical testing machine (Instron, Norwood, MA, USA) was used to conduct the three-point testing. The three-point bending to failure test method was based on ASTM D790 (2010). The beams were placed on a support span of 40 mm, with the periosteal side facing the span. The applied load was measured using a calibrated 100 N load cell. Instron Bluehill software controlled the test and recorded time, beam deflection and load. From the load and deflection data, a stress–strain curve (S-e curve) was created. Elastic modulus (E), yield strength (Sy) and strain (εy), ultimate strength (US), failure strain (εf) and work-to-fracture (Wfx) were determined from the calculated S-e curves (Burton et al. 2014; Willett et al. 2013).
Thermo-mechanical testing using hydrothermal isometric tension testing (HIT)
After mechanical testing, portions of each beam away from the fracture site were decalcified with EDTA solution for 4 weeks. The samples were prepared to assess the thermal stability and connectivity of the bone collagen by using a custom-designed HIT instrument suited to our bone collagen specimens. These tests were conducted as previously reported (Burton et al. 2014; Willett et al. 2015). After decalcification, the bone collagen was trimmed with a razor blade to dimensions of roughly 1.5 × 1.5 × 20 millimetres. The tissue sample was held at a fixed length while attached to the load cell (Interface MB-5, Durham Instruments, Pickering, ON). While held, the tissue was then placed in a bath of distilled water heated from room temperature to 90 °C at a rate of 1.5 °C per minute. At a certain temperature (Td), the collagen is driven to denature (melt) and an increase in tension is measured. This is followed by a period of increasing tension due to the driving force favoring an amorphous structure and finally failure may occur before reaching 90 °C. Maximum Isometric stress (MIS) and the Maximum Slope all reflect the connectivity and extent of crosslinking of the collagen network (Zioupos et al. 1999).
High performance liquid chromatography (HPLC) for collagen crosslink contents
The HPLC method was established and previously published in Burton et al. (2014). After conducting the mechanical testing, a small piece of bone (~50 mg) away from the fracture surface was used to determine the amounts of mature enzymatically-derived pyridinoline crosslinks and the glyco-oxidation crosslink, Pentosidine, and these contents were normalized to bone collagen content according to Burton et al. (2014).
Scanning electron microscopy of fracture surfaces
After mechanical testing, the fracture surfaces were examined using a scanning electron microscope after cutting them carefully from the two resulting specimen fragments with a diamond wire saw (Model 3241, Well). The samples were prepared for scanning electron microscope (XL30 ESEM; Philips, USA). The fracture surfaces of the samples were examined by imaging the pulled out collagen fibres that correspond to the tensile failure site, which are indicators for ductility and toughness of the specimen (Burton et al. 2014; Willett et al. 2015). Since the beams were consistently placed with the periosteal side facing downwards, the tensile regions could be easily identified. The imaging was performed with the accelerating voltage set to 20 kV, working distance ~16 mm and a spot size of 4.
Experiment 2: correlation between free radical content and mechanical properties of irradiated and ribose treated group
Cortical bone beams were cut from human femurs (males between 59 and 67 years) with final dimensions of 2 × 4 × 50–60 mm. The beams were separated into 4 different treatment groups: Normal (not irradiated control), Irradiated control and ribose pre-treated + irradiated with a concentration of 1.2 M (R1.2) and a 1.2 M ribose pre-treated group + irradiation + washout (R1.2 W). The washout was performed to remove the unbound ribose in the bone. The 1.2 W group was soaked in PBS for two cycles, each in 37 °C for 24 h. The PBS solution was changed between the two cycles. Benedict’s reagent was used to confirm that no reducing sugar was detectable in the PBS solution, indicating that all unbound ribose was washed out of the bone (Benedict 1909).
