Abstract
Aims
This review summarizes the findings of preclinical studies evaluating the pleiotropic effects of ticagrelor. These include attenuation of ischemia–reperfusion injury (IRI), inflammation, adverse cardiac remodeling, and atherosclerosis. In doing so, it aims to provide novel insights into ticagrelor’s mechanisms and benefits over other P2Y12 inhibitors. It also generates viable hypotheses for the results of seminal clinical trials assessing ticagrelor use in acute and chronic coronary syndromes.
Methods and Results
A comprehensive review of the preclinical literature demonstrates that ticagrelor protects against IRI in the setting of both an acute myocardial infarction (MI), and when MI occurs while on chronic treatment. Maintenance therapy with ticagrelor also likely mitigates adverse inflammation, cardiac remodeling, and atherosclerosis, while improving stem cell recruitment. These effects are probably mediated by ticagrelor’s ability to increase local interstitial adenosine levels which activate downstream cardio-protective molecules. Attenuation and augmentation of these pleiotropic effects by high-dose aspirin and caffeine, and statins respectively may help explain variable outcomes in PLATO and subsequent randomized controlled trials (RCTs).
Conclusion
Most RCTs and meta-analyses have not evaluated the pleiotropic effects of ticagrelor. We need further studies comparing cardiovascular outcomes in patients treated with ticagrelor versus other P2Y12 inhibitors that are mindful of the unique pleiotropic advantages afforded by ticagrelor, as well as possible interactions with other therapies (e.g., aspirin, statins, caffeine).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor, i.e., prasugrel, ticagrelor, or clopidogrel, is the cornerstone of treatment for patients who present with acute coronary syndrome (ACS) or undergo percutaneous coronary intervention (PCI) [1]. The latest European Society of Cardiology and American College of Cardiology/American Heart Association guidelines recommend a higher potency P2Y12 receptor inhibitor (ticagrelor or prasugrel) over clopidogrel in patients with an ST-elevation myocardial infarction (STEMI) treated with primary PCI or non-ST-elevation myocardial infarction (NSTEMI) managed with either invasive or conservative measures [2,3,4,5]. The preference for ticagrelor over clopidogrel is informed by the landmark Platelet Inhibition and Patient Outcomes (PLATO) randomized controlled trial (RCT), which demonstrated superiority of ticagrelor to reduce a composite outcome of death from vascular causes, myocardial infarction (MI), or stroke, consistent in STEMI patients treated with primary PCI, and in NSTEMI managed with or without revascularization [6,7,8,9]. While ticagrelor did not increase the risk of major bleeding according to the primary endpoint definition, there was a higher rate of major bleeding not related to coronary-artery bypass grafting, including more instances of fatal intracranial bleeding [6].
However, most subsequent RCTs and meta-analyses comparing ticagrelor and clopidogrel failed to replicate the positive results seen in PLATO [10,11,12,13,14,15,16,17,18]. Notably, these studies did not account for two crucial observations from the PLATO trial. First, the positive effects of ticagrelor were absent in those who received maintenance therapy with high-dose (HD) compared to low-dose (LD) aspirin, a finding that was mostly confined to patients enrolled in North America [19]. Second, these effects were also attenuated in those off lipid-lowering drugs [6]. Understanding these interactions may provide the key to uncovering why subsequent trials and meta-analyses comparing ticagrelor to clopidogrel have yielded mostly neutral results.
Patients in RCTs assessing P2Y12 inhibitors receive a loading dose followed by maintenance treatment and evaluated for major adverse cardiovascular events (MACE) months to years after initiation of therapy. Save for some landmark analyses, this study design is not conducive to distinguishing between acute versus chronic effects of ticagrelor, nor temporal interactions with other therapies [20]. Preclinical studies can better establish a temporal relationship between treatment and effects, as well as provide evidence of the possible mechanisms that mediate the observed outcomes [21]. This review aims to synthesize preclinical findings on the benefits of ticagrelor with those from the seminal RCTs and meta-analyses. In doing so, we provide insights into the mechanisms of action of ticagrelor, its benefits over other P2Y12 inhibitors, and the failure of RCTs to reproduce the original results of PLATO.
The mechanisms that may underlie the advantages of ticagrelor over clopidogrel include faster and more complete platelet inhibition shortly after the first dose, and more efficient and sustained platelet inhibition during chronic treatment. Additionally, ticagrelor possesses unique adenosine-mediated pleiotropic effects that may also explain its greater efficacy, i.e., greater vasodilation of infarcted vessels, protection against ischemia–reperfusion injury (IRI), and reduced inflammation when ticagrelor is initiated after an acute MI or if an MI occurs on chronic treatment; and attenuation of recurrent cardiovascular events with maintenance ticagrelor therapy due to mitigation of inflammation, atherosclerosis, and adverse cardiac remodeling (Fig. 1). Furthermore, interactions with commonly used background therapy may attenuate or augment these non-platelet-mediated effects of ticagrelor.
Does Greater Platelet Inhibition from Acute Loading with Ticagrelor Explain Its Benefits?
Adenosine diphosphate (ADP) triggers platelet degranulation, thromboxane production, and eventual activation of the glycoprotein IIb/IIIa receptor leading to platelet aggregation. Clopidogrel is a prodrug that requires two-step activation by the liver to its active metabolite before irreversibly antagonizing the ADP-binding site on the P2Y12 receptor. In contrast, ticagrelor directly and reversibly binds to a site on the P2Y12 receptor separate from the ADP-binding site which inhibits the ADP-induced receptor conformational change and G-protein activation preventing ADP downstream signaling. Consequently, ticagrelor inhibits platelet aggregation 0.5–2 h after ingestion compared to clopidogrel which takes 2–6 h before onset of action. Furthermore, ticagrelor leaves the receptor intact upon dissociation [22]. Theoretically, more potency and efficacy of platelet inhibition with ticagrelor loading can lead to faster and greater attenuation of atherothrombosis which improves coronary artery perfusion and may lead to acute benefits in the setting of a STEMI.
However, findings from preclinical trials do not support this hypothesis. A single dose of intraperitoneal ticagrelor 5 min prior to reperfusion reduced infarct size (IS) measured 24 h after reperfusion in rats more than clopidogrel. Ticagrelor and clopidogrel had similar levels of platelet inhibition and comparable bleeding times measured 2 h after reperfusion. This acute benefit translated to improved myocardial function with ticagrelor over clopidogrel 4 weeks post-MI [23]. Regardless of a similar platelet inhibitory effect, ticagrelor added to thrombolytic therapy significantly reduced the rate of coronary re-occlusion and limited IS minutes to hours after MI induction in dogs as compared to clopidogrel [24].
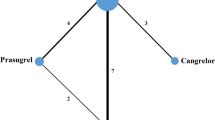
A single dose of ticagrelor 10 min before reperfusion or cangrelor 10 min before reperfusion followed by a continuous infusion equally limited IS at 2 h and 3 days postreperfusion [25]. A recent RCT that compared the effects on IS of a loading dose of ticagrelor to cangrelor followed by maintenance therapy with ticagrelor in patients presenting with a STEMI, corroborated these findings; despite cangrelor producing more potent platelet inhibition compared to ticagrelor at time of PCI, there was no improvement in coronary reperfusion or IS 12 weeks later [26]. Cangrelor is an intravenous (IV) P2Y12 inhibitor that achieves maximal platelet inhibition within 15 min of administration [22]. If greater speed, potency, and efficacy of platelet inhibition were responsible for attenuation of IS, one would expect cangrelor to reduce IS more than ticagrelor. This was not observed, suggesting that greater platelet inhibition does not likely account for reduced IS with ticagrelor in comparison to clopidogrel therapy demonstrated by Ye and Wang [23, 24]. Indeed, minimizing delayed microvascular damage, which evolves over several hours after coronary reperfusion may be more important than platelet inhibition at time of PCI [27].
