Abstract
Aim
In recent decades, there has been a revolutionary decrease in cancer-related mortality and an increase in survival due to the introduction of novel targeted drugs. Nevertheless, drugs targeting human epidermal growth factor receptor 2 (HER-2), angiogenesis, and other tyrosine kinases also come with unexpected cardiac side effects, including heart failure, hypertension, arterial thrombosis, and arrhythmias, and have mechanisms that are unlike those of classic chemotherapeutic agents. In addition, it is challenging to address some problems, as the existing guidelines need to be more specific, and further large-scale clinical trials and experimental studies are required to confirm the benefit of administering cardioprotective agents to patients treated with targeted therapies. Therefore, an improved understanding of cardiotoxicity becomes increasingly important to minimize the pernicious effects and maximize the beneficial effects of targeted agents.
Methods
“Cardiotoxicity”, “targeted drugs”, “HER2”, “trastuzumab”, “angiogenesis inhibitor”, “VEGF inhibitor” and “tyrosine kinase inhibitors” are used as keywords for article searches.
Results
In this article, we report several targeted therapies that induce cardiotoxicity and update knowledge of the clinical evidence, molecular mechanisms, and management measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer therapy has made steady progress, which has considerably increased survival and brought hope to patients with cancer. Paradoxically, along with increased survival and reduced morality, another critical factor has also emerged—cardiotoxicity. Beginning with cardiomyopathy induced by anthracyclines, it has been increasingly important for oncologists to alleviate the detrimental side effects induced by cancer therapies, and related studies are emerging one after another. To date, the cardiotoxicity of certain classic chemotherapeutic agents, including anthracycline, cisplatin, and paclitaxel, is well established. In contrast to traditional cancer therapies, novel targeted agents interfere with specific molecular pathways, which has revolutionized the treatment of cancer patients. Drugs targeting angiogenesis, HER2, or tyrosine kinases also cause inevitable adverse events such as heart failure, hypertension, arterial thrombosis, and arrhythmias due to effects off of their intended cancer tissue or molecular targets. Nevertheless, there are a lack of precise mechanisms and specific therapeutic measures for cardiac adverse effects, which would improve the understanding for both oncologists and cardiologists. This review focuses on the molecular mechanisms of cardiotoxicity induced by some targeted agents. The purpose of this article is to stimulate awareness of the emerging cardiotoxicity of novel targeted anticancer drugs and provide novel insights into cardiovascular disease.
HER2-Targeting Drugs
Heart Failure (HF)
Clinical Evidence
Breast cancer has gained the attention of oncologists due to its high prevalence and mortality rate [1]. Human epidermal growth factor receptor 2 (HER2) is a proto-oncogene correlated with a poor prognosis in breast cancer and is overexpressed in approximately 15–20% of breast cancers [2, 3]. Trastuzumab (TRZ), a monoclonal antibody (mAb) that targets HER2, prolongs survival for patients with HER2-positive breast cancer but unexpectedly causes cardiac dysfunction, particularly in combination with anthracycline [4]. Several clinical trials and meta-analyses have proven the relevance between trastuzumab and cardiac dysfunction [4,5,6]. A meta-analysis reported that the incidence of left ventricular ejection fraction (LVEF) decrease and congestive heart failure (CHF) was 7.5% (95% confidence interval (CI) 4.2–13.1) and 1.9% (95% CI 1.0–3.8), respectively, among patients receiving trastuzumab. Trastuzumab was associated with a higher risk of LVEF decrease (relative risk (RR) = 2.13, 95% CI [1.31–3.49]) and CHF (RR 4.19 [2.73–6.42]). Notably, the risk of CHF is greatly increased (RR 4.27 [2.75–6.61]) when trastuzumab is used in combination with anthracycline [4]. Moreover, up to 27% of patients develop cardiac dysfunction during concurrent treatment with trastuzumab and anthracycline; however, these high rates are not observed when the drugs are administered sequentially [7]. In addition, pertuzumab, lapatinib, and some other HER2-targeting therapies also potentially induce heart failure. Paradoxically, combination anti-HER2 therapy is not associated with increased cardiotoxicity, as shown in the CLEOPATRA trial [8]. Anthracyclines and trastuzumab are the most commonly used breast cancer therapies, and they cause different kinds of cardiomyopathy. Anthracycline-induced cardiomyopathy is generally irreversible and is correlated with cardiomyocyte necrosis and heart failure symptoms. However, with early detection and timely treatment, anthracycline-induced cardiomyopathy could also be reversible. However, we could not conclude that cardiotoxicity is cured, as we cannot predict what would happen without further supportive treatment. Trastuzumab-induced cardiomyopathy is not as closely related to symptomatic heart failure, which can be reversible after drug discontinuation. Nevertheless, studies have revealed that the cardiotoxicity induced by trastuzumab could sometimes persist many years after the withdrawal of the therapy [9,10,11,12].
Molecular Mechanism
The precise mechanism by which HER inhibitors induce cardiotoxicity remains unclear and may involve complicated cellular pathways. HER2 (also called erythroblastic leukemia viral oncogene homolog 2 (ErbB2)) and its ligand neuregulin (NRG) are responsible for embryonic heart formation, survival of cardiomyocytes, and maintenance of cardiac function in the adult heart [13,14,15,16]. After binding to ligands, HER receptors form homodimers or heterodimers, which trigger tyrosine kinase function and recruit downstream effectors. The downstream signaling pathways mainly include the MEK/Erk, PI3K/Akt, and Src/FAK pathways [17, 18]. The PI3K/Akt pathway increases nitric oxide (NO) production and reduces reactive oxygen species (ROS) accumulation via activation of endothelial nitric oxide synthase (eNOS) [15, 19, 20]. Additionally, PI3K/Akt signaling can inhibit cardiocyte apoptosis and promote myocyte survival by altering the expression of proteins of the Bcl-2 family [21, 22]. The MEK/Erk and Src/FAK pathways improve myocardial structure, while inhibition of related pathways results in the disarray of myofibers and sarcomeres [17, 19]. Recent studies indicated that a recently discovered cardiac-specific myosin light-chain kinase (cMLCK) may be involved in this process by modulating muscle contraction and sarcomere organization [23, 24]. Moreover, neuregulin is reported to regulate β-adrenergic activity via activation of eNOS and inhibitory parasympathetic activity, which slows the process from compensatory hypertrophy to heart failure by inhibiting excessive β-adrenergic stimulation and ventricular remodeling [13, 25, 26].