EPR and three-point bending
The sample size of each sub-group was n = 15. Beams were cut, wrapped in saline soaked gauze and stored in a −80 °C freezer. Normal controls were kept frozen while the rest were sent for irradiation (~30 kGy on dry ice). After irradiation, these specimens were again stored at −80 °C to ensure optimal retention of free radicals. Subsequently, each specimen was placed into the EPR cavity so that the entire cavity was filled. EPR spectra was collected using a Bruker EMX EPR Spectrometer running at 9.5 GHz (X band) using an ER4119HS cavity, running at −80 °C to ensure stable free radical content. EPR scans were done between 320 and 344 mT to ensure all relevant peaks were captured. The separation of the organic (collagen) and inorganic (mineral) components of the bone has been performed based on Breen and Battista (1995). The peaks of the mineral component are found between 334 and 336 mT. A baseline correction was applied, and subtracted from original spectra. The free radical count was found by taking the double integral of the spectra from 329 to 340 mT. Upon completion, beams were removed and stored in saline soaked gauze at −20 °C.
Statistical analysis
For experiment 1 and 2, a one-way repeated measures ANOVA (RM-ANOVA) was used to detect differences between the means of each group. RM considers each sample within its matched set, which controls for inter-donor and inter-bone site variance. A Holms-Sidak post hoc analysis at a 95% confidence level was used for multiple comparisons between groups when significance was detected using RM. Pearson correlation (with two-tailed t test) was used to test for correlations between the work-to-fracture and connectivity of the bone (normalized to non-irradiated bone). All the tests were conducted using IBM SPSS Statistics (SPSS 22.0, Chicago,IL, USA).
Results
Experiment 1: dose dependent treatment of human bone
Three-point bending
γ-irradiation detectably decreased the work-to-fracture by 29% (p < 0.001) and the strain to failure by 19% (p < 0.001). Ribose pre-treatment improved the mechanical properties of irradiation-sterilized human bone in a concentration-dependent manner (Fig. 1; Table 1). The 1.2 M pre-treatment (R1.2) provided >100% of ultimate strength of normal controls and protected 76% of the work-to-fracture (toughness) lost in the irradiated controls. The 0.6 M treatment showed a protection of the work-to-fracture (33%) over the irradiated group with a statistically significant difference. The 0.06 M pre-treatment didn’t detectably protect the bone’s mechanical properties from irradiation. The ribose treatment starting from a concentration of 0.3 M showed a detectable trend towards protection. The yield stress was not affected by irradiation or any of the ribose pre-treatments, except for the R1.2x group, which was statistically different from the I-group (p = 0.034).
Representative stress–strain curves for the different treatment groups. Normal = untreated control, irradiated = irradiated control, R0.06 = 0.06 M ribose treated group + irradiation, R0.3 = 0.3 M ribose treated group + irradiation, R0.6 = 0.6 M ribose treated group + irradiation, R1.2 = 1.2 M ribose treated group + irradiation, R1.2x = 1.2 M ribose treated group without irradiation
HIT
Similarly to the results of the mechanical properties, ribose pre-treatment improved the thermo-mechanical properties of irradiation sterilized human bone collagen in a concentration-dependent manner (Fig. 2). The 1.2 M pre-treatment provided ~100% protection of thermal stability and connectivity of the bone collagen network. We found a strong positive correlation between bone’s toughness and collagen connectivity (r = 0.87, p < 0.05; Fig. 3). Differences in denaturation temperature, Td, were statistically detectable across all groups, with a 10% decrease (6.4 °C; p < 0.0001) due to irradiation and 100% protection (p < 0.0005) due to the 1.2 M ribose pre-treatment. Maximum slope also experienced a statistically detectable (p < 0.0002) reduction due to irradiation of 41%. Again, connectivity was protected with the ribose pre-treatment. The effect was such that the N and R0.6, R1.2 and R1.2x groups were not detectably different (p ≈ 1) thus protection of this measure was 100%. See Table 2 for the summary of the thermo-mechanical data of the different treatment groups.