Ticagrelor, Adenosine, and Ischemia–Reperfusion Injury
IRI is myocardial damage that occurs after reperfusion due to oxidative stress, excess intracellular calcium, and inflammation [28]. Animal studies have shown that IRI may account for up to 50% of final IS [29]. Thus, attenuating IRI may be as consequential as shortening ischemic time.
Adenosine mitigates IRI and apoptosis, in addition to improving myocyte regeneration, contractility, and electrical stability [22]. Adenosine activates various receptors on endothelial cells and cardiomyocytes which lead to increases in cyclic adenosine monophosphate (cAMP) and nitric oxide production. These molecules induce vasodilation during ischemia which leads to improved metabolic function in both endothelium and coronary smooth muscle. Ischemic preconditioning, the phenomenon of repeated brief episodes of ischemia and reperfusion preceding sustained ischemia, also protects against IRI via adenosine-mediated activation of adenosine triphosphate (ATP)-sensitive potassium channels [30]. Adenosine attenuates production of free radicals and pro-inflammatory mediators during ischemia and reperfusion. In animal models, these effects of adenosine reduce myocardial stunning and improve long-term cardiac function [31].
Unique among the P2Y12 inhibitors, ticagrelor has direct effects on adenosine metabolism (Fig. 2). Ticagrelor binds to equilibrate nucleoside transporter-1 (ENT-1) which blocks reuptake of adenosine into the cells and facilitates subsequent degradation to inosine [32]. In turn, higher levels of adenosine in the interstitial space at the site of ischemia mediates local vasodilation and reduction of free radicals and pro-inflammatory molecules that facilitate IRI [31]. Downstream, adenosine receptor activation leads to cyclooxygenase-2 (COX-2) activation, in addition to pro-survival kinases, e.g., Akt, extracellular signal-regular kinase (ERK) 1/2, and endothelial nitric oxide synthase (eNOS), which mediate cardioprotective effects [33, 34]. Adenosine receptor activation is the proposed mechanism for the sensation of dyspnea, a well-described side effect of ticagrelor, which in some cases leads to discontinuation of the medication [32, 35].
The adenosine-mediated pleiotropic effects of ticagrelor and its interactions with statins, aspirin, and caffeine. ATP adenosine triphosphate, Plt platelet, RBC red blood cell, P2Y12 P2Y12, ADP adenosine diphosphate, ENT-1 equilibrate nucleoside transporter-1, E5’N ecto-5’-nucleotidase, ADA adenosine deaminase, COX-2 cyclooxygenase 2, AMPK adenosine monophosphate-activated protein kinase
Statins upregulate 5′-nucleotidase, which leads to more adenosine export into the interstitial space [36, 37]. HD-aspirin administered prior to reperfusion—at doses comparable to those used in the clinical setting—dose-dependently inhibits COX-2 and production of cardioprotective eicosanoids and prostaglandins. This correlates with dose-dependent attenuation of the IS-limiting effects of atorvastatin in rats [38].
Ticagrelor Exhibits Adenosine-Mediated Protection Against Ischemia–Reperfusion Injury When Administered Prior to Reperfusion
As previously discussed, ticagrelor administered to rats 5 min prior to reperfusion reduced IS 24 h after reperfusion and preserved left ventricular (LV) function at 4 weeks compared to clopidogrel. This correlated with higher levels of adenosine and pro-survival kinases in ticagrelor-treated rats [23]. Adenosine levels have been shown to be higher in patients with ACS treated with ticagrelor as compared to clopidogrel due to inhibition of adenosine uptake by red blood cells [39]. It is possible that higher levels of adenosine in STEMI patients may protect against IRI acutely.
In a rat model, co-administration of ticagrelor and a caspase-1 inhibitor with a potent anti-inflammatory effect (VX-765) had significant additive effects of reducing IS at both 120 min and 3 days after reperfusion when given 5–10 min prior to reperfusion. Conversely, ischemic postconditioning in conjunction with ticagrelor administration had no additive effect, suggesting that P2Y12 inhibitors already condition the heart in a similar manner rendering further postconditioning redundant [25].
In a dog study, IV ticagrelor exhibited a significant reduction in rates of coronary re-occlusion, quicker return to baseline coronary blood flow with reduced cyclic flow variation and reduced IS after 120 min of reperfusion compared to clopidogrel. All dogs received loading with 325 mg aspirin orally and tissue plasminogen activator (tPA) with reperfusion [24]. Another study demonstrated that ticagrelor dose-dependently inhibited adenosine uptake by erythrocytes and augmented the hyperemic response to temporary occlusion or direct intracoronary adenosine infusion [40]. Thus, the authors concluded that the improvement in coronary blood flow was possibly due to adenosine-mediated vasodilation induced by ticagrelor [24]. A summary of animal trials evaluating the effects of ticagrelor on IRI is available in Table 1.
Maintenance Ticagrelor Treatment Prevents Future Cardiovascular Events
The benefits of chronic ticagrelor therapy over clopidogrel may be attributed to its ability to prevent future cardiac events after an MI. The mechanisms by which this may occur include greater platelet inhibition in the long term leading to less recurrent ischemia, attenuation of inflammation thereby mitigating both adverse cardiac remodeling, and atherosclerosis progression [41].
Platelet Inhibition
In the chronic setting, it is also possible that greater long-term platelet inhibition can prevent atherothrombosis and subsequent coronary ischemia. Nanhwan demonstrated reduced IS after 24 h of reperfusion in rats pretreated with ticagrelor compared to clopidogrel 7 days prior to MI [34]. Birnbaum yielded similar results comparing ticagrelor to prasugrel after 3 days of dosing [42]. Superiority of ticagrelor over prasugrel to improve LV function measured by echocardiography at days 14 and 28 started 7 days after ischemia and reperfusion was further demonstrated [43]. Another animal model found attenuation of atherosclerosis progression in rats treated with ticagrelor or rosuvastatin compared to clopidogrel after 14 weeks of treatment [44]. All these results were seen in the setting of similar levels of platelet inhibition with ticagrelor compared to other P2Y12 inhibitors. Thus, just as with acute ticagrelor loading, greater potency and efficacy with regards to platelet inhibition in the chronic setting does not appear to explain the benefits of reduced IS, attenuation of adverse remodeling, mitigation of atherosclerotic progression, or protection from IRI when reinfarction occurs on chronic ticagrelor treatment. However, in the clinical setting, better anti-platelet effects might contribute to improved clinical outcomes.
Attenuation of Inflammation and Adverse Cardiac Remodeling
Adenosine also protects against adverse cardiac remodeling. An increased neurohormonal response after infarction induces a release of catecholamines and growth factors which lead to fibrosis, beta-adrenoceptor-mediated myocardial hypercontractility, and myocyte hypertrophy [45]. Adenosine reduces the release of catecholamines and calcium overload, augments coronary blood flow, and prevents platelet and leukocyte activation. Adenosine also inhibits renin release and tumor necrosis factor (TNF)-α production in experimental models, processes that contribute to adverse cardiac remodeling [46, 47].
Ye assessed the effects of chronic ticagrelor treatment on myocardial function in rats when initiated after completion of IRI. They demonstrated that 4w of dosing started 24 h after reperfusion normalized LV internal diameter in diastole and systole, and preserved LV ejection fraction similar to a loading dose of ticagrelor before reperfusion. In the group of rats treated with both a loading dose and chronic treatment after reperfusion, there was an additive effect of improving these echocardiographic parameters. Increased levels of adenosine and pro-survival kinases, a reduction in fibrosis and lower levels of inflammatory cytokines (TNF-α, interleukin (IL)-1β, and IL-18) were also observed in ticagrelor-treated rats. None of these positive effects were seen with clopidogrel [23]. This study suggests that regardless of protection from IRI, chronic ticagrelor therapy attenuates inflammation, prevents adverse cardiac remodeling, and preserves myocardial function via an adenosine-mediated pathway.