TRZ binds to domain IV of the extracellular segment of HER2 receptors and blocks HER2 activity, while pertuzumab, another anti-ErbB2 mAb, binds to domain II and inhibits dimerization. Lapatinib, a dual tyrosine kinase inhibitor (TKI), blocks HER2 and epidermal growth factor receptor (EGFR) signaling [3]. It is believed that mitochondrial dysfunction is essential for TRZ-induced cardiomyopathy. The inhibition of HER2 results in a decreased antioxidant reserve and an accumulation of ROS by downregulating the expression of eNOS, and accumulation of ROS leads to successful mitochondrial impairment [27]. Moreover, inhibition of HER2 and the PI3K-Akt pathway leads to upregulation of the proapoptotic Bcl-2 family protein Bcl-xS and downregulation of the antiapoptotic protein Bcl-xL, which activates the mitochondrial apoptosis pathway and leads to mitochondrial impairment and disorder of cellular energetics [21, 28, 29]. Subsequently, mitochondrial dysfunction causes decreased contractility and cell injury, which are important features of heart failure. In addition, inhibition of HER2 downregulates the cMLCK, MEK/Erk, and Src/FAK pathways, which gives rise to a disordered myocardial structure. Inhibition of HER2 also fails to counterbalance excessive β-adrenergic reactions, predisposing patients to cardiac hypertrophy and ventricular remodeling [25, 26]. Recently, several studies have indicated another novel mechanism by which inhibition of HER2 activates the Erk/mTOR signaling cascade, which leads to autophagy inhibition and ROS accumulation [30,31,32]. The histological findings reveal myofiber damage, mitochondrial swelling, and interstitial infiltration, unlike the typical biopsy changes observed with anthracyclines (vacuoles, necrosis, myocyte death) [22]. In summary, mitochondrial dysfunction, not necrosis or cell death, is of vital importance to the cardiomyopathy induced by HER2 inhibitors.
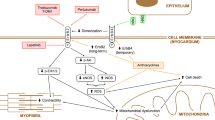
In clinical practice, the incidence of cardiotoxicity is significantly increased in patients receiving trastuzumab in combination with doxorubicin [4, 33], supporting the hypothesis that trastuzumab amplifies the cardiotoxicity of anthracycline treatment. In anthracycline treatment, the activation of HER2 by NRG decreases ROS levels, preserves mitochondrial dehydrogenase function, maintains calcium homeostasis, and inhibits apoptosis [21, 22, 34, 35]. A “dual-hit” model may better explain why trastuzumab augments doxorubicin-induced cardiopathy [34]. On one hand, anthracyclines activate cardiac stress pathways through several mechanisms, including inhibition of topoisomerase 2β and production of reactive oxygen species [36]. In addition, HER2 inhibition increases the production of ROS and accelerates apoptosis, which aggravates oxidative stress and cell injury. On the other hand, HER2 inhibition disturbs HER2/NRG survival and protective pathways and increases the susceptibility of cardiocytes to stress. The detailed mechanism is shown in Fig. 1.
Mechanisms of HER2 inhibitor-induced heart failure. Trastuzumab binds to domain IV of the extracellular segment of the HER2 receptors, which inhibits the downstream HER2 pathway. The inhibition of PI3K/Akt signaling results in accumulation of ROS and activation of apoptosis, which subsequently leads to mitochondrial impairment. The decreased activity of MEK/Erk and Src/FAK pathway results in the disarray of myofibers and sarcomeres. Furthermore, mitochondrial dysfunction and myocardial structure disorder lead to heart failure. Anthracycline, a classical chemotherapeutic drug, could augment the cardiotoxicity of trastuzumab by aggravating mitochondrial dysfunction and cell injury. HER2, human epidermal growth factor receptor 2; ROS, reactive oxygen species
Management
Existing guidelines recommend that patients treated with HER2 inhibitors have a thorough clinical evaluation and comprehensive assessment of risk factors [37,38,39,40]. Routine cardiac function monitoring is needed before and during trastuzumab therapy. Echocardiography, with high practicability and no radiation exposure, has become the most widely used strategy for monitoring cardiac function in patients treated with trastuzumab [38, 41, 42]. Global longitudinal strain (GLS) has been identified as an echocardiographic marker for the early prediction of cardiotoxicity induced by trastuzumab [42, 43]. In addition, cardiac magnetic resonance imaging (cMRI) and serum biomarkers such as brain natriuretic peptide (BNP) and troponin are used as complementary tools to monitor cardiac function [44]. Exercise is strongly recommended to exert its protective role by reducing ROS production, increasing adenosine 5′-monophosphate-activated protein kinase (AMPK) activity, and activating NRG [45, 46]. In a retrospective study, the CECCY and OVERCOME trials suggested the benefit of beta-blockers, angiotensin-converting enzyme inhibitors (ACEis), and aldosterone receptor blockers (ARBs), which are also recommended as reasonable first-line choices in the guidelines [39, 47,48,49]. However, large-scale clinical trials are needed to guide treatment for trastuzumab-induced HF. A recent clinical trial suggests the benefit of statins, mainly due to their anti-inflammatory and antioxidant effects [50,51,52,53]. In addition, ranolazine and melatonin show cardioprotective profiles in animal models by reducing oxidative stress [54, 55]. However, further clinical evidence is required to confirm their effects.