Representative HIT curves for the different treatment groups. Normal = untreated control, irradiated = irradiated control, R0.06 = 0.06 M ribose treated group + irradiation, R0.3 = 0.3 M ribose treated group + irradiation, R0.6 = 0.6 M ribose treated group + irradiation, R1.2 = 1.2 M ribose treated group + irradiation, R1.2x = 1.2 M ribose treated group without irradiation
Work-to-fracture versus connectivity for all groups tested in three-point bending (r = 0.86, p < 0.05). The averages of the normalized values are shown here and the error bars represent the standard error of the mean. N = untreated control, I = irradiated control, R0.06 = 0.06 M ribose treated group + irradiation, R0.3 = 0.3 M ribose treated group + irradiation, R0.6 = 0.6 M ribose treated group + irradiation, R1.2 = 1.2 M ribose treated group + irradiation, R1.2x = 1.2 M ribose treated group without irradiation
HPLC
Pentosidine crosslinks were quantified using HPLC in order to determine if ribose pre-treatment was in fact crosslinking collagen. The concentration of Pentosidine in the sample was normalized to the amount of collagen in the sample using a colorimetric assay for hydroxyproline. Figure 4 shows the Pentosidine content of the different treatment groups. γ-Irradiation sterilization alone did not produce a detectable change in the collagen crosslinks measured using HPLC. Pentosidine content was small in both Normal and Irradiated controls (0.85 vs. 0.88 pmol/nmoles Collagen) in comparison to the ribose treated groups. All treated groups are statistically different than the Normal and Irradiated group (p < 0.001). The Pentosidine concentrations among R0.3, R0.6 and R1.2 were not statistically different from each other.
Pentosidine content in human bone for different treatment groups. The error bars represent the standard deviation. Normal = untreated control, irradiated = irradiated control, R0.06 = 0.06 M ribose treated group + irradiation, R0.3 = 0.3 M ribose treated group + irradiation, R0.6 = 0.6 M ribose treated group + irradiation, R1.2 = 1.2 M ribose treated group + irradiation, R1.2x = 1.2 M ribose treated group without irradiation. *All versus N and I p < 0.001. #R0.06 versus R0.3 and R0.6 p < 0.05. ^Rx versus R0.3, R0.6, R1.2 p < 0.05
SEM
Representative SEM images are shown in Fig. 5. The fracture surfaces of the different treatment groups show distinguished altered features. The tensile regions (periosteal side) on the fracture surfaces of the γ-irradiated group are flatter and less rough than the normal and ribose treated ones. Overall, there was less definition of lamellae and torn fibrils. At some areas of the fracture surface the morphology suggested a slightly melted structure (indicated with a white arrow in Fig. 5). The surface from the normal group displayed more overall depth of roughness. The ribose pre-treated groups appeared to contain similar features to both the normal and irradiated groups. There are rough features as in the normal group, but also smoother featureless areas as in the irradiated group (Fig. 5).
SEM images of tensile fracture surfaces resulting from three-point bending of human specimens (scale bar 20 microns). N = normal control (not irradiated); I = irradiated control; R = ribose pre-treated and irradiated specimen with different concentrations, R0.06 = 0.06 M ribose treated group + irradiation, R0.3 = 0.3 M ribose treated group + irradiation, R0.6 = 0.6 M ribose treated group + irradiation, R1.2 = 1.2 M ribose treated group + irradiation, R1.2x = 1.2 M ribose treated group without irradiation. White arrow indicates “melted” structure
Experiment 2: correlation between free radical content and mechanical properties of irradiated and ribose treated group
3-point bending and EPR
As expected, 30 kGy of γ-irradiation created large free radical contents in both the organic and mineral phases of the conventionally irradiated bone. Non-irradiated controls contained barely detectable amounts. The 1.2 M ribose pre-treatment and 1.2 M-Washout resulted in 40 and 22% less free radical content immediately after irradiation, respectively, in comparison to the I-group (p < 0.0001, Fig. 6). Conventional irradiation greatly decreased the work-to-fracture of the bone by 45% (p < 0.001). 1.2 M ribose pre-treatment protected the work-to-fracture compared to irradiated bone by 62% (p = 0.001). The 1.2 M-Washout was not statistically different from the 1.2 M ribose treatment group (Fig. 6).
