Abstract
Purpose
Heart failure (HF) is a major complication of acute myocardial infarction (AMI). Transplantation of bone marrow mononuclear cells (BM-MNC) in the setting of AMI has been proposed as a means for myocardial tissue regeneration. Several trials have explored the outcomes of these cells on surrogate end points such as left ventricular ejection fraction (LVEF) in patients with AMI. However, the data regarding the clinical efficacy are infrequent. Here, we performed a meta-analysis investigating the effect of BM-MNCs injection on the rate of hospitalization for HF in the long-term follow-up period.
Methods
PubMed, Scopus, and Cochrane databases were queried with various combinations of keywords through May 2, 2022. A random-effects meta-analysis was performed to calculate risk ratio (RR) and 95% confidence interval (CI) of hospitalization for HF, all-cause mortality, and stroke rate. Subgroup analyses for hospitalization based on time and cell dose were performed.
Results
A total of 2150 patients with AMI across 22 trials were included for quantitative synthesis. At long-term follow-up, AMI patients treated with an intracoronary injection of BM-MNCs were less likely to be hospitalized for heart failure compared to the control group receiving standard treatment (RR = 0.54, 95% CI = [0.37; 0.78], p = 0.002). There was no association between BM-MNC therapy and all-cause mortality (RR = 0.69, 95% CI = [0.47; 1.01], p = 0.05) and stroke (RR = 1.12, 95% CI= [0.24; 5.21], p = 0.85).
Conclusion
Autologous injection of BM-MNC in the setting of AMI may be associated with decreased risk of hospitalization of heart failure in the long term. However, its effect on all-cause mortality and stroke rate is questionable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence suggests that the occurrence of heart failure (HF) following acute myocardial infarction (AMI) is a strong predictor of both all-cause mortality and cardiac-related mortality [1]. In addition to the patients diagnosed with in-hospital HF after AMI, 13–30% of cases develop HF in the first year after hospital discharge [2]. Introduction of percutaneous coronary intervention (PCI) in the setting of AMI has been associated with decreased incidence of HF since early invasive reperfusion therapy can prevent myocardial necrosis by restoring the blood flow to the compromised tissue [3]. Various variables and risk factors such as advanced age, female gender, previous history of infarction, several biochemical markers, and systolic function indices have been linked to increased risk for post AMI-HF [2]. It has been demonstrated that a 5% decline in left ventricular ejection fraction (LVEF) appears to be a predictor of HF in AMI (hazard ratio = 1.07 (1.03–1.11)) [4].
Due to its remarkable regeneration properties for myocardial tissue [5], stem cell therapy has entered clinical studies in patients with AMI to investigate its potential effects on left ventricular function indices and clinical events. Although the results from a previous meta-analysis have shown that BM-MNC therapy in the setting of AMI is associated with an increase of LVEF by 2.5% compared to controls [6], it remains unclear if improvement in cardiac function indices can be translated into prevention of HF in a long–term follow-up period. Also, the probable effect of timing and dosage of stem cell therapy on the outcomes is not fully elucidated. Thus, conducting a meta-analysis on the potential preventive effect of BM-MNC therapy after AMI on clinical outcomes is imperative.
Methods
Search Strategy
Digital databases, including PubMed, Scopus, and Cochrane, were queried using the combination of medical subject headings (MeSH) and keywords for identification of potential relevant studies. These keywords included “acute myocardial infarction,” “stem cell,” “bone marrow mononuclear cell,” “heart failure,” and “hospitalization.” The search was not restricted with any time frame. Based on our PICO (participants, intervention, comparison, and outcome) approach to the search, the potentially eligible studies were all the randomized controlled trials (RCT) investigating the long-term effect of autologous intracoronary injection of BM-MNCs in AMI participants on hospitalization for heart failure. Eligible participants were all the patients > 18 years, diagnosed with ST-segment acute myocardial infarction and treated with either successful coronary angioplasty with stent implantation or thrombolytics. The intervention group should be compared to a control group of patients diagnosed with AMI receiving standard therapy for AMI based on guidelines with/without intracoronary injection of placebo. The primary study endpoint for this study was long-term rates of hospitalization due to heart failure in AMI patients. Other outcomes of interest included all-cause mortality, stroke, and in short-term (4–6 months) change in left ventricular ejection fraction (LVEF) after stem cell therapy. Titles and abstracts of the extracted studies were screened by two authors, AH and HH. Then full text reviewing of the eligible studies were performed. Any disagreements through the screening process were resolved with consensus and discussion with AA.