VEGF Inhibitors
Vascular endothelial growth factor inhibitors (VEGFi), also known as angiogenesis inhibitors, bring promising advances to the development of cancer chemotherapies. Nonetheless, VEGF inhibitors also cause unexpected complications, particularly hypertension (HTN) [56]. Broadly, VEGFi are classified as monoclonal antibodies and tyrosine kinase inhibitors. Bevacizumab and ramucirumab are mAbs against VEGF and its receptors. Drugs such as sunitinib and sorafenib are small-molecule TKIs that target the intracellular tyrosine kinase activity of VEGF receptors, inhibiting intracellular signaling and downstream pathways.
Hypertension
Clinical Evidence
Hypertension is one of the well-known cardiovascular adverse events induced by anti-VEGF therapies that has been proven in a series of clinical trials. In a meta-analysis including 77 RCTs, anti-VEGF treatment was found to significantly induce hypertension (odds ratio (OR) 5.28 [4.53–6.15]) and severe hypertension (OR 5.59 [4.67–6.69]) [57]. Matthias Totzeck reported similar results in a meta-analysis assessing the risk of HTN related to bevacizumab [58]. Another meta-analysis indicated a rather lower risk of HTN related to tyrosine kinase inhibitors (RR 3.78 [3.15–4.54]) [59]. The elevation in blood pressure occurs rapidly, within hours to days after the administration of anti-VEGF agents. Monoclonal antibodies such as bevacizumab can have cardiotoxic effects that last for several weeks after stopping therapy compared to small-molecule TKIs, for which the effects of HTN are typically mitigated within 1 week [60, 61]. In addition, the risk of HTN is dose-related and increases when bevacizumab is used in combination with other TKIs. In contrast to traditional off-target side effects, hypertension involves mechanism-dependent, on-target toxicity, reflecting potent inhibition of VEGF activity. Therefore, HTN is a potential biomarker for assessing the effectiveness and proper dosing of anti-VEGF therapy [62, 63].
Molecular Mechanism
The VEGF family includes five distinct proteins. Of these isoforms, VEGF-A plays a central role in angiogenesis and is the most biologically active [64, 65]. Three tyrosine kinase receptors bind to VEGF, and among these, VEGF-A binding to VEGF receptor-2 (VEGFR-2) is the most important [66]. VEGF binds to VEGFR and initiates the downstream signaling pathway, which regulates a series of biological actions. As mentioned above, the PI3K/Akt pathway increases NO generation via activation of eNOS, inhibits apoptotic activity, and promotes cell survival [67,68,69]. In addition, phospholipase C-γ (PLCγ) induces the synthesis of another vasodilator, prostacyclin (PGI2), through the p42/44 mitogen-activated protein kinase (MAPK)/cPLA2 pathway [69, 70]. PLCγ also stimulates the activation of diacylglycerol (DAG) and inositol trisphosphate (IP3) production, which is associated with acutely increased intracellular calcium [69, 71,72,73], while calcium influx mediates both vascular permeability and the activation of eNOS [73,74,75]. The downstream signaling pathway RAF/MEK/Erk is responsible for endothelial cell migration, proliferation, and homeostasis [72, 75, 76]. Furthermore, VEGF, once known as vascular permeability factor, could potently build up vascular permeability by increasing intracellular calcium and the production of NO, activating src kinase, and activating the Erk pathway [74, 75, 77].
Vascular dysfunction is crucial in the development of HTN, and many studies support that VEGF inhibitors reduce the level of vasodilators, including NO and PGI2, but increase the production of endothelin-1 (ET-1), promoting vasoconstriction and inhibiting vasodilation [78,79,80,81]. N-Nitro-l-arginine methyl ester (L-NAME), an NO synthesis inhibitor, reverses the elevation in blood pressure (BP) [82]. Steeghs reported a decreased capillary density and reduced capillary dilatory capacity after bevacizumab treatment, which can be reversed after the withdrawal of VEGFi [83]. Capillary rarefaction and decreased dilatory capacity consequently increase cardiac afterload and elevate blood pressure. It is worth noting that VEGFi-induced HTN is salt-sensitive and is characterized by interstitial sodium accumulation. Under normal conditions, sodium accumulation is sensed by dermal macrophages, which activates VEGFC/VEGFR3 signaling and promotes the formation of lymph vessels to remove excessive sodium, while the inhibition of VEGF pathways disrupts this physiological process and results in the retention of sodium and water [65, 80, 84]. Moreover, it is controversial whether oxidative stress could lead to HTN when VEGF signaling is blocked. Neves and her colleagues demonstrated that VEGFi increased ROS and peroxynitrite (ONOO–) formation while decreasing activation of the eNOS–NO pathway [79]. Nevertheless, some antioxidant therapies failed to reduce BP in sunitinib-induced hypertension in animal models, casting doubt on this theory [85]. Renal function and the renin-angiotensin-aldosterone system (RAAS) are vital for the maintenance of blood pressure; however, data are scarce regarding whether blockade of VEGF leads to activation of the RAAS and whether renal injury is caused by hypertension or a side effect of VEGF inhibition [65, 80, 86]. The detailed mechanism is shown in Fig. 2.