In another study, rats were administered daily oral doses of ticagrelor, HD-aspirin, ticagrelor and HD-aspirin, or prasugrel 7 days after infarction and reperfusion. Ticagrelor and HD-aspirin independently attenuated the decrease in systolic function and remodeling measured on days 14 and 28, decreased levels of markers of fibrosis, and increased levels of the eicosanoid, 15-epi-lipoxin-A4. These effects were not seen with prasugrel. Furthermore, only ticagrelor-treated rats had higher levels of progenitor stem cell markers and showed cardiac regeneration in the infarcted tissue. These benefits were found to be attenuated when HD-aspirin and ticagrelor were administered together [43]. The results suggest that HD-aspirin may block the effects of maintenance ticagrelor therapy to limit adverse cardiac remodeling and induce cardiomyocyte regeneration by inhibiting the production of cardioprotective eicosanoids.
Atherosclerosis
Atherogenesis involves complex interactions between lipids, endothelial cells, and the immune system [48, 49]. The following studies have shown that ticagrelor mitigates the progression of atherosclerosis through adenosine-mediated attenuation of pro-inflammatory cytokines.
Preusch demonstrated a significant reduction in the relative area of the necrotic core and increase in fibrous cap thickness in mice with advanced atherosclerotic lesions in the aortic sinus treated with ticagrelor for 25 weeks. An in vitro analysis revealed significant reduction in the prevalence of apoptotic macrophages and their uptake of oxidized low-density lipoprotein (LDL) [50]. Another study showed this reduction of oxidized LDL was dose-dependent, and that ticagrelor also decreased expression of proprotein convertase subtilisin/kexin type (PCSK9), a powerful regulator of LDL receptor degradation [51]. Less macrophage infiltration into the atherosclerotic intima and lower serum levels of pro-atherosclerotic markers were also observed in mice fed a high-fat diet treated with ticagrelor compared to clopidogrel for 16w [52]. These studies highlight not only ticagrelor’s ability to mitigate atherogenesis, but also its increased efficacy over clopidogrel via non-platelet-mediated lowering of pro-inflammatory cytokines.
Ye assessed whether daily HD-aspirin can also block the anti-atherogenic effects of ticagrelor. After 14 weeks of treatment, aspirin, ticagrelor, and rosuvastatin each independently increased 15-epi-lipoxin-A4 and decreased IL-1β, IL-6, TNF-α, as well as atherosclerotic plaque area in diabetic mice. The combination of rosuvastatin and ticagrelor augmented these effects, while aspirin interfered with the attenuation of inflammatory cytokine levels by both ticagrelor and rosuvastatin. Clopidogrel did not exhibit any of these beneficial effects [44]. Table 2 provides a summary of animal trials that evaluated the effects of ticagrelor and statins to reduce cardiovascular events.
Ticagrelor and Atorvastatin Pretreatment Protect Against Ischemia–Reperfusion Injury
As previously discussed, 7-day pretreatment with ticagrelor reduced myocardial IS in rats as compared to clopidogrel despite similar levels of platelet inhibition. This was associated with higher levels of adenosine, cytosolic phospholipase (cPLA2), an enzyme necessary to liberate AA, COX-2, 15-epi-lipoxin-A4, and eNOS in myocardium. Co-administration of an adenosine receptor antagonist, COX-2 inhibitor, or HD-aspirin with ticagrelor 1 h before ischemia–reperfusion mitigated the reduction in IS and production of these molecules in a dose-dependent manner. However, no attenuation was seen when a COX-1 inhibitor or LD-aspirin were given [34]. This study indicates that HD-aspirin loading given as standard of care in the setting of ACS likely blocks the ability of maintenance ticagrelor therapy to protect from IRI.
The combination of rosuvastatin and ticagrelor, but not prasugrel, had an additive effect of increasing adenosine levels and reducing IS more than either rosuvastatin or ticagrelor alone. Ticagrelor and rosuvastatin also increased expression of COX-2, 15-epi-lipoxin-A4, Akt, ERK 1/2, and eNOS. These outcomes were not observed in the group given ticagrelor and rosuvastatin in addition to an adenosine receptor antagonist 1 h prior to infarction [42]. Both ticagrelor and rosuvastatin (not prasugrel) also significantly attenuated the increase of caspase-1 following ischemia–reperfusion. Taken together with the results of the previous study that showed HD-aspirin administered to rats pretreated with atorvastatin 15 min prior to reperfusion blunted its reduction of IS [38], this suggests that aspirin loading prior to PCI could block the IS-limiting effects of both chronic ticagrelor and statins.
Another murine study evaluated whether caffeine blocks the pleiotropic effects of statins. IS was significantly reduced in rats pretreated for 3 days with atorvastatin and sugar water or decaffeinated coffee, but not caffeinated coffee when measured after coronary artery occlusion and 4 h of reperfusion on day 4 [53]. This was associated with inhibition of atorvastatin-mediated phosphorylation of Akt and subsequently less eNOS activation. The results of this study are evidence that the effects of statins to defend against IRI may be attenuated by adenosine receptor antagonism by caffeine via interference of the production of cardioprotective pro-survival kinases. Like statins, protection against IRI by ticagrelor has been shown to be adenosine mediated. It is conceivable that caffeine may also attenuate the pleiotropic effects of ticagrelor, but this remains to be sufficiently addressed.
Rats treated with ticagrelor initiated after induction of initial infarction had significantly reduced IS and lower levels of nuclear factor-kappa B (NF-kB), galectin-3, IL-6, and TNF-α when reinfarction occurred at 24 h, 3 days, and 7 days later. Pretreatment with a known NF-kB agonist, dextran sodium sulfate, for 7 days prior to initial MI attenuated these protective effects. Thus, chronic ticagrelor therapy was demonstrated to reduce IRI from recurrent infarction by causing less activation of NF-kB in the ischemic myocardium [54]. Adenosine can inhibit NF-kB [55]. Thus, whether reduction of NF-kB represents a separate mechanism by which ticagrelor improves myocardial function in the setting of IRI, or it is further evidence of its adenosine-mediated effects has yet to be determined.
Studies of IRI utilizing large-animal models—with a higher translatability potential—yielded similar results to those seen in murine models. Cardiac MRI (CMR) evidenced that administration of a single dose of 180 mg ticagrelor prior to infarction in pigs reduced IS, edema formation and necrosis at 24 h post-MI to a larger extent than loading with clopidogrel. Concomitant administration of an adenosine receptor antagonist abolished these protective effects. Both drugs were given at doses that displayed comparable platelet inhibition, excluding any platelet-related effects [56]. In a second porcine study, a 180 mg loading dose of ticagrelor prior to MI induction followed by 90 mg twice daily reduced IS, scar formation, edema, and attenuated impairment in LV ejection faction 3 days after reperfusion—effects that persisted at 42 days. These benefits were not detected in pig hearts treated with clopidogrel. As in prior rat models, ticagrelor led to increased adenosine levels, and greater activation of COX-2 and adenosine monophosphate activated protein kinase (AMPK) in the ischemic myocardium [57]. AMPK is an energy sensor that exerts cardio-protection primarily via upstream activation of adenosine. Thus, acute and chronic ticagrelor treatment attenuates IRI and adverse cardiac remodeling via adenosine-mediated mechanisms in large animals, strengthening the case for ticagrelor’s pleiotropic benefits and potential interactions that may affect these in the clinical setting.
Other Pleiotropic Effects
In an in vitro study, ticagrelor demonstrated bactericidal activity against gram-positive organisms, including drug resistant strains, i.e., glycopeptide intermediate-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus. Although bactericidal concentrations were not reached systemically in patients receiving typical dosages for treating cardiovascular disease, the authors concluded that antibacterial activity at infection sites may still be achieved through local, possibly platelet-driven, drug accumulation [58]. In another study, extracellular vesicles derived from ticagrelor-pretreated rat cardiomyocyte precursor cells in vitro significantly decreased hyperglycemia-induced oxidative stress and prevented the development of apoptosis and endoplasmic reticulum stress in these cells. This suggests another non-platelet-mediated effect of ticagrelor to protect against diabetic cardiomyopathy through extracellular vesicular modulation [59]. Furthermore, a novel drug-target interaction between ticagrelor, and vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) receptors which may serve as potential targets for the development of new diagnostic and therapeutic tools for neuronal, metabolic, inflammatory, and malignant diseases was described. Cangrelor did not show a similar effect on VIP and PACAP receptors likely due to its slightly different molecular structure [60].