Data Extraction
Raw data comprising the primary and secondary outcomes were extracted from the eligible RCTs by two authors (AH and HH) and disagreements were resolved through discussion. Detailed study characteristics included the name of first author, publication year, trial design, type of therapy used for control arm (placebo vs. no placebo), time and dosage of stem cell therapy, follow-up period, data related to sex and age, number of events in both intervention and control group at the longest available follow-up, values of LVEF at baseline, final, and the absolute change after 4–6 months of follow-up. All the data were validated by the corresponding author.
Quality of the Included Studies
We employed Cochrane Collaboration’s tool for assessing risk of bias in randomized trials [7] for risk assessment of the included RCTs. Each included study was closely investigated in terms of random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), outcome assessment blinding (detection bias), presence of incomplete outcome data (attrition), and selective reporting of outcomes (reporting bias). For each type of bias, studies were marked as low, high, or unclear risk of bias. The overall quality of the studies were summarized and entered into Review Manager (RevMan 5.1.7) Software. All the studies with moderate and high risk were included for quantitative synthesis.
Statistical Analysis
Random-effects meta-analysis was performed for all the included analyses. For dichotomous data, risk ratio (RR) with 95% confidence interval (CI) were calculated using the Mantel-Haenszel (MH) method. Pre-specified subgroup analyses based on the time of injection (early: BM-MNC therapy ≤ 10 days after diagnosis of AMI, late: BM-MNC therapy after 10 days of the diagnosis of AMI) and stem cell dose (high dose: ≥ 108 cells injected, low dose: < 108 cells injected) were performed for the primary outcome. Another subgroup analysis was performed for LVEF based on the imaging modality used in each trial. For continuous data, the absolute change of LVEF from baseline after 4–6 months was extracted. In case of missing an absolute change, this endpoint was calculated with the correlation coefficient formula using the baseline and final values of the endpoint. Then, the mean difference (MD) and its 95% CI were computed using the inverse variance (IV) method. Pooled effects with CI that did not cross the zero line (p<0.05) were considered to be statistically significant. For assessing the rate of heterogeneity, the I2 statistical method was observed, and in the case of p < 0.05, heterogeneity was labeled as statistically significant. We assessed the publication bias graphically by illustrating the funnel plot and numerically by Egger’s test, in which a p<0.05 was considered to have publication bias. This meta-analysis was carried out using RStudio Software Version 1.3.959.
Results
Search Results and Study Characteristics
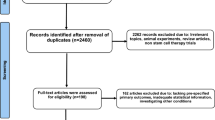
The initial comprehensive search through PubMed, Cochrane databases, and Scopus identified 2983 records for further screening. After removal of 536 duplicates and 2224 irrelevant studies, full texts of 223 studies were retrieved for the final stage of screening. Of these, 201 articles were excluded based on the inclusion criteria. Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the step-by-step search strategy.
A total of 2150 patients diagnosed with ST-segment elevation MI pooled from 22 trials were included in the study, of these 1271 (59%) received intracoronary injection of BM-MNCs and 879 (41%) participants were placed in the control group who had standard care for AMI according to guidelines with or without placebo injection. The mean (95% CI) age of patients was 57.28 (56.09–58.47) versus 57.66 (56.25–59.08) years for the intervention and control group, respectively. The mean (95% CI) baseline LVEF was 44.91% (42.36–47.46) and 45.26% (42.43–48.08) for the intervention and control group, respectively. The follow-up duration for clinical events ranged between 6–60 months. The comparator arm received the standard treatment for AMI in 12 trials [8,9,10,11,12,13,14,15,16,17,18,19], whereas in other studies the control group received an injection of placebo with or without undergoing bone marrow aspiration (Tables 1 and 2).
Risk of Bias in Individual Studies
Although the eligible studies were chosen from randomized controlled trials, several trials lacked a specific method mentioned for random sequence generation and also allocation concealment. Thus, the overall selection bias was rated as low or unclear. The majority of the studies blinded the groups from outcome assessors, except one study [20], contrary to blinding of patients and personnel in which masking was not done for either patients, personnel or both in at least seven trials [9, 12, 13, 16,17,18, 20], which were scored as high risk for performance bias. Attrition bias or incomplete outcome data was at high risk for six of the included studies [13, 15, 17, 18, 20, 21], and the rest of the studies were at low risk. Also, except two studies [8, 22], the rest of the trials were at low risk of selective reporting of the data. The visual assessment of the risk of bias is depicted in Fig. 2.