Mechanisms of VEGFi-induced hypertension. Bevacizumab binds to VEGF, blocking downstream pathway activity and inhibiting angiogenesis. The inhibition of the PI3K/Akt pathway leads to a decreased level of vasodilators (NO and PGI2) and an increased level of ET-1, promoting vasoconstriction and causing endothelial dysfunction. In addition, inhibition of angiogenesis results in capillary rarefaction, which increases cardiac afterload. Both pathological changes may be the potential mechanisms of VEGFi-induced hypertension. However, it is controversial whether oxidative stress could impair endothelial function and lead to hypertension. VEGF, vascular endothelial growth factor; NO, nitric oxide; PGI2, prostacyclin; ET-1, endothelin-1
Management
The prevention and management of VEGFi-induced HTN are driven primarily by the guidelines [87, 88]. Primarily, a comprehensive evaluation should be implemented to assess cardiovascular risk factors and baseline blood condition. Active BP monitoring throughout VEGFi treatment is of considerable importance. Following standard HTN guidelines, the BP target before and during anticancer therapies is <140/90 mmHg. For high-risk groups such as patients with chronic kidney disease or diabetes (DM), the threshold is slightly lower (<130/80 mmHg) [89]. Lifestyle recommendations include exercise, cessation of smoking, reduced alcohol intake, and a low-salt diet. ACEis, ARBs, or calcium channel blockers (CCBs) are recommended as first-line options [39, 40, 87, 88]. A retrospective study with 4736 patients indicated that angiotensin system inhibitors significantly improved survival in metastatic renal cell carcinoma patients treated with VEGFi compared to those treated with other antihypertensives [90]. Notably, nondihydropyridine calcium channel blockers should be used cautiously because they inhibit cytochrome P450 3A4 enzyme (CYP3A4) and may potentiate the toxicity of anti-VEGF therapy [91].
Heart Failure (HF)
Clinical Evidence
Both anti-VEGF monoclonal antibodies and tyrosine kinase inhibitors are relevant to left ventricular dysfunction and heart failure. In the case of bevacizumab, a high risk of high-grade CHF was observed in cancer patients treated with bevacizumab (RR 1.98 [1.30–3.02]) according to a meta-analysis [92]. The risk is relatively high for anti-VEGF TKIs compared to other therapies, and tyrosine kinase inhibitor therapy significantly increased the risk of left ventricular systolic dysfunction (RR 2.53 [1.79–3.57]), partly due to its multitarget effect [59].
Molecular Mechanism
The mechanisms of VEGFi-related heart failure are not fully understood. First, VEGFi-induced endothelial dysfunction and HTN increase cardiac afterload, which increases the burden on the heart. As mentioned above, inhibition of the PI3K-Akt pathway increases ROS levels via activation of eNOS and promotes apoptosis, which leads to mitochondrial injury. The PI3K-Akt pathway may also be responsible for physiological and pathological cardiac growth, while disruption of coordinated tissue growth may accelerate the progression from adaptive cardiac hypertrophy to HF [93]. In addition, from clinical data, we found that anti-VEGF TKIs tend to have a higher risk of HF than anti-VEGF mAbs [59, 92], indicating that their multiple-target effects may contribute to HF. Sunitinib is a TKI that targets VEGFR, platelet-derived growth factor receptors (PDGFRs), and c-KIT [94]. HF induced by sunitinib may be partly due to the inhibition of PDGFR, as it promotes cell survival and regulates the compensatory cardiac response to pressure overload-induced stress [95, 96]. Moreover, inhibition of AMPK may also contribute to the mechanisms. Studies show that sunitinib administration could decrease AMPK activity and that AMPK signaling is vital in maintaining cardiac energy homeostasis, inhibiting anabolic processes, and promoting catabolic processes under conditions of energy stress [94, 97, 98]. Arad found an uncommon familial form of hypertrophic cardiomyopathy featuring mutations in the PRKAG2 gene, which encodes the γ2 regulatory subunit of AMPK [99]. Another study found that AMPKα2 knockout mice exhibited poorer left ventricular function, increased left ventricular hypertrophy, and higher mortality under pressure overload conditions than under non-pressure overload conditions [100]. In brief, inhibition of not only angiogenesis but also other off-targets, such as PDGFR or AMPK, may be potential mechanisms that lead to heart failure. The detailed mechanism is shown in Fig. 3.
Mechanisms of VEGFi-induced heart failure and artery thrombosis. Sorafenib or sunitinib are TKIs targeting VEGFR and PDGFR. Inhibition of VEGF induces hypertension and increases cardiac afterload, bringing burden to the heart. Moreover, the inhibition of the PI3K/Akt pathway leads to activation of apoptosis and accumulation of ROS, which may impair the function of mitochondria. While inhibition of off-target like PDGFR and AMPK are associated with disruption of energy homeostasis, inhibition of cell survival, and decreased response to cardiac stress. Each factor may be relevant to the development of heart failure. As for artery thrombosis, the decreased level of NO and PGI2 promotes platelet aggregation and increases TXA2, which predisposes to ATEs. Furthermore, decreased NO production may lead to oxidative stress, which is an important mechanism of AS. Moreover, inhibition of angiogenesis could impair vascular integrity and lead to thrombosis. Cancer patients themselves are at a hypercoagulant status as cancer cells secret some pro-coagulant factors. Patients treated with VEGFi with a history of ATEs are at a much higher risk because the angiogenesis inhibition impairs the ability of vascular recovery, which accelerates the recurrence. TKIs, tyrosine kinase inhibitors; PDGFR, platelet-derived growth factor receptor; AMPK, adenosine 5′-monophosphate-activated protein kinase; NO, nitric oxide; PGI2, prostacyclin; TXA2, thromboxane A2; AS, atherosclerosis; ATEs, artery thrombosis events
Thrombosis
Clinical Evidence
It has been reported that bevacizumab is associated with a higher incidence of arterial thrombosis events (ATEs), particularly cardiac and cerebral ischemia. In a meta-analysis including 20,050 patients, bevacizumab was reported to be associated with a high risk of arterial adverse events (RR 1.37 [1.10–1.70]), and high-dose bevacizumab significantly increased the risk of cardiac and cerebral ischemia (RR 4.4 [1.59–12.70] and RR 6.67 [2.17–20.66]) [58]. Another meta-analysis reported a high risk (OR 2.26 [1.38–3.68]) [52] for VEGF TKIs, and the overall incidence was 1.5% (95% CI, 1.0% to 2.3%). Notably, the highest incidence was reported for cediranib (3.2%, 0.5–19.6%) and pazopanib (2.4%, 1.6–3.7%), while only pazopanib and sorafenib significantly increased the risk of ATE in the subgroup analysis [101]. A pooled analysis indicated that the strongest independent risk factor for ischemic events with VEGFi therapy was a history of ATE (hazard ratio (HR) 3.65 [1.9–6.9]). It is worth noting that elderly patients (> 65 years old) with a history of ATE had an 8.3-fold higher risk of ischemic events than controls in the subgroup analysis [102]. Nevertheless, the risk is uncertain for venous thrombosis. Bevacizumab significantly increased the risk of venous thromboembolism (VTE), with an RR of 1.33 (95% CI, 1.13–1.56) reported in a meta-analysis including 7956 patients [103]. Another systematic review reported a neutral result (RR 0.89 [0.66–1.20]) in patients with metastatic carcinoma [102]. The CALGB trial also indicated that there was no increase in venous thrombosis in the bevacizumab arm [104]. Moreover, patients treated with TKIs showed a similar incidence of venous thrombosis as controls (RR 1.02 [0.74–1.4]) in a meta-analysis [59].