Analyzing Clinical Trial Outcomes in the Context of Preclinical Study Results
In a retrospective review of 1754 patients who presented with STEMI, troponin release was reduced in those treated with ticagrelor as compared to clopidogrel (adjusted 48 h area under the curve ratio: 0.67, 95% confidence interval (CI): 0.47–0.94). This correlated with lower rates of a composite endpoint of cardiac death or hospitalization for heart failure within 12 months in STEMI patients loaded with ticagrelor (hazard ratio (HR): 0.63, CI: 0.42–0.94) but not prasugrel (HR: 0.84, CI: 0.43–1.63), prior to primary PCI. Given the results of a concomitant rat study demonstrating reduced IS with ticagrelor but not prasugrel or clopidogrel administration after induction of acute MI, the authors concluded that the cardioprotective effects of ticagrelor in reducing IS may contribute to the clinical benefit observed in STEMI patients undergoing primary PCI [61].
Atlantic
In the 2014 multicenter double-blind RCT, Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary Artery (ATLANTIC), of 1862 patients with ongoing STEMI of less than 6-h duration, administration of a 180 mg loading dose of ticagrelor en route to the hospital did not improve the coprimary endpoints of pre-PCI coronary reperfusion of the culprit artery nor ST-segment elevation resolution on electrocardiogram (ECG) 1 h post-PCI in patients as compared to ticagrelor administration in the catheterization laboratory. The original hypothesis of the study was that earlier ticagrelor administration would cause faster and stronger platelet inhibition, and lead to quicker resolution of acute coronary occlusion. The authors explained the lack of efficacy in the primary outcomes by pointing to a clinically non-significant time-to-PCI difference in both groups; the median time from randomization to angiography was 48 min, and the median time difference between the prehospital and in hospital group was only 31 min [62].
As has been demonstrated by preclinical trials, ticagrelor’s acute cardioprotective effects are mostly attributed to protection from IRI rather than more potent and efficacious platelet inhibition. Given the effects of increased coronary blood flow and reduced IS demonstrated with ticagrelor loading in several animal studies, it would be expected that these findings would correlate with quicker resolution of ST-segment elevation or improved reperfusion. However, it is possible that 31 min was not enough time to make a clinical difference in protection from IRI. Another plausible yet unexplored explanation inferred from the preclinical trial findings is that HD chewable aspirin that was administered to all patients as standard of care blocked the ability of ticagrelor to attenuate IRI through the COX-2 pathway.
There were significantly more occurrences of the co-primary endpoints in patients treated with morphine [62]. One explanation is that delayed gastric absorption due to opioid-induced decreased gastric motility. This was corroborated by a sub-study of the ATLANTIC trial which demonstrated that platelet inhibition was unaffected by prehospital ticagrelor administration at the time of initial angiogram due to the short transfer delay, but that morphine administration was associated with delayed onset of platelet inhibition at 1 and 6 h post-PCI [63]. Another RCT of 195 patients who presented with STEMI and received crushed aspirin and ticagrelor prior to PCI found that IV acetaminophen in comparison to IV fentanyl was associated with significantly higher ticagrelor plasma levels, but no difference in platelet reactivity [64]. These trials raise the possibility that lower rates of ST resolution seen in the ATALNTIC trial in patients who received morphine may be due to other non-platelet-mediated interactions between morphine and ticagrelor.
Clinical Trials Evaluating Infarct Size and Adverse Cardiac Remodeling
Ubaid found that despite greater platelet inhibition by cangrelor compared to ticagrelor at time of balloon inflation during primary PCI, there was no improvement in coronary reperfusion or IS 13 weeks later in 100 STEMI patients [26]. Theoretically, more potent and efficacious platelet inhibition by cangrelor as compared to ticagrelor could result in improved reperfusion of infarcted tissue at the time of PCI which would manifest in reduced IS weeks to months later. As this was not seen, it is possible that other non-platelet-mediated mechanisms of ticagrelor may have compensated for its relatively weaker potency and efficacy of platelet inhibition resulting in no perceived difference in IS. Given the results of the preclinical trials showing that ticagrelor protects against IRI when initiated shortly after an acute MI [23,24,25], it may be expected that patients in the ticagrelor group, who received the drug several hours prior to those in the cangrelor group, would have had reduced IS. However, all patients who received cangrelor prior to primary PCI were also loaded with ticagrelor after intervention. Furthermore, all patients received HD-aspirin prior to randomization, which could have inhibited ticagrelor’s ability to protect against IRI as suggested by the findings of Nanhawan et al. [34].
In another RCT of 203 patients presenting with STEMI, IS was smaller and myocardial salvage greater at 3 days when ticagrelor or prasugrel were administered prior to primary PCI as compared to clopidogrel [65]. A retrospective analysis of a RCT found similar results at 3-month follow-up [66] However, these trials did not separately analyze patients given prasugrel and ticagrelor, and so conclusions about the effects of the individual therapies cannot be discerned. Furthermore, Ubaid and Sabbah evaluated IS at 3 months in patients who received both acute and chronic ticagrelor therapy [26, 66]. Therefore, individual effects of acute protection from IRI and chronic effects of ticagrelor to prevent cardiac remodeling as demonstrated in the animal studies cannot be determined.
Resembling the findings of Ye [23], ticagrelor in comparison to clopidogrel was also associated with significant reductions in N-terminal pro-hormone B-type natriuretic peptide levels and a numerical index of LV remodeling at 6 months in 110 patients presenting with first-time STEMI treated with primary PCI [67]. This was despite similar levels of platelet inhibition with ticagrelor and clopidogrel therapy, and use of both HD-aspirin prior to and LD-aspirin after PCI [67]. It is notable that ticagrelor still attenuated adverse cardiac remodeling in the setting of concomitant LD-aspirin. This is like the findings of Nanhwan. that LD-aspirin given prior to reperfusion had no effect on IS attenuation by maintenance ticagrelor [34]. No significant difference was observed in pathological LV remodeling (defined as LV remolding index > 20%), but sample size was low, as was incidence of heart failure with reduced ejection fraction [68].
Insights from the PLATO Trial
The benefits of ticagrelor demonstrated in the PLATO trial were not homogenously distributed. Subgroup analysis revealed that ticagrelor was associated with greater MACE in the North American (HR: 1.25) and US populations (HR: 1.27) compared to the rest of the world (HR: 0.84). While the possibility of chance occurrence could not be definitively ruled out via statistical analysis, HD-aspirin (≥ 300 mg daily) was associated with more MACE than LD (≤ 100 mg daily) in patients who received ticagrelor. These patterns were absent in those who received clopidogrel [19].
As a result of this observation, the US Food and Drug Administration warn that “maintenance doses of aspirin > 100 mg reduce the effectiveness of [ticagrelor] and should be avoided” [69]. Mahaffey proposed that aspirin at daily doses of > 80 mg may have attenuated ticagrelor’s antiplatelet effects via inhibition of endothelial release of prostaglandins, in a dose-dependent fashion [19, 33]. However, there are no specific recommendations regarding the loading dose of aspirin. Findings from the preclinical trials indicate that aspirin loading likely blocks the acute effects of ticagrelor to protect against IRI, while chronic HD-aspirin may mitigate protection against adverse cardiac remodeling, atherosclerosis, and IRI from reinfarction. Indeed, “the potential adverse effect of aspirin in attenuating protection has not yet been considered seriously in this regard” [70].
The PLATO investigators also observed that patients who were administered ticagrelor exhibited a significant reduction in the primary endpoint while on concomitant therapy with lipid-lowering drugs versus those who were not as compared to clopidogrel [6]. The study did not specify which lipid-lowering agents were used, though presumably these were statins [71]. Via increasing extracellular adenosine levels, statin medications augment ticagrelor’s ability to protect against atherosclerosis and IRI. Thus, patients on both ticagrelor and statins likely had the added benefit of protection from complications of IRI and future cardiovascular events, including reduced atherosclerotic burden, reflected by a lower event rate compared to patients not taking statins.