a Free radical content of the different treatment groups. The error bars represent the standard deviation. Normal = untreated control, irradiated = irradiated control, R1.2 = 1.2 M ribose treated group + irradiation, R1.2 W = 1.2 M ribose treated group + washout the loose ribose + irradiation. One way repeated measures ANOVA. a N versus all: p < 0.0001, I versus all: p < 0.0001, R1.2 versus all p < 0.001, R1.2 W versus all: p < 0.0001. b Work-to-fracture of the different treatment groups. #Versus N: p < 0.0001, ^Versus N: p < 0.05, *Versus I: p < 0.001, R1.2 versus R1.2 W no statistically difference
Discussion
The objective of this study was to evaluate changes in the mechanical properties of cortical bone as a result of γ-irradiation and ribose treatment, and to more completely characterize the properties of bone in order to better understand the mechanisms by which ribose protects bone’s mechanical properties. Our data show that the modified collagenous phase created by ribose pre-treatment improves the post-yield mechanical properties of irradiation-sterilized human bone allograft. The modulus and yield stress were not affected by irradiation of bone or any of the pre-treatments, except the R1.2x group. These properties are considered to be mainly influenced by the mineral in bone, since they reflect the stiffness and the transition point from pre-yield behaviour to post-yield behaviour, respectively (Turner 2006; Wang et al. 2002). The post-yield mechanical properties improved with increasing ribose pre-treatment concentration. The Pentosidine amounts in the Normal and Irradiated controls occur naturally with age due to the formation of advanced glycation end-products (Sroga et al. 2015). Interestingly, the Pentosidine amount in R0.3, R0.6 and R1.2 showed no significant difference (Fig. 4) but the mechanical properties were superior in the R1.2 group (Table 1). This leads to the assumption that Pentosidine is not, or at least not the only protective component for the bone’s mechanical and thermo-mechanical properties as the mechanical properties do not correlate with crosslinking amount. In collagen, Pentosidine is the resultant crosslink formed between arginine and lysine (Dong et al. 2011). Pentosidine is not the only crosslink or adduct formed as a result of non-enzymatic glycation but it is relatively easily measured and hence has become the marker both in vivo and in vitro AGEs. As there is only x-amount of lysine and arginine sites, we believe we saturated the crosslink formation. Another possible reason for the limited increase of Pentosidine among the ribose treated groups is the increased viscosity of the ribose solution with higher concentrations, which might cause diffusion barriers inside the bone. Other AGEs have been shown to accumulate in bone tissue including vesperlysine, methylglyoxal-derived lysine dimer, glyoxal-derived lysine dimer, imidazolone and N(epsilon)-carboxymethyllysine (Vashishth 2009). Versperlysine, for example, is a potential AGE that could be formed between two lysine side-chains in proteins due to the ribose treatment (Nakamura et al. 1997). However, their correlation with the mechanical properties has not been fully investigated/understood yet. This requires further studies. The R1.2x (ribose incubation without irradiation) showed improved mechanical and thermo-mechanical properties over the non-irradiated group. This suggests that a modification to the connectivity is done prior irradiation. There are two possible mechanisms that could provide protection to bone: (1) the increase in overall network connectivity resulting from the large number of ribose crosslinks, in addition to Pentosidine, that may compensate for chain scissions that occur during irradiation. (2) Collagen stabilization resulting from ribose crosslinking may protect the collagen from the chain scission that occurs during irradiation sterilization. This requires further studies. Interestingly, many studies have reported degraded post-yield mechanical properties after treating the bone with ribose at body temperature for longer period of time (up to 38 days) (Catanese III et al. 1999; Tang and Vashishth 2011; Vashishth 2009; Vashishth et al. 2001), in comparison to our previously published (Willett et al. 2015; Woodside and Willett 2016) and current results. The proposed mechanism for the degradation is due to