Rate of Hospitalization Due to Heart Failure
The pooled estimate from 1217 patients receiving intracoronary BM-MNCs and 865 participants in the control group receiving standard therapy for AMI showed that BM-MNC therapy was associated with a significantly lower rate of hospitalization for heart failure in the long-time follow-up compared to the control arm (RR = 0.54, 95% CI = [0.37; 0.78], p = 0.0025) with no level of heterogeneity (I2 = 0.00%, p = 0.74) (Fig. 3A). Subgroup analyses of time and cell dosage revealed that BM-MNC therapy was more effective when performed before 11 days after AMI (early) and with high dosage (≥108 cells) as the group with early injection of BM-MNCs was less likely to be hospitalized for heart failure compared to late group (early group: RR = 0.51, 95% CI = [0.34; 0.77], I2 = 0.00% and late group: RR = 0.78, 95% CI = [0.28; 2.21], I2 = 0.00%) (Fig. 3B) and also an injection of a high dose of cells was associated with lower hospitalization rate contrary to the low dose group (high-dose group: RR = 0.50, 95% CI = [0.34; 0.73], I2 = 0.00% and low-dose group: RR = 0.92, 95% CI = [0.20; 4.30], I2 = 0.00%) (Fig. 3C).
(A) Forest plot of risk ratio (RR) with 95% confidence interval (CI) of long-term hospitalization rate for heart failure in AMI patients receiving intracoronary BM-MNCs compared to the control group receiving optimal medical treatment with/without placebo injection; (B) Subgroup analysis of RR of hospitalization based on time of the injection after PCI (Early, transplantation of BM-MNCs ≤10 days; Late >10 days); (C) Subgroup analysis of RR of hospitalization based on BM-MNC dosage (High ≥108 cells; Low <108 cells)
All-cause Mortality
A total of 20 trials [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] reported the data regarding all-cause mortality rate in the follow-up period with a total of 2022 AMI patients comprising 1177 patients in the intervention group and 845 patients in the control group. Two of the included trials reported no case of mortality during the follow-up period [20, 24]. There was no significant difference between the intervention and control groups in terms of all-cause mortality with no sign of heterogeneity (RR = 0.69, 95% CI = [0.47; 1.01], p = 0.05, I2 = 0.00%) (Fig. 4).
Stroke
Using a pooled analysis from seven trials [9,10,11,12, 16, 17, 25] with 1082 patients, no significant difference was observed in stroke rate for AMI patients with or without stem cell therapy (RR = 1.12, 95% CI = [0.24; 5.21], p= 0.85, I2 = 13.9%) (Fig. 5).
Left Ventricular Ejection Fraction
Studies used different modalities for measuring ventricular indices such as echocardiography [9,10,11, 13, 15, 20, 26, 27], cardiac magnetic resonance (CMR) [12, 14, 17, 18, 21,22,23,24, 28], left ventricular (LV) angiography [25], and single photon emission computed tomography (SPECT) [8, 19]. The most frequent modality used for assessing the cardiac function was CMR followed by echocardiography. Based on the results of 20 studies (1593 participants), BM-MNC therapy revealed a treatment benefit in terms of LVEF improvement compared with control arm (MD = 1.47%, 95% CI = [0.39; 2.55], p = 0.01, I2 = 44.0%) (Fig. 6).
Publication Bias and Sensitivity Analysis
Visual assessment of the funnel plot illustrated for the primary endpoint showed an overall symmetrical distribution of the studies on each side of the vertical axis (Fig. 7). Also, Egger’s test showed no sign of publication bias for asymmetry intercept (p = 0.33). For sensitivity analysis, we removed each single study from all the analyses to see their impact on the summary of results and no significant change was observed for all the endpoints.
Discussion
Here, we have conducted a meta-analysis specifically focused on clinical outcomes of BM-MNCs therapy after AMI. The results of our study showed that this intervention both improved the myocardial function indices and reduced the HF incidence. However, the effect on all-cause mortality appeared to be marginally non-significant.
Utilization of stem cells in clinical trials for AMI and HF has proposed favorable results on cardiac function. The most probable mechanism of action of stem cells for HF is by secreting cardio-protective factors that can induce vascular growth and remodeling and also prevent myocardial tissue fibrosis [29]. BM-MNC, which is the most frequent stem cell used in clinical trials for patients with AMI or HF, has shown promising effects on LVEF and LVESV. Results from a meta-analysis showed that in short term follow-up, BM-MNCs improve LVEF both in patients with AMI and also ischemic cardiomyopathy, particularly when injected with less than 600 million cells. Notably, this improvement in LVEF was not translated into decreased incidence of major adverse cardiovascular events (MACE) both in short and long term [30].