The Effects of Caffeine on Ticagrelor
Caffeine is a widely used non-specific adenosine receptor antagonist [72]. In a prespecified analysis of 21,162 patients in the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction (PEGASUS-TIMI 54) trial, caffeine was not associated with an excess risk for MACE, sudden cardiac death, or atrial fibrillation [73]. However, all patients received daily LD-aspirin which—albeit less compared to HD—still exhibits COX-2 inhibition [74]. A small prospective study of 23 patients treated with ticagrelor for ACS found that caffeine did not alleviate dyspnea. However, the investigators concluded that the adenosine hypothesis could not be refuted by these results, since the randomized assessment of adenosine antagonism in this setting could not be accomplished [75]. Adenosine antagonism, HD-aspirin, and caffeine have all been demonstrated in animal models to attenuate ticagrelor’s protection against IRI and progression of atherosclerosis [23, 34, 38, 43, 56]. Theoretically, concomitant use of multiple agents that interfere with ticagrelor-mediated production of cardioprotective molecules, i.e., aspirin and caffeine, may have sufficiently inhibited these pleiotropic effects.
Ticagrelor in Acute and Chronic Coronary Syndrome
The PEGASUS-TIMI 54 trial demonstrated that in addition to LD-aspirin, ticagrelor started a median 1.7 years after an MI is associated with reduced cardiovascular death, recurrent MI, and stroke, but increased risk of major bleeding as compared to placebo. Patients who received ticagrelor 90 mg or 60 mg twice daily had significantly lower rates of recurrent MI (275, 4.40% and 285, 4.53%, respectively), as compared to LD-aspirin plus placebo (338, 5.25%) with a HR = 0.84, 95% CI = 0.72–0.98; p = 0.03 [76]. Another RCT found similar results in patients with type 2 diabetes and chronic coronary syndrome without a previous history of MI or stroke [77]. As suggested by the findings of the preclinical trials, these results may have been due to added attenuation of atherosclerosis and adverse cardiac remodeling by ticagrelor. If so, these cardio-protective benefits correlated with less recurrent MI and cardiovascular death in the clinical setting. Table 3 presents the findings of RCTs of ticagrelor.
In a post hoc analysis of the Ticagrelor Monotherapy After 3 Months in the Patients Treated With New Generation Sirolimus-eluting Stent for Acute Coronary Syndrome (TICO) trial, which included 3056 participants, reduction of the primary end point (a composite of major bleeding, death, MI, stent thrombosis, stroke, or target-vessel revascularization) seen with ticagrelor monotherapy after 3-month DAPT versus ticagrelor-based 12-month DAPT increased with age with a change point of 64 years of age. Significant decreases in rates of both major bleeding and major adverse cardiovascular and cerebrovascular events were observed in patients ≥ 64, but not < 64 years old [78]. A meta-analysis of 3 RCTs including 4175 participants undergoing complex PCI also demonstrated a significant reduction in the risk of cardiovascular death (incidence rate ratio (IRR): 0.52, 95% CI: 0.28–0.96; p = 0.04) likely driven by a numerical trend toward lower rates of MI (IRR: 0.79; CI: 0.61–1.01; p = 0.06) and definite or probable stent thrombosis (IRR: 0.77, CI: 0.34–1.75; p = 0.53; I2 = 0%) with short-course (1–3 months) ticagrelor-based DAPT followed by ticagrelor monotherapy as compared to standard DAPT [79]. Thus, ticagrelor monotherapy after a short course of ticagrelor-based DAPT appears to be safer and more efficacious than 12 months of standard DAPT with either clopidogrel or ticagrelor in preventing recurrent MI and stent thrombosis, especially in patients with advanced age and high atherosclerotic burden.
Ischemic Stroke and Peripheral Artery Disease
In the Examining Use of tiCagreLor In paD (EUCLID) trial of 13,885 patients with symptomatic peripheral artery disease, ticagrelor was not shown to be superior to clopidogrel for the reduction of the primary endpoint of adjudicated cardiovascular death, MI, or ischemic stroke or major bleeding events as compared to clopidogrel after a mean follow-up of 30 months [80]. However, ticagrelor was associated with a significant reduction of ischemic stroke (OR: 0.78, 95% CI: 0.62–0.98; p = 0.03) which may be evidence of its ability to mitigated vascular inflammation and attenuate atherosclerotic progression. In a subgroup analysis of a RCT that compared 30-day use of aspirin plus either ticagrelor or placebo in patients with mild-to-moderate acute non-cardioembolic ischemic stroke or transient ischemic attack (TIA) and who were not undergoing thrombolysis or thrombectomy, net clinical impact factor favored the combination of aspirin and ticagrelor in the first week (absolute risk reduction: 0.97%, 95% CI: 0.17–1.77%) and remained constant throughout the 30 days [81].
On the other hand, another RCT of 13,199 patients with either a non-severe ischemic stroke or high-risk TIA presumed to not be due to a cardioembolic source and who did not receive IV or intraarterial thrombolysis did not find a significant difference between ticagrelor and aspirin administered 24 h after symptom onset with regard to the primary endpoint of stroke, MI, or death (HR: 0.89, 95% CI: 0.78–1.01; p = 0.07), or secondary endpoints of ischemic stroke (HR: 0.87, 0.76–1.01; p = 0.046) and ischemic stroke, MI, or cardiovascular death (HR: 0.89, 0.78–1.01; p = 0.07) [82]. However, patients were only followed for 90 days. Given the borderline CIs, it is possible a longer follow-up time as demonstrated in the EUCLID trial may have may have resulted in a statistically significant reduction of ischemic stroke, MI, and/or cardiovascular death. A future network meta-analysis will examine the efficacy of DAPT with ticagrelor versus clopidogrel in preventing recurrent stroke and mortality up to 30 days after an initial cerebrovascular ischemic event [83].
Antimicrobial Effects of Ticagrelor
Several studies have attempted to assess whether the observation that ticagrelor exhibits antimicrobial effects in vitro has real-world consequences. In an RCT, ticagrelor as compared with clopidogrel treatment did not significantly alter the risk of infections during hospitalization among 2116 STEMI patients undergoing PCI but was associated with a slightly lower risk of in-hospital all-cause death and major adverse cardiovascular and cerebrovascular events [84]. However, an observational cohort study of 26,606 Dutch patients who underwent urgent or emergent PCI found a reduced absolute 1-year risk difference of S. aureus bacteremia (− 0.19%, 95% CI: − 0.32 to − 0.05%; p = 0.006), sepsis (0.50%, − 0.86 to − 0.14%; p < 0.007) and pneumonia (− 1.43%, − 2.03 to − 0.82%; p < 0.001) when they received ticagrelor versus clopidogrel [85]. This may explain the lower mortality risk following pulmonary adverse events and sepsis in ACS patients treated with ticagrelor as compared to clopidogrel in a subgroup analysis of the PLATO trial [86].
A subgroup analysis of A Clinical Study Comparing Two Forms of Anti-platelet Therapy After Stent Implantation (GLOBAL LEADERS) found that 1 month of DAPT followed by 23 months of ticagrelor monotherapy was associated with a significant lower incidence of the primary endpoint of all-cause mortality or new Q-wave MI at 2 years as compared with 24 months of DAPT in the cohort of patients with white blood cell (WBC) counts lower than the median of 7.8 × 109 cells/L (2.8% vs. 4.2%; HR: 0.67, 95% CI: 0.52–0.86) but not that with WBC counts greater than the median (4.8% vs. 4.7%; HR: 1.01, 0.82–1.25; P interaction = 0.013). The authors concluded that a higher inflammatory state at the time of index procedure likely reflected by increased WBC counts, may attenuate the anti-ischemic benefits of ticagrelor monotherapy observed in patients with lower WBC counts [87]. Adenosine also plays a role in modulating inflammatory cytokines and leukocyte chemotaxis [88]. Perhaps adenosine diverted toward these tasks depleted adenosine levels needed to facilitate the pleiotropic effects of ticagrelor at time of injury and reperfusion such as local vasoconstriction and protection against IRI.