the glycation end-product crosslinking. It is widely believed that the loss of collagen ductility due to advanced glycation end-product crosslinking is causing the bone to get weak and brittle. Herein, we propose that the increased crosslinking compensates for the loss of scission sites that occur during irradiation. Other degrading mechanism that could take place with longer incubation time is dehydrating the bone due to the hydrophilic properties of a sugar. This requires further studies. The strength, ductility and toughness are protected in irradiated bone pre- treated with ribose due to the increased stability and connectivity of the collagen network resulting from crosslinking. Figure 3 shows a strong positive correlation of connectivity, measured with HIT, and work-to-fracture of the cortical bone. This data suggest that it may be possible to achieve mechanical performance equal to or greater than that of normal bone by continuing to increase connectivity. Nevertheless, the different treatment conditions in the above mentioned studies (Catanese III et al. 1999; Tang and Vashishth 2011; Vashishth 2009; Vashishth et al. 2001) make it difficult to compare with achieved results in this study. Incubation times of up to 38 days at body temperature require further study, as they could lead to different degradation mechanisms and therefore to inferior mechanical properties.
Another possible hypothesis is that ribose is protecting the bone from free radicals that occur during irradiation. It is known that γ-irradiation causes radiolysis of water molecules and therefore the generation of free radicals (Akkus et al. 2005; Nguyen et al. 2007). This seems to be the major contributor to the loss of collagen connectivity and bone toughness, as previously reported (Akkus et al. 2005; Houben 1971; Seto et al. 2008, 2009). This study demonstrates that the ribose pre-treatment reduces the number of free radicals formed in the bone and free ribose may play a role in reducing free radical content and presumably damage (Fig. 6). Interestingly, the difference in free radical content between 1.2 and 1.2 M-Washout (p < 0.0001) did not correlate with differences in mechanical properties of the bone, as the work-to-fracture showed no significant difference between the two ribose treated groups. This leads to the conclusion that the free radical content by itself may not be a sufficient correlate for changes in the mechanical properties of irradiation-sterilized bone, although further studies are required.
The SEM images of the fracture surfaces of the normal and ribose-treated group show evidence of tearing collagen fibrils, which is noticeably diminished in the irradiated group. Similar results has been reported in Akkus et al. (2005) and Nguyen et al. (2007). The melted and amorphous structure of the irradiated group suggests that the integrity of the collagen matrix is damaged through scission sites caused by irradiation; the collagen fibres fail to transfer loads and collapse prematurely. On the other hand, the ribose-treated groups show intermediate roughness and to some extent pulled out fibres, which indicates intact collagen structure. The fractography is further evidence that the ribose protects the collagen structure from scission sites created by γ-irradiation.
This study shows that ribose pre-treatment is a promising approach to protect the mechanical properties of irradiation-sterilized bone allografts. The treatment’s effect on the fatigue life still remains to be examined. Furthermore, it needs to be determined if the presence of ribose within the bone tissue would interfere with the effectiveness of the γ-irradiation sterilization against bacteria.
Conclusions
In this study, we have shown that the mechanical properties of irradiation-sterilized cortical bone allografts can be protected by incubating the bone in ribose prior to irradiation. The exact mechanisms at play require future study. The next steps include: (a) assessing the potential of this technology to determine if the presence of ribose within the bone tissue will interfere with the effectiveness of the gamma-irradiation sterilization against bacteria, and (b) fatigue testing of bone tissue treated with the methods reported herein. An improved understanding of the ribose treatment and its protective mechanism may lead to novel ways of avoiding graft fracture.