Molecular Mechanism
VEGF activity (angiogenesis) is involved in endothelial cell proliferation, survival, and maintenance of vascular integrity, while blockage of VEGF signaling impairs the integrity and regenerative capacity of endothelial cells, subsequently leading to thrombosis [105, 106]. In addition, inhibition of the PI3K/Akt pathway reduces the production of NO and PGI2, predisposing patients to thromboembolic events on account of their ability to inhibit platelet aggregation and antagonize thromboxane A2 (TXA2). Cancer patients are more prone to thrombosis because of the procoagulant factors secreted by tumor cells, which lead to hypercoagulability. Moreover, VEGFi-induced endothelial dysfunction facilitates coronary vasospasm, which causes cardiac ischemia [107]. Oxidative stress has been identified as a critical mechanism in atherosclerosis (AS), which leads to the formation of oxidized LDL (ox-LDL) and foam cells, platelet activation, vascular injury, and plaque instability [108, 109]. While VEGF inhibitors increase ROS generation and decrease activation of the eNOS–NO pathway, the imbalance between antioxidants and oxidants predisposes patients to atherosclerosis. According to some experimental results, VEGF improves cardiac function and accelerates microvasculature recovery after MI. Thus, angiogenesis induced by VEGF has become a promising mechanism for post-myocardial infarction (MI) recovery [110,111,112]. Therefore, it is not difficult to explain why patients with a history of ATE are more prone to recurrence. The mechanism correlated with VTE is similar, including impaired vascular integrity, inhibition of NO, and hypercoagulability caused by cancer. In addition, experimental results indicate that inhibition of PAI-1 may play important roles in the mechanisms of bevacizumab-induced VTE [113]. The detailed mechanism is shown in Fig. 3.
Management
First, the risk of ATE should be addressed with each patient before the initiation of VEGFi treatment. Throughout treatment with VEGF inhibitors, a baseline 12-lead electrocardiogram (ECG) is highly recommended to monitor both ischemic ECG changes and QT interval prolongation [39]. If ischemic ST-T changes are noted, cardiac evaluations, including serum troponin levels, coronary computed tomography angiography, or even coronary angiography, are advised. Cessation of treatment is generally advised when patients develop angina or suffer an acute myocardial infarction. As mentioned above, endothelial dysfunction and oxidative stress are strongly associated with the development of ATE; hence, lifestyle changes such as exercising and quitting smoking are strongly advised to improve endothelial and vascular health, although they may not be easily implemented in cancer patients. Exercise training could increase shear stress, which is a potent natural inducer of eNOS and the PI3K/Akt pathway [114]. Moreover, exercise promotes endothelium-dependent vascular dilatation and rejuvenates cardiovascular reserve and the endothelial response to vascular growth factors [115, 116]. Regarding medication therapy, a pooled analysis reported that aspirin could significantly reduce the incidence of ATEs in the high-risk group of patients ≥ 65 years with a prior ATE history but nevertheless caused an increased number of grade 3 and 4 bleeding events [102]. In consideration of the potential bleeding risk of VEGFi [106], dual antiplatelet therapy is not recommended unless under conditions of drug-eluting stent placement. Statin therapy is strongly advised as it improves vascular health, NO bioavailability, and eNOS activity by activating PI3K/Akt signaling [117, 118]. According to the guidelines, the detection of VTE generally depends on clinical symptoms. Whether to choose anticoagulation is an individualized decision that considers the bleeding risk and life expectancy. Warfarin and non-vitamin K antagonist oral anticoagulants (NOACs) are both advised, and there is no difference in VTE recurrence or bleeding between the two kinds of agents [39, 40].
Tyrosine Kinase Inhibitors (TKIs
Atrial Fibrillation (AF)
Clinical Evidence
There are emerging studies reporting an association of TKIs, including ponatinib, sunitinib, sorafenib, and ibrutinib, with AF; however, the exact incidence of AF is rarely known. In contrast, ibrutinib, a novel Bruton tyrosine kinase (BTK) inhibitor, is strongly associated with AF, which is reported in 6–16% of patients treated with ibrutinib [119]. A meta-analysis indicated that ibrutinib significantly increased the risk of AF (RR 4.69 [2.17–7.64]) [120].