The major focus of the clinical studies investigating the impact of BM-MNCs in AMI has been on cardiac function indices such as LVEF and clinical outcomes have not been the primary outcome of interest so far. BAMI trial was the first phase III randomized controlled trial recruiting a total of 375 patients, 185 patients in the intervention group receiving an intracoronary injection of BM-MNCs 2–8 days after PCI and 190 participants in the control arm who received only medical treatment, which primarily aimed to evaluate if stem cell therapy can reduce all-cause mortality and data regarding clinical outcomes, including all-cause mortality, hospitalization for heart failure, and stroke rate, were collected after 2 years of follow-up. The hazard ratio of all-cause mortality and rehospitalization for HF were both in favor of the BM-MNC group (0.85 vs. 0.33, respectively) [16]. The TIME trial was another RCT exploring the effect of BM-MNC therapy 3–7 days after PCI in 85 patients with anterior MI and moderate ventricular dysfunction (LVEF ≤ 45%) [23]. Contrary to the results of the BAMI trial, their 2-year cohort results revealed that hospitalization following HF was higher in the stem cell group compared to the placebo group (5 hospitalizations in the stem cell group and 2 in the placebo group). Considering these controversies, conducting a meta-analysis on this topic seemed essential to clarify the situation. Thus, we performed a meta-analysis on the effect of BM-MNC therapy on hospitalization rate for HF. Notably, we found that stem cell therapy can decrease the relative risk of HF hospitalizations by 46% in the longest available follow-up (median follow-up of 12 months) when compared to optimal medical treatment. This impact was more pronounced when BM-MNCs were infused shortly after AMI (≤ 10 days) and in higher doses (≥ 108 cells). For other outcomes, BM-MNC therapy was not associated with a significant reduction in all-cause mortality (RR = 0.69) and stroke rate (RR = 1.12). In accordance with previous meta-analyses [6, 30], we detected an increase of LVEF by 1.46% after 4–6 months following AMI. The novel finding of this study was the fact that for the first time, improvement in LVEF was translated into a long-term clinical outcome, which was the incidence of HF needing hospitalization. Because the occurrence of HF is a strong predictor of mortality in patients with AMI [31], the main finding of this study supports the potential preventive effect of stem cells on heart failure after AMI. In a similar previous meta-analysis [32], stem cell transplantation with BM-MNCs did not result in a significant decreased odds of HF hospitalization (odds ratio = 0.84). There may be some explanations to this issue. Since the study by de Jong et al. [32], several new trials were conducted, and two of them [16, 25] had sample sizes of over 200 patients with long-term follow-up durations of 2 and 5 years. Also, in the mentioned study, the two groups were compared with a median follow-up duration of 6 months, whereas in our meta-analysis the median follow-up was 12 months. The longer follow-up periods for assessing the clinical events may provide more reliable and comprehensive results. Furthermore, the primary endpoint of that study was not HF incidence and consequently study selection and inclusion criteria were not based on that, while our study was specifically designed to answer this question.
There were some limitations to our analysis that should be taken into account. As with any meta-analysis, limitations to the method include heterogeneity across trials. In particular, there are differences in terms of treatment characteristics, including the cell dosage used, cell isolation protocols, storage methods, timing of delivery, and imaging modalities. There were heterogeneity among studies regarding trial designs and their methodology. Furthermore, the primary outcome of many studies was LVEF, and these studies were not designed specifically to monitor major cardiovascular events .
Conclusion
In conclusion, injection of BM-MNC in patients with AMI may contribute to a significantly lower risk of long-term hospitalization for HF, especially when administered in high doses and shortly after the reperfusion therapy. However, this treatment does not reduce stroke rate and all-cause mortality. BM-MNC therapy could also result in significant improvements in LVEF in the short-term follow-up period compared to the patients receiving standard therapy. The results of this meta-analysis showed that transplantation of BM-MNCs can have a substantial effect on clinical outcomes although the great impact of advances in coronary angioplasty and medical therapy on lowering the rates of mortality and potentially other major cardiac events is undeniable.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Gerber Y, Weston SA, Enriquez-Sarano M, Berardi C, Chamberlain AM, Manemann SM, Jiang R, Dunlay SM, Roger VL. Mortality associated with heart failure after myocardial infarction: a contemporary community perspective. Circ Heart Fail. 2016;9(1):e002460.
Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, Adámková V, Wohlfahrt P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Failure. 2021;8(1):222–37.
Desta L, Jernberg T, Löfman I, Hofman-Bang C, Hagerman I, Spaak J, Persson H. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Failure. 2015;3(3):234–42.
Lewis EF, Velazquez EJ, Solomon SD, Hellkamp AS, McMurray JJ, Mathias J, Rouleau JL, Maggioni AP, Swedberg K, Kober L, White H, Dalby AJ, Francis GS, Zannad F, Califf RM, Pfeffer MA. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J. 2008;29(6):748–56.
Donndorf P, Strauer BE, Haverich A, Steinhoff G. Stem cell therapy for the treatment of acute myocardial infarction and chronic ischemic heart disease. Curr Pharm Biotechnol. 2013;14(1):12–9.
Delewi R, Hirsch A, Tijssen JG, Schächinger V, Wojakowski W, Roncalli J, Aakhus S, Erbs S, Assmus B, Tendera M. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35(15):989–98.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011;343:d5928.
Meluzín J, Janousek S, Mayer J, Groch L, Hornácek I, Hlinomaz O, Kala P, Panovský R, Prásek J, Kamínek M, Stanícek J, Klabusay M, Korístek Z, Navrátil M, Dusek L, Vinklárková J. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128(2):185–92.
Lamirault G, de Bock E, Sébille V, Delasalle B, Roncalli J, Susen S, Piot C, Trochu JN, Teiger E, Neuder Y, Le Tourneau T, Manrique A, Hardouin JB, Lemarchand P. Sustained quality of life improvement after intracoronary injection of autologous bone marrow cells in the setting of acute myocardial infarction: results from the BONAMI trial. Qual Life Res Int J Qual Life Asp Treat Care Rehab. 2017;26(1):121–5.
Hu X, Huang X, Yang Q, Wang L, Sun J, Zhan H, Lin J, Pu Z, Jiang J, Sun Y, Xiang M, Liu X, Xie X, Yu X, Chen Z, Tse HF, Zhang J, Wang J. Safety and efficacy of intracoronary hypoxia-preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: The CHINA-AMI randomized controlled trial. Int J Cardiol. 2015;184:446–51.
Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, Forfang K, Aakhus S. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95(24):1983–9.
Delewi R, van der Laan AM, Robbers LF, Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, Tio RA, Waltenberger J, Ten Berg JM, Doevendans PA, Gehlmann HR, van Rossum AC, Piek JJ, Zijlstra F. Long term outcome after mononuclear bone marrow or peripheral blood cells infusion after myocardial infarction. Heart. 2015;101(5):363–8.
Skalicka H, Horak J, Kobylka P, Palecek T, Linhart A, Aschermann M. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction and left ventricular dysfunction: a 24-month follow up study. Bratisl Lek Listy. 2012;113(4):220–7.
Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30(24):2978–84.
Plewka M, Krzemińska-Pakuła M, Peruga JZ, Lipiec P, Kurpesa M, Wierzbowska-Drabik K, Korycka-Wołowiec A, Kasprzak JD. The effects of intracoronary delivery of mononuclear bone marrow cells in patients with myocardial infarction: a two year follow-up results. Kardiol Pol. 2011;69(12):1234–40.
Mathur A, Fernández-Avilés F, Bartunek J, Belmans A, Crea F, Dowlut S, Galiñanes M, Good MC, Hartikainen J, Hauskeller C, Janssens S, Kala P, Kastrup J, Martin J, Menasché P, Sanz-Ruiz R, Ylä-Herttuala S, Zeiher A. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41(38):3702–10.
Sürder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, Soncin S, Turchetto L, Radrizzani M, Zuber M, Windecker S, Moschovitis A, Bühler I, Kozerke S, Erne P, Lüscher TF, Corti R. Effect of bone marrow-derived mononuclear cell treatment, early or late after acute myocardial infarction: twelve months CMR and long-term clinical results. Circ Res. 2016;119(3):481–90.