References
Akkus O, Rimnac CM (2001) Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res 19:927–934. doi:10.1016/S0736-0266(01)00004-3
Akkus O, Belaney RM (2005) Sterilization by gamma radiation impairs the tensile fatigue life of cortical bone by two orders of magnitude. J Orthop Res 23(1054–1058):2005. doi:10.1016/J.Orthres.2005.03.003
Akkus O, Belaney RM, Das P (2005) Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res 23(838–845):2005. doi:10.1016/J.Orthres.2005.01.007
Benedict SR (1909) A reagent for the detection of reducing sugars. J Biol Chem 5:485–487
Breen SL, Battista JJ (1995) Radiation dosimetry in human bone using electron paramagnetic resonance. Phys Med Biol 40:2065–2077. doi:10.1088/0031-9155/40/12/005
Burton B (2013) Improving the mechanical properties of irradiation sterilized bone allografts master of applied science Thesis, Institute of Biomaterials and Biomedical Engineering University of Toronto
Burton B, Gaspar A, Josey D, Tupy J, Grynpas MD, Willett TL (2014) Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization. Bone 61C:71–81. doi:10.1016/j.bone.2014.01.006
Catanese J, III, Bank RA, Tekoppele JM, Keaveny TM (1999) Increased cross-linking by non-enzymatic glycation reduces the ductility of bone and bone collagen bioengineering conference. ASME 42:267–268
Currey JD, Foreman J, Laketic I, Mitchell J, Pegg DE, Reilly GC (1997) Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res 15:111–117. doi:10.1002/Jor.1100150116
Dong XN, Qin A, Xu JK, Wang XD (2011) In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone 49:174–183. doi:10.1016/j.bone.2011.04.009
Enneking WE, Campanacci DA (2001) Retrieved human allografts—a clinicopathological study. J Bone Joint Surg Am 83A:971–986
Goldberg VM (2008) Biology of bone allograft and clinical application. In: Pietrzak W (ed) Musculoskeletal tissue regeneration: biological materials and methods, vol 5. Humana Press, New York, pp 81–92
Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN, Implants CB (2001) Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am 83A:98–103
Houben JL (1971) Free radicals produced by ionizing radiation in bone and its constituents. Int J Radiat Biol Relat Stud Phys Chem Med 20:373–389
Kawaguchi S, Hart RA (2015) The need for structural allograft biomechanical guidelines. J Am Acad Orthop Surg 23:119–125. doi:10.5435/JAAOS-D-14-00263
Komender A (1976) Influence of preservation on some mechanical properties of human haversian bone. Mater Med Pol 1:13–17
Lietman SA, Tomford WW, Gebhardt MC, Springfield DS, Mankin HJ (2000) Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res 375:214–217
Mitchell EJ, Stawarz AM, Kayacan R, Rimnac CM (2004) The effect of gamma radiation sterilization on the fatigue crack propagation resistance of human cortical bone. J Bone Joint Surg Am 86A:2648–2657
Nakamura K, Nakazawa Y, Ienaga K (1997) Acid-stable fluorescent advanced glycation end products: vesperlysines A, B, and C are formed as crosslinked products in the maillard reaction between lysine or proteins with glucose. Biochem Biophys Res Commun 232:227–230. doi:10.1006/Bbrc.1997.6262
Nguyen H, Morgan DA, Forwood MR (2007) Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank 8:93–105. doi:10.1007/s10561-006-9020-1
Norman TL, Wang Z (1997) Microdamage of human cortical bone: incidence and morphology in long bones. Bone 20:375–379. doi:10.1016/S8756-3282(97)00004-5
Ritchie RO, Kinney JH, Kruzic JJ, Nalla RK (2005) A fracture mechanics and mechanistic approach to the failure of cortical bone. Fatigue Fract Eng Mater Struct 28:345–371. doi:10.1111/J.1460-2695.2005.00878.X