Molecular Mechanism
The underlying mechanisms remain unclear. McMullen indicated that reduced activity of the PI3K-Akt pathway was associated with a higher susceptibility to AF, while ibrutinib inhibited the BTK and PI3K-Akt pathways [121, 122]. Experimental data also led to another hypothesis that AF was related to enhanced ROS signaling, which led to abnormal Ca2+ release and atrial remodeling [123, 124]. In addition, ibrutinib exhibited its antitumor effect via inhibition of BKT, which also had other off-target effects, including Tec protein tyrosine kinase (TEC), epidermal growth factor receptor (EGFR), and interleukin-2-inducible T-cell kinase (ITK). The second-generation BTK inhibitor acalabrutinib did not increase the incidence of AF, as it was more selective, indicating that off-target effects may also play a part [122, 125, 126]. In contrast to the second-generation BTK inhibitor, ibrutinib exhibited direct cell-specific effects on atrial cardiomyocytes (CMs), which had a much shorter action potential duration and a higher propensity toward AF than ventricular CMs [127]. Finally, ibrutinib was also correlated with an increased incidence of hypertension, which is a risk factor for AF [120]. The detailed mechanisms are displayed in Table 1.
Management
The current guideline gives a recommendation for treating atrial fibrillation in cancer patients; however, specific ideas on the management of ibrutinib-induced AF are scarce [39, 40]. First, risk factors including hypertension, coronary heart disease, diabetes, valvular heart disease, cardiomyopathy, and hyperthyroidism should be fully assessed and well controlled. Baseline cardiac function needs to be monitored, including BNP and echocardiography. Decisions on rate or rhythm control should be patient centered and symptom directed. Beta-blockers or CCBs may help with rate control, while type Ic and type III antiarrhythmic drugs are effective for rhythm control. In patients with a CHA2DS2-VASc score ≥2, oral anticoagulation is generally advised for thromboembolic prophylaxis [128]. However, the problem becomes complicated due to various factors. On one hand, BTK inhibitors are approved for the treatment of mantle cell lymphoma and chronic lymphocytic leukemia, which may be accompanied by low platelet count and anticoagulant system dysfunction. On the other hand, ibrutinib is associated with a high risk of overall bleeding (RR 2.72 [1.62–6.58]). Warfarin is restricted in combination with ibrutinib, while DOACs are recommended due to their superior safety for bleeding if anticoagulants are strongly indicated [129,130,131].
QT Interval Prolongation
Clinical Evidence
Many TKIs have negligible clinically relevant effects on the QT interval, including sunitinib, sorafenib, pazopanib, lapatinib, dasatinib and nilotinib and vandetanib [132, 133]. Among these drugs, vandetanib has the highest incidence and the most significant prolongation of the corrected QT (QTc) interval. In a meta-analysis with 2188 patients from nine RCTs of vandetanib, the total incidence of all-grade and high-grade QTc interval prolongation was 16.4% and 3.7%, respectively [134]. Another meta-analysis indicated that vandetanib was associated with a high risk for all-grade (RR 7.90 [4.03–15.50]) and high-grade QTc interval prolongation (RR 3.12 [1.01–9.63]) [135].
Molecular Mechanism
The QT interval on the ECG is a measurement of the overall duration of ventricular action potential. Multiple forms of ion channel disorders could lead to an increased positive intracellular charge, which prolongs ventricular repolarization and gives rise to a prolonged QT interval. Long QT syndrome (LQTS), based on its pathogenesis, is classified as congenital long QT syndrome (cLQTS) and acquired LQTS (aLQTS). cLQTS derives from congenital mutations that disturb the function of ion channels that are critical components of the action potential. cLQT1 (changes in KCNQ1 that reduce slowly activating delayed rectifier K+ repolarization current (IKs)), cLQT2 (changes in hERG that reduce rapidly activating delayed rectifier K+ repolarization current (IKr)), and cLQT3 (changes in SCN5A that increase the sodium current (INa)) account for 95% of the identified mutations [136, 137]. aLQTS refers to long QT syndrome induced by drugs [138]. The generally accepted mechanism of aLQTS is the direct block of a vital repolarizing potassium current, IKr, which is encoded by hERG [139, 140]. Drugs binding to the pore-forming subunit of the ion channel encoded by KCNH2 and disrupting channel trafficking are believed to be the major mechanism for IKr inhibition [141, 142]. However, Lu indicated that unlike traditional mechanisms of aLQTS, inhibition of the PI3K/Akt pathway may be the key mechanism by which TKIs induce QT interval prolongation [143]. PI3K and Akt, with their downstream effectors, modulate multiple ion channels [144, 145]. Blocking PI3K signaling results in an increase in the persistent sodium current (INaP) as well as a decrease in the L-type calcium ion current (ICaL) and potassium currents (IKr and IKs), and the combined changes in IKr and INaP accounted for more than 70% of the overall prolongation [145]. Interestingly, diabetes is also relevant to QT interval prolongation because diabetes downregulates cardiac PI3K signaling and alters INa [146]. In conclusion, rather than direct blockade of the channel, inhibition of PI3K/Akt signaling and subsequent changes in ion current result in the final QT prolongation. All the mechanisms mentioned above are listed in Table 1.
Management
Following the currently available data [39, 147, 148], several recommendations could be suggested: (1) a baseline ECG should be obtained for patients initiating anticancer treatment that prolongs the QT interval; (2) electrolytes should be carefully monitored, and electrolyte abnormalities must be corrected; (3) drug-drug interactions should be considered to avoid using drugs that are proven to induce QT interval prolongation; (4) cessation of anticancer drugs should be carefully monitored if the QTc is >500 ms or ΔQTc (change from baseline) >60 ms during monitoring; and (5) β-blockers are first-line treatment for symptomatic and asymptomatic LQTS; however, the limited clinical evidence could prove the beneficial effects of these drugs on patients treated with anticancer medication.