Ticagrelor as Compared to Prasugrel
In the landmark Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT 5) trial, the primary endpoint, a composite of death, MI, or stroke at 1 year, occurred in 184 of 2012 patients (9.3%) in the ticagrelor group and in 137 of 2006 patients (6.9%) in the prasugrel group (HR: 1.36, 95% CI: 1.09–1.70; p = 0.006). There was no significant difference in major bleeding events [89]. A study of platelet reactivity variability throughout the day in patients 4 days after an acute MI demonstrated that ticagrelor has greater diurnal variability in platelet aggregation than prasugrel, likely due to the continuous increase of platelet inhibition after the morning maintenance dose. Despite this difference, both drugs provided adequate platelet aggregation inhibition throughout the day, including prior to the morning dose. The results challenge the hypothesis that less platelet inhibition by prasugrel at the time of the morning dose may be responsible for higher rates of stent thrombosis in the early morning hours, again suggesting that ticagrelor may possess further non-platelet-mediated anti-ischemic benefits beyond those of prasugrel [90]. In fact, in patients with an NSTEMI undergoing PCI administered incremental doses of adenosine, coronary blood flow velocity is augmented in those who receive ticagrelor as compared to prasugrel suggesting that ticagrelor’s effects to increase adenosine levels may lead to greater perfusion in the setting of ischemia [91].
Meta-analyses
Most subsequent RCTs and meta-analyses comparing ticagrelor and clopidogrel (in addition to aspirin) found no significant difference in all-cause mortality, CV death, or MACE, but a slightly higher risk of major bleeding, especially in older patients [10,11,12,13,14,15,16,17,18]. Hong demonstrated that while all-cause mortality (driven primarily by lower rates of major bleeding) appears to be lower with ticagrelor monotherapy started after initial treatment with DAPT for 1–3 months as compared to continuation of DAPT, rates of MACE at 1–2-years were not significantly different [15]. However, a recent meta-analysis showed that both ticagrelor and clopidogrel monotherapy was associated with greater secondary prevention of recurrent MACE as compared to aspirin monotherapy in patients months-to-years post-PCI, coronary bypass, or stroke without an increased risk of major bleeding events [92]. Though there was no significant difference between ticagrelor and clopidogrel, no RCT analyzed directly compared the two agents. Concomitant use of statins and caffeine which may augment and attenuate the pleiotropic effects of ticagrelor, were also not evaluated. This may be one possible explanation for the lack of superiority of ticagrelor as compared to clopidogrel demonstrated.
In a network meta-analysis of 7 RCTs that reported separately the results of adults older > 70 years for at least the primary endpoint, i.e., a composite of death, MI, and stroke, comprising 14,485 patients, prasugrel was associated with a similar occurrence of the primary endpoint based on a Surface Under the Cumulative Ranking curve Analysis (SUCRA) of 54.5 as compared to ticagrelor (32.9) and clopidogrel (12.6). Ticagrelor was associated with the lowest risk of stent thrombosis (SUCRA: 55.6) as compared to prasugrel (42.4) or clopidogrel (27.8) [93]. If more efficacious platelet inhibition were responsible, one would suspect for prasugrel to also result in less chance of stent thrombosis than ticagrelor. This was not seen, suggesting an alternative explanation, e.g., an enhanced anti-inflammatory atherosclerotic environment.
Implications of the Findings Presented in This Review on Future Studies
Most RCTs and meta-analyses comparing ticagrelor and clopidogrel did not evaluate concomitant use of aspirin, statins, or caffeine (Table 4). Thus, the conclusion of these RCTs and meta-analyses should not be considered definitive. The findings presented in this review can be used to direct the design of future studies aimed at assessing the effects of ticagrelor as compared to other P2Y12 inhibitors used to treat cardiovascular diseases. To begin, large animal models are needed to corroborate the findings of rodent studies that HD aspirin may attenuate the effects of ticagrelor to protect against IRI, adverse cardiac remodeling, and atherosclerosis. They should also assess the interaction between ticagrelor and statins, caffeine, opiates, and ischemic postconditioning (of which aspirin has also been shown to limit the IS-reducing effects) on these mechanisms [70]. If large animal models confirm the findings of the preclinical trials discussed in this review, it may be time for RCTs to compare patients who present with ACS receiving ticagrelor versus other P2Y12 inhibitors with and without loading or maintenance dose aspirin. Guided selection of P2Y12 inhibitor therapy and attention to genotypic variations in metabolism may further optimize ischemic benefits and limit bleeding with P2Y12 inhibitor monotherapy peri-PCI [94, 95].
Limitations
The doses of ticagrelor in the animal studies were often several times higher than those used in the clinical trials discussed. As pharmacotherapies tend to exhibit greater pleiotropic effects at greater concentrations, it is unknown whether the effects of attenuation of infarct size, atherosclerosis, inflammation, and adverse cardiac remolding are exhibited in patients with clinically indicated doses. Differences in physiology and metabolism of the animals studied and humans further compound this issue. Furthermore, many of the studies discussed are post hoc and subgroup analyses of RCTs and thus meant to generate hypothesis rather than answer specific clinical questions. Regardless, the work within this manuscript provides a comprehensive review of the pleiotropic effects identified in preclinical studies, which can guide further discussion and design of future clinical trials aimed at assessing the efficacy of ticagrelor as compared to other P2Y12 inhibitors.
Conclusion
When administered prior to reperfusion, small and large animal studies demonstrate that ticagrelor likely protects against IRI. Preclinical trials also show that chronic treatment with ticagrelor mitigates adverse cardiac remodeling and the development of atherosclerosis, while also protects against IRI from recurrent ischemia. These effects are likely mediated by ticagrelor’s ability to increase local interstitial adenosine levels which activate downstream anti-inflammatory prostaglandins, eicosanoids and AMPK. RCTs suggest that these benefits may confer reduced rates of recurrent infarction and cardiovascular death.
HD-aspirin and adenosine-antagonism have been demonstrated to block these benefits of ticagrelor, as well as statin’s ability to enhance them. Attenuation of ticagrelor’s adenosine-mediated pleiotropic effects by aspirin likely explains the differential of outcomes among PLATO participants who received HD- versus LD-aspirin, and statin versus no statin therapy. Most RCTs and meta-analyses have not accounted for these interactions. We need more preclinical and RCTs comparing cardiovascular outcomes in patients who present with ACS treated with ticagrelor versus other P2Y12 inhibitors that are mindful of the unique pleiotropic advantages afforded by ticagrelor, and possible interactions with other therapies (e.g., aspirin, statins, and caffeine).
Data Availability
N/A.
Code Availability
N/A.
References
Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. Euro Intervention. 2022;17:e1371–96.
Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77.
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228.
O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2013;82:E1-27.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–41.
Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283–93.
Lindholm D, Varenhorst C, Cannon CP, et al. Ticagrelor vs clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J. 2014;35:2083–93.
Gupta R, Malik AH, Briasoulis A, Joshi AM, Guthier DG, Popli T, et al. Comparative safety and effectiveness of loading doses of P2Y12 inhibitors in patients undergoing elective PCI: a network meta-analysis. Cardiovasc Drugs Ther. 2021. https://doi.org/10.1007/s10557-021-07270-3.
Zhao X, Zhang J, Guo J, et al. Comparison of safety and efficacy between clopidogrel and ticagrelor in elderly patients with acute coronary syndrome: a systematic review and meta-analysis. Front Pharmacol. 2021;12:743259.
Abusnina W, Al-Abdouh A, Bizanti A, et al. Ischemic and bleeding outcomes of potent P2Y12 inhibitor antiplatelet agents versus clopidogrel in elderly patients with acute coronary syndrome: a meta-analysis of randomized trials. Cardiovasc Revasc Med. 2022;38:54–60.