San Roman JA, Sánchez PL, Villa A, Sanz-Ruiz R, Fernandez-Santos ME, Gimeno F, Ramos B, Arnold R, Serrador A, Gutiérrez H, Martin-Herrero F, Rollán MJ, Fernández-Vázquez F, López-Messa J, Ancillo P, Pérez-Ojeda G, Fernández-Avilés F. Comparison of different bone marrow-derived stem cell approaches in reperfused STEMI. A multicenter, prospective, randomized, open-labeled TECAM trial. J Am Coll Cardiol. 2015;65(22):2372–82.
Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, Passerini F, Tommasi L, Rossi A, Capucci A. Bone marrow cell transplantation improves cardiac, autonomic, and functional indexes in acute anterior myocardial infarction patients (cardiac study). Eur J Heart Fail. 2010;12(2):172–80.
Huang R, Yao K, Sun A, Qian J, Ge L, Zhang Y, Niu Y, Wang K, Zou Y, Ge J. Timing for intracoronary administration of bone marrow mononuclear cells after acute ST-elevation myocardial infarction: a pilot study. Stem Cell Res Ther. 2015;6(1):112.
Wollert KC, Meyer GP, Müller-Ehmsen J, Tschöpe C, Bonarjee V, Larsen AI, May AE, Empen K, Chorianopoulos E, Tebbe U, Waltenberger J, Mahrholdt H, Ritter B, Pirr J, Fischer D, Korf-Klingebiel M, Arseniev L, Heuft HG, Brinchmann JE, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J. 2017;38(39):2936–43.
Wöhrle J, Merkle N, Mailänder V, Nusser T, Schauwecker P, von Scheidt F, Schwarz K, Bommer M, Wiesneth M, Schrezenmeier H, Hombach V. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010;105(6):804–12.
Traverse JH, Henry TD, Pepine CJ, Willerson JT, Chugh A, Yang PC, Zhao DXM, Ellis SG, Forder JR, Perin EC, Penn MS, Hatzopoulos AK, Chambers JC, Baran KW, Raveendran G, Gee AP, Taylor DA, Moyé L, Ebert RF, Simari RD. TIME trial: effect of timing of stem cell delivery following ST-elevation myocardial infarction on the recovery of global and regional left ventricular function: final 2-year analysis. Circ Res. 2018;122(3):479–88.
Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Bostrom N, Kadidlo D, Lesser JR, Jagadeesan V, Garberich R, Henry TD. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. Am Heart J. 2010;160(3):428–34.
Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Sedding D, Yu J, Corti R. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35(19):1275–83.
Benedek I, Bucur O, Benedek T. Intracoronary infusion of mononuclear bone marrow-derived stem cells is associated with a lower plaque burden after four years. J Atheroscler Thromb. 2014;21(3):217–29.
Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, Savolainen ER, Ukkonen H, Pietilä M, Airaksinen JK, Knuuti J, Mäkikallio TH. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29(22):2723–32.
Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. Jama. 2011;306(19):2110–9.
Nguyen PK, Rhee JW, Wu JC. Adult stem cell therapy and heart failure, 2000 to 2016: a systematic review. JAMA Cardiol. 2016;1(7):831–41.
Yang D, O’Brien CG, Ikeda G, Traverse JH, Taylor DA, Henry TD, Bolli R, Yang PC. Meta-analysis of short- and long-term efficacy of mononuclear cell transplantation in patients with myocardial infarction. Am Heart J. 2020;220:155–75.
Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–53.
de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circul Cardiovasc Intervent. 2014;7(2):156–67.
Mathur A, Sim DS, Choudry F, Veerapen J, Colicchia M, Turlejski T, Hussain M, Hamshere S, Locca D, Rakhit R, Crake T, Kastrup J, Agrawal S, Jones DA, Martin J. Five-year follow-up of intracoronary autologous cell therapy in acute myocardial infarction: the REGENERATE-AMI trial. ESC Heart Fail. 2022;9(2):1152–9.
Funding
This study was not supported by any grant from funding agencies.
Author information
Authors and Affiliations
Contributions
AA contributed to the concept and design of the study. AH contributed to the screening process and statistical analysis. The first draft of the manuscript was written by AH and HH. AA and AH revised the final draft. AA, AH, and HH contributed to manuscript writing and preparing the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Publish
Not applicable
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosseinpour, A., Hosseinpour, H. & Attar, A. Preventive Effect of Bone Marrow Mononuclear Cell Transplantation on Acute Myocardial Infarction-Induced Heart Failure: A Meta-analysis of Randomized Controlled Trials. Cardiovasc Drugs Ther 37, 1143–1153 (2023). https://doi.org/10.1007/s10557-022-07359-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07359-3