Conclusions
In this review, we concentrate on the molecular mechanisms of cardiotoxicity induced by certain targeted drugs and identify several important signaling pathways, including PI3K-Akt and MEK-Erk signaling, as well as some potential mechanisms, such as endothelial dysfunction and mitochondrial impairment. Notably, the PI3K-Akt pathway exerts complicated biological effects by increasing NO production via activation of eNOS, inhibiting apoptosis, and modulating multiple ion channels and taking part in mechanisms of heart failure, hypertension, arterial thrombosis, and even arrhythmias. Currently, the management of cardiotoxicity induced by antitumor agents mainly depends on early detection, careful monitoring, and empirical treatment, and there is still a lack of specific recommendations by guidelines. Future treatments for targeted drug-induced cardiotoxicity depend on a deeper understanding of mechanisms and further clinical evidence.
Data Availability
All data generated or used during the study are available in a repository or online in accordance with funder data retention policies (provide full citations that include url or DOIs).
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Waks AG, Winer EP. Breast cancer treatment: a review. Jama. 2019;321(3):288–300.
Leemasawat K, Phrommintikul A, Chattipakorn SC, Chattipakorn N. Mechanisms and potential interventions associated with the cardiotoxicity of ErbB2-targeted drugs: insights from in vitro, in vivo, and clinical studies in breast cancer patients. Cell Mol Life Sci. 2019;77:1571–89.
Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev. 2011;37(4):312–20.
Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–9.
Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73.
Leemasawat K, Phrommintikul A, Chattipakorn SC, Chattipakorn N. Mechanisms and potential interventions associated with the cardiotoxicity of ErbB2-targeted drugs: insights from in vitro, in vivo, and clinical studies in breast cancer patients. Cell Mol Life Sci. 2020;77(8):1571–89.
Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–30.
Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23(13):2900–2.
Barish R, Gates E, Barac A. Trastuzumab-induced cardiomyopathy. Cardiol Clin. 2019;37(4):407–18.
Riccio G, Coppola C, Piscopo G, Capasso I, Maurea C, Esposito E, et al. Trastuzumab and target-therapy side effects: is still valid to differentiate anthracycline type I from type II cardiomyopathies? Hum Vaccin Immunother. 2016;12(5):1124–31.
Maurea N, Coppola C, Piscopo G, Galletta F, Riccio G, Esposito E, et al. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J Cardiovasc Med (Hagerstown). 2016;17(Suppl 1):S19–26.
Rupert CE, Coulombe KL. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark Insights. 2015;10(Suppl 1):1–9.
D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17(5):627–38.
Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116(8):954–60.
Parodi EM, Kuhn B. Signalling between microvascular endothelium and cardiomyocytes through neuregulin. Cardiovasc Res. 2014;102(2):194–204.
Jiang Z, Zhou M. Neuregulin signaling and heart failure. Curr Heart Fail Rep. 2010;7(1):42–7.
Vermeulen Z, Segers VF, De Keulenaer GW. ErbB2 signaling at the crossing between heart failure and cancer. Basic Res Cardiol. 2016;111(6):60.
Pentassuglia L, Sawyer DB. The role of Neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res. 2009;315(4):627–37.
Lee JH, Yoo JY, Kim HB, Yoo HI, Song DY, Min SS, et al. Neuregulin1 attenuates H(2)O(2)-induced reductions in EAAC1 protein levels and reduces H(2)O(2)-induced oxidative stress. Neurotox Res. 2019;35(2):401–9.
Rohrbach S, Muller-Werdan U, Werdan K, Koch S, Gellerich NF, Holtz J. Apoptosis-modulating interaction of the neuregulin/erbB pathway with anthracyclines in regulating Bcl-xS and Bcl-xL in cardiomyocytes. J Mol Cell Cardiol. 2005;38(3):485–93.
Jie B, Zhang X, Wu X, Xin Y, Liu Y, Guo Y. Neuregulin-1 suppresses cardiomyocyte apoptosis by activating PI3K/Akt and inhibiting mitochondrial permeability transition pore. Mol Cell Biochem. 2012;370(1-2):35–43.
Liu YQ, Yang M, Duan CH, Su GB, Wang JH, Liu YF, et al. Protective role of neuregulin-1 toward doxorubicin-induced myocardial toxicity. Genet Mol Res. 2014;13(2):4627–34.
Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res. 2010;88(2):334–43.
Samson R, Baydoun H, Jaiswal A, Le Jemtel TH. Cardiac adrenergic nervous system and left ventricular remodeling. Am J Med Sci. 2015;350(4):321–6.
Okoshi K, Nakayama M, Yan X, Okoshi MP, Schuldt AJ, Marchionni MA, et al. Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation. 2004;110(6):713–7.
Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, et al. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem. 2009;284(4):2080–7.
Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxidative Med Cell Longev. 2018;2018:7582730–15.
Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I. Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways. BMC Cancer. 2008;8:129.
Mohan N, Shen Y, Endo Y, ElZarrad MK, Wu WJ. Trastuzumab, but not pertuzumab, dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther. 2016;15(6):1321–31.
Mohan N, Jiang J, Wu WJ. Implications of autophagy and oxidative stress in trastuzumab-mediated cardiac toxicities. Austin Pharmacol Pharm. 2017;2(1):1005.
Janser FA, Tschan MP, Langer R. The role of autophagy in HER2-targeted therapy. Swiss Med Wkly. 2019;149:w20138.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84.
Belmonte F, Das S, Sysa-Shah P, Sivakumaran V, Stanley B, Guo X, et al. ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;309(8):H1271–80.
Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, et al. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41(5):845–54.
Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: A 'dual-hit'. Exp Clin Cardiol. 2011;16(3):70–4.
Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34(10):1030–3.
Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 1. J Am Coll Cardiol. 2017;70(20):2536–51.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–801.
Raschi E, Diemberger I, Cosmi B, De Ponti F. ESC position paper on cardiovascular toxicity of cancer treatments: challenges and expectations. Intern Emerg Med. 2018;13(1):1–9.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
Negishi T, Miyazaki S, Negishi K. Echocardiography and cardio-oncology. Heart Lung Circ. 2019;28(9):1331–8.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 Pt A):2751–68.
Löffler AI, Salerno M. Cardiac MRI for the evaluation of oncologic cardiotoxicity. J Nucl Cardiol. 2018;25(6):2148–58.
Thirupathi A, de Souza CT. Multi-regulatory network of ROS: the interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J Physiol Biochem. 2017;73(4):487–94.
Cai MX, Shi XC, Chen T, Tan ZN, Lin QQ, Du SJ, et al. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 2016;149:1–9.
Ohtani K, Ide T, Hiasa KI, Sakamoto I, Yamashita N, Kubo M, et al. Cardioprotective effect of renin-angiotensin inhibitors and β-blockers in trastuzumab-related cardiotoxicity. Clin Res Cardiol. 2019;108(10):1128–39.
Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61(23):2355–62.
Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: The CECCY Trial. J Am Coll Cardiol. 2018;71(20):2281–90.
Calvillo-Argüelles O, Abdel-Qadir H, Michalowska M, Billia F, Suntheralingam S, Amir E, et al. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can J Cardiol. 2019;35(2):153–9.
Cho DH, Lim IR, Kim JH, Kim MN, Kim YH, Park KH, et al. Protective effects of statin and angiotensin receptor blocker in a rat model of doxorubicin- and trastuzumab-induced cardiomyopathy. J Am Soc Echocardiogr. 2020;33(10):1253–63.
Kabel AM, Elkhoely AA. Targeting proinflammatory cytokines, oxidative stress, TGF-β1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomed Pharmacother. 2017;93:17–26.
Davis MK, Virani SA. Statins in cardio-oncology: holy grail or epiphenomenon. Can J Cardiol. 2019;35(2):142–4.
Ozturk M, Ozler M, Kurt YG, Ozturk B, Uysal B, Ersoz N, et al. Efficacy of melatonin, mercaptoethylguanidine and 1400W in doxorubicin- and trastuzumab-induced cardiotoxicity. J Pineal Res. 2011;50(1):89–96.
Riccio G, Antonucci S, Coppola C, D'Avino C, Piscopo G, Fiore D, et al. Ranolazine attenuates trastuzumab-induced heart dysfunction by modulating ROS production. Front Physiol. 2018;9:38.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–34.
Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2017;53:120–7.
Totzeck M, Mincu RI, Rassaf T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20 000 patients. J Am Heart Assoc. 2017;6(8):e006278.
Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: a meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25(5):482–94.
Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12(6):409–25.
Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102(9):596–604.
Budolfsen C, Faber J, Grimm D, Krüger M, Bauer J, Wehland M, et al. Tyrosine kinase inhibitor-induced hypertension: role of hypertension as a biomarker in cancer treatment. Curr Vasc Pharmacol. 2019;17(6):618–34.
Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–38.
Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian J Morphol Embryol. 2018;59(2):455–67.
Lankhorst S, Saleh L, Danser AJ, van den Meiracker AH. Etiology of angiogenesis inhibition-related hypertension. Curr Opin Pharmacol. 2015;21:7–13.
Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49.
Galvano A, Guarini A, Iacono F, Castiglia M, Rizzo S, Tarantini L, et al. An update on the conquests and perspectives of cardio-oncology in the field of tumor angiogenesis-targeting TKI-based therapy. Expert Opin Drug Saf. 2019;18(6):485–96.
Nagai A, Sado T, Naruse K, Noguchi T, Haruta S, Yoshida S, et al. Antiangiogenic-induced hypertension: the molecular basis of signaling network. Gynecol Obstet Investig. 2012;73(2):89–98.
Li M, Kroetz DL. Bevacizumab-induced hypertension: clinical presentation and molecular understanding. Pharmacol Ther. 2018;182:152–60.
Neagoe PE, Lemieux C, Sirois MG. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J Biol Chem. 2005;280(11):9904–12.
Lankhorst S, Kappers MH, van Esch JH, Danser AH, van den Meiracker AH. Mechanism of hypertension and proteinuria during angiogenesis inhibition: evolving role of endothelin-1. J Hypertens. 2013;31(3):444–54 discussion 54.
Kruzliak P, Novák J, Novák M. Vascular endothelial growth factor inhibitor-induced hypertension: from pathophysiology to prevention and treatment based on long-acting nitric oxide donors. Am J Hypertens. 2014;27(1):3–13.
Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–25.
Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–71.
Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87(2):262–71.
Matsumoto K, Ema M. Roles of VEGF-A signalling in development, regeneration, and tumours. J Biochem. 2014;156(1):1–10.
Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27.
Lankhorst S, Danser AH, van den Meiracker AH. Endothelin-1 and antiangiogenesis. Am J Physiol Regul Integr Comp Physiol. 2016;310(3):R230–4.
Neves KB, Rios FJ, van der Mey L, Alves-Lopes R, Cameron AC, Volpe M, et al. VEGFR (vascular endothelial growth factor receptor) inhibition induces cardiovascular damage via redox-sensitive processes. Hypertension. 2018;71(4):638–47.
Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension. 2018;71(2):e1–8.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40.
Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54(3):652–8.
Steeghs N