Tarantini G, Ueshima D, D’Amico G, et al. Efficacy and safety of potent platelet P2Y12 receptor inhibitors in elderly versus nonelderly patients with acute coronary syndrome: a systematic review and meta-analysis. Am Heart J. 2018;195:78–85.
Baldetti L, Melillo F, Moroni F, et al. Meta-analysis comparing P2Y12 inhibitors in acute coronary syndrome. Am J Cardiol. 2020;125:1815–22.
Hong SJ, Ahn CM, Kim JS, et al. Effect of ticagrelor monotherapy on mortality after percutaneous coronary intervention: a systematic review and meta-analysis of randomized trials including 26 143 patients. Eur Heart J Cardiovasc Pharmacother. 2022;8:48–55.
Ma Y, Zhong PY, Shang YS, et al. Comparison of ticagrelor with clopidogrel in East Asian patients with acute coronary syndrome: a systematic review and meta-analysis of randomized clinical trials. J Cardiovasc Pharmacol. 2022;79:632–40.
Sun M, Cui W, Li L. Comparison of clinical outcomes between ticagrelor and clopidogrel in acute coronary syndrome: a comprehensive meta-analysis. Front Cardiovasc Med. 2021;8:818215.
Bergh N, Myredal A, Nivedahl P, et al. Efficacy and safety of clopidogrel versus ticagrelor as part of dual antiplatelet therapy in acute coronary syndrome-a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2022;79:620–31.
Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544–54.
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15.
Birnbaum GD, Birnbaum I, Ye Y, Birnbaum Y. Statin-induced cardioprotection against ischemia-reperfusion injury: potential drug-drug interactions. Lesson to be learnt by translating results from animal models to the clinical settings. Cardiovasc Drugs Ther. 2015;29:461–7.
Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143–53.
Ye Y, Birnbaum GD, Perez-Polo JR, et al. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1805–14.
Wang K, Zhou X, Huang Y, et al. Adjunctive treatment with ticagrelor, but not clopidogrel, added to tPA enables sustained coronary artery recanalisation with recovery of myocardium perfusion in a canine coronary thrombosis model. Thromb Haemost. 2010;104:609–17.
Audia JP, Yang XM, Crockett ES, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y. Basic Res Cardiol. 2018;113:32.
Ubaid S, Ford TJ, Berry C, et al. Cangrelor versus ticagrelor in patients treated with primary percutaneous coronary intervention: impact on platelet activity, myocardial microvascular function and infarct size: a randomized controlled trial. Thromb Haemost. 2019;119:1171–81.
Allencherril J, Alam M, Levine G, et al. Do we need potent intravenous antiplatelet inhibition at the time of reperfusion during ST-segment elevation myocardial infarction? J Cardiovasc Pharmacol Ther. 2019;24:215–24.
Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35.
Asanuma H, Kitakaze M. Is aspirin loading before primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction necessary?. Cardiovasc Drugs Ther 2022.
Ishida T, Yarimizu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock. 1997;8:86–94.
Kitakaze M, Minamino T, Node K, et al. Adenosine and cardioprotection in the diseased heart. Jpn Circ J. 1999;63:231–43.
Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63:2503–9.
Badimon L, Vilahur G, Rocca B, Patrono C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc Res. 2021;117:2001–15.
Nanhwan MK, Ling S, Kodakandla M, et al. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol. 2014;34:2078–85.
Granger CB, Berger PB. Understanding the Adverse Effects of Ticagrelor in Practice. JAMA Cardiol. 2016;1.
Atar S, Ye Y, Lin Y, et al. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2. Am J Physiol Heart Circ Physiol. 2006;290:H1960-1968.
Sanada S, Asanuma H, Minamino T, et al. Optimal windows of statin use for immediate infarct limitation: 5’-nucleotidase as another downstream molecule of phosphatidylinositol 3-kinase. Circulation. 2004;110:2143–9.
Birnbaum Y, Lin Y, Ye Y, et al. Aspirin before reperfusion blunts the infarct size limiting effect of atorvastatin. Am J Physiol Heart Circ Physiol. 2007;292:H2891-2897.
Bonello L, Laine M, Kipson N, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63:872–7.
Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–74.
Adali MK, Buber I, Kilic O, Turkoz A, Yilmaz S. Ticagrelor improves systemic immune-inflammation index in acute coronary syndrome patients. Acta Cardiol. 2021;1–7.
Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30:539–50.
Birnbaum Y, Tran D, Chen H, et al. Ticagrelor improves remodeling, reduces apoptosis, inflammation and fibrosis and increases the number of progenitor stem cells after myocardial infarction in a rat model of ischemia reperfusion. Cell Physiol Biochem. 2019;53:961–81.
Ye Y, Nylander S, Birnbaum Y. Unraveling the interaction of aspirin, ticagrelor, and rosuvastatin on the progression of atherosclerosis and inflammation in diabetic mice. Cardiovasc Drugs Ther. 2017;31:489–500.
Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8.
Lagerkranser M, Sollevi A, Irestedt L, Tidgren B, Andreen M. Renin release during controlled hypotension with sodium nitroprusside, nitroglycerin and adenosine: a comparative study in the dog. Acta Anaesthesiol Scand. 1985;29:45–9.
Parmely MJ, Zhou WW, Edwards CK, et al. Adenosine and a related carbocyclic nucleoside analogue selectively inhibit tumor necrosis factor-alpha production and protect mice against endotoxin challenge. J Immunol. 1993;151:389–96.
Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307–11.
Manduteanu I, Simionescu M. Inflammation in atherosclerosis: a cause or a result of vascular disorders? J Cell Mol Med. 2012;16:1978–90.
Preusch MR, Rusnak J, Staudacher K, et al. Ticagrelor promotes atherosclerotic plaque stability in a mouse model of advanced atherosclerosis. Drug Des Devel Ther. 2016;10:2691–9.
Xia X, Li J, Liang X, et al. Ticagrelor suppresses oxidized low-density lipoprotein-induced endothelial cell apoptosis and alleviates atherosclerosis in ApoE-/- mice via downregulation of PCSK9. Mol Med Rep. 2019;19:1453–62.
Halim H, Pinkaew D, Chunhacha P, et al. Ticagrelor induces paraoxonase-1 (PON1) and better protects hypercholesterolemic mice against atherosclerosis compared to clopidogrel. PLoS ONE. 2019;14:e0218934.
Ye Y, Abu Said GH, Lin Y, et al. Caffeinated coffee blunts the myocardial protective effects of statins against ischemia-reperfusion injury in the rat. Cardiovasc Drugs Ther. 2008;22:275–82.
Liu X, Wang Y, Zhang M, et al. Ticagrelor reduces ischemia-reperfusion injury through the NF-κB-dependent pathway in rats. J Cardiovasc Pharmacol. 2019;74:13–9.
Li C, Ha T, Liu L, Browder W, Kao RL. Adenosine prevents activation of transcription factor NF-kappa B and enhances activator protein-1 binding activity in ischemic rat heart. Surgery. 2000;127:161–9.
Vilahur G, Gutiérrez M, Casani L, et al. Protective effects of ticagrelor on myocardial injury after infarction. Circulation. 2016;134:1708–19.
Vilahur G, Gutiérrez M, Casani L, et al. P2Y12 antagonists and cardiac repair post-myocardial infarction: global and regional heart function analysis and molecular assessments in pigs. Cardiovasc Res. 2018;114:1860–70.
Lancellotti P, Musumeci L, Jacques N, et al. Antibacterial activity of ticagrelor in conventional antiplatelet dosages against antibiotic-resistant gram-positive bacteria. JAMA Cardiol. 2019;4:596–9.
Bitirim CV, Ozer ZB, Aydos D, et al. Cardioprotective effect of extracellular vesicles derived from ticagrelor-pretreated cardiomyocyte on hyperglycemic cardiomyocytes through alleviation of oxidative and endoplasmic reticulum stress. Sci Rep. 2022;12:5651.