Salehpour A, Butler DL, Proch E, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A (1995) Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of coat bone-patellar tendon-bone allografts. J Orthop Res 13:898–906. doi:10.1002/Jor.1100130614
Seto A, Gatt CJ, Dunn MG (2008) Radioprotection of tendon tissue via crosslinking and free radical scavenging. Clin Orthop Relat Res 466:1788–1795. doi:10.1007/S11999-008-0301-9
Seto A, Gatt CJ, Dunn MG (2009) Improved Tendon Radioprotection by Combined Cross-linking and Free Radical Scavenging. Clin Orthop Relat Res 467:2994–3001. doi:10.1007/S11999-009-0934-3
Sroga GE, Siddula A, Vashishth D (2015) Glycation of human cortical and cancellous bone captures differences in the formation of maillard reaction products between glucose and ribose. PLoS ONE 10:e0117240. doi:10.1371/journal.pone.0117240
Tang SY, Vashishth D (2011) The relative contributions of non-enzymatic glycation and cortical porosity on the fracture toughness of aging bone. J Biomech 44:330–336. doi:10.1016/j.jbiomech.2010.10.016
Thompson RC Jr, Garg A, Clohisy DR, Cheng EY (2000) Fractures in large-segment allografts. Clin Orthop Relat Res 370:227–235
Turner CH (2006) Bone strength: current concepts. Ann NY Acad Sci 1068:429–446. doi:10.1196/Annals.1346.039
Vashishth AK (2009) Advanced glycation end-products and bone fractures. IBMS BoneKEy 6:268–278
Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP (2001) Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28:195–201. doi:10.1016/S8756-3282(00)00434-8
Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7. doi:10.1016/S8756-3282(01)00697-4
Willett TL, Sutty S, Gaspar A, Avery N, Grynpas M (2013) In vitro non-enzymatic ribation reduces post-yield strain accommodation in cortical bone. Bone 52:611–622. doi:10.1016/j.bone.2012.11.014
Willett TL, Burton B, Woodside M, Wang Z, Gaspar A, Attia T (2015) Gamma-irradiation sterilized bone strengthened and toughened by ribose pre-treatment. J Mech Behav Biomed Mater 44C:147–155. doi:10.1016/j.jmbbm.2015.01.003
Woodside M, Willett TL (2016) Elastic–plastic fracture toughness and rising J(R)-curve behavior of cortical bone is partially protected from irradiation–sterilization-induced degradation by ribose protectant. J Mech Behav Biomed Mater 64:53–64. doi:10.1016/j.jmbbm.2016.07.001
Zimmermann EA, Gludovatz B, Schaible E, Busse B, Ritchie RO (2014) Fracture resistance of human cortical bone across multiple length-scales at physiological strain rates. Biomaterials 35(5472–5481):2014. doi:10.1016/J.Biomaterials.03.066
Zioupos P, Currey JD, Hamer AJ (1999) The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res 45:108–116. doi:10.1002/(Sici)1097-4636(199905)45:2<108:Aid-Jbm5>3.0.Co;2-A
Zioupos P, Gresle M, Winwood K (2008) Fatigue strength of human cortical bone: age, physical, and material heterogeneity effects. J Biomed Mater Res, Part A 86A:627–636. doi:10.1002/Jbm.A.31576
Acknowledgements
This work was funded by the Canadian Institute of Health Research, the Natural Science and Engineering Research Council of Canada, and scholarships from the University of Toronto Institute for Biomaterials and Biomedical Engineering and Toronto Musculoskeletal Centre. The authors also acknowledge the contributions made by Mr. Jindra Tupy and Mr. Doug Holmyard. We acknowledge the contributions of our tissue-banking partner, Mount Sinai Allograft Technologies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attia, T., Woodside, M., Minhas, G. et al. Development of a novel method for the strengthening and toughening of irradiation-sterilized bone allografts. Cell Tissue Bank 18, 323–334 (2017). https://doi.org/10.1007/s10561-017-9634-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9634-5