Langer I, Latek D. Drug repositioning for allosteric modulation of VIP and PACAP receptors. Front Endocrinol (Lausanne). 2021;12:711906.
Hjortbak MV, Olesen KKW, Seefeldt JM, et al. Translation of experimental cardioprotective capability of P2Y12 inhibitors into clinical outcome in patients with ST-elevation myocardial infarction. Basic Res Cardiol. 2021;116:36.
Montalescot G, Van ’t Hof AW, Lapostolle F, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016–27.
Silvain J, Storey RF, Cayla G, et al. P2Y12 receptor inhibition and effect of morphine in patients undergoing primary PCI for ST-segment elevation myocardial infarction. Private-Atlantic Stud Thromb Haemost. 2016;116:369–78.
Tavenier AH, Hermanides RS, Ottervanger JP, et al. Impact of opioids on P2Y12 receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial. Eur Heart J Cardiovasc Pharmacother. 2022;8:4–12.
Khan JN, Greenwood JP, Nazir SA, et al. Infarct size following treatment with second- versus third-generation P2Y12 antagonists in patients with multivessel coronary disease at ST-segment elevation myocardial infarction in the CvLPRIT study. J Am Heart Assoc 2016;5.
Sabbah M, Nepper-Christensen L, Køber L, et al. Infarct size following loading with Ticagrelor/Prasugrel versus Clopidogrel in ST-segment elevation myocardial infarction. Int J Cardiol. 2020;314:7–12.
Park Y, Koh JS, Lee JH, et al. Effect of ticagrelor on left ventricular remodeling in patients with ST-segment elevation myocardial infarction (HEALING-AMI). JACC Cardiovasc Interv. 2020;13:2220–34.
Vilahur G, Arzanauskaite M, Sutelman P. Ticagrelor in post-STEMI adverse ventricular remodeling: there is more than meets the platelet. JACC Cardiovasc Interv. 2020;13:2235–7.
Brilinta™ (ticagrelor) tablets: a P2Y(12) platelet inhibitor indicated to reduce the rate of thrombotic cardiovascular events in patients with acute coronary syndrome (ACS). P T 2012;37:4–18.
Ye R, Jneid H, Alam M, et al. Do we really need aspirin loading for STEMI? Cardiovasc Drugs Ther 2022.
Carroll MD, Kit BK, Lacher DA, Yoon SS. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013:1–8
Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3-15.
Furtado RHM, Venkateswaran RV, Nicolau JC, et al. Caffeinated beverage intake, dyspnea with ticagrelor, and cardiovascular outcomes: insights from the PEGASUS-TIMI 54 trial. J Am Heart Assoc. 2020;9:e015785.
Ellis EF, Wright KF, Jones PS, Richardson DW, Ellis CK. Effect of oral aspirin dose on platelet aggregation and vascular prostacyclin (PGI2) synthesis in humans and rabbits. J Cardiovasc Pharmacol. 1980;2:387–97.
Lindholm D, James S, Andersson J, et al. Caffeine and incidence of dyspnea in patients treated with ticagrelor. Am Heart J. 2018;200:141–3.
Bonaca MP, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;373:1274–5.
Steg PG, Bhatt DL, Simon T, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381:1309–20.
Kim BG, Hong SJ, Kim BK, et al. Age-dependent effect of ticagrelor monotherapy versus ticagrelor with aspirin on major bleeding and cardiovascular events: a post hoc analysis of the TICO randomized trial. J Am Heart Assoc. 2021;10:e022700.
Condello F, Sturla M, Terzi R, Polimeni A, Stefanini GG. Walking the line with ticagrelor: meta-analysis comparing the safety and efficacy of ticagrelor monotherapy after a short course of ticagrelor-based dual antiplatelet therapy versus standard therapy in complex percutaneous coronary intervention. J Clin Med. 2021;10.
Hiatt WR, Fowkes FG, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376:32–40.
Wang Y, Pan Y, Li H, et al. Time course for benefit and risk of ticagrelor and aspirin in acute ischemic stroke or transient ischemic attack. Neurology. 2022;99(1):e46–54. https://doi.org/10.1212/WNL.0000000000200355.
Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35–43.
Zitikyte G, Roy DC, Dhaliwal S, et al. Ticagrelor vs Clopidogrel in addition to Aspirin in minor ischemic stroke/ transient ischemic attack-protocol for a systematic review and network meta-analysis. PLoS ONE. 2021;16:e0250553.
Lian XJ, Dai YN, Xue JH, et al. Ticagrelor and the risk of infections during hospitalization in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention. Atherosclerosis. 2021;331:6–11.
Butt JH, Fosbøl EL, Gerds TA, et al. Ticagrelor and the risk of Staphylococcus aureus bacteraemia and other infections. Eur Heart J Cardiovasc Pharmacother. 2022;8:13–9.
Storey RF, James SK, Siegbahn A, et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014;25:517–25.
Ono M, Tomaniak M, Koenig W, et al. Impact of white blood cell count on clinical outcomes in patients treated with aspirin-free ticagrelor monotherapy after percutaneous coronary intervention: insights from the GLOBAL LEADERS trial. Eur Heart J Cardiovasc Pharmacother. 2022;8:39–47.
Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol. 2011;61:95–114.
Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–34.
Mogabgab O, Wiviott SD, Cannon CP, et al. Circadian variation of stent thrombosis and the effect of more robust platelet inhibition: a post hoc analysis of the TRITON-TIMI 38 trial. J Cardiovasc Pharmacol Ther. 2013;18:555–9.
Alexopoulos D, Moulias A, Koutsogiannis N, et al. Differential effect of ticagrelor versus prasugrel on coronary blood flow velocity in patients with non-ST-elevation acute coronary syndrome undergoing percutaneous coronary intervention: an exploratory study. Circ Cardiovasc Interv. 2013;6:277–83.
Aggarwal D, Bhatia K, Chunawala ZS, et al. P2Y12 inhibitor versus aspirin monotherapy for secondary prevention of cardiovascular events: meta-analysis of randomized trials. Eur Heart J Open. 2022;2
Montalto C, Morici N, Munafò AR, et al. Optimal P2Y12 inhibition in older adults with acute coronary syndromes: a network meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2022;8:20–7.
Galli M, Benenati S, Capodanno D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 2021;397:1470–83.
Xie Q, Xiang Q, Liu Z, et al. Effect of CYP2C19 genetic polymorphism on the pharmacodynamics and clinical outcomes for patients treated with ticagrelor: a systematic review with qualitative and quantitative meta-analysis. BMC Cardiovasc Disord. 2022;22:111.
Funding
The study was funded by a grant from the John S. Dunn Chair in Cardiology Research and Education. Award number: B2017/BMD-3738.
Author information
Authors and Affiliations
Contributions
Professor Birnbaum provided the concept for and guidance of the project. The bulk of the manuscript and analysis was written by Drs. Jeffrey Triska, Neil Maitra, and Faris Haddadin. Dr. Matthew Deshotels also helped write the manuscript and configuration of the tables. Drs. Dominick Angiolillo, Gemma Vilahur, Hani Jneid, Dan Atar, and Birnbaum provided expert commentary and significant edits to the manuscript. All authors read and agreed with the final version of the manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics Approval
N/A.
Consent to Participate
N/A.
Consent for Publication
N/A.
Conflict of Interest
Dominick Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, and Sanofi. He also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. Gemma Vilahur is the recipient of an unrestricted research grant from Astra Zeneca. Dan Atar declares that he received a speaker honoraria and consultancy fees from AstraZeneca and Bayer Healthcare and research grants to the institution from Bayer Healthcare. Yochai Birnbaum received research grants from Astra Zeneca for ticagrelor and dapagliflozin. The remaining authors have no declarations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Triska, J., Maitra, N., Deshotels, M.R. et al. A Comprehensive Review of the Pleiotropic Effects of Ticagrelor. Cardiovasc Drugs Ther 38, 775–797 (2024). https://doi.org/10.1007/s10557-022-07373-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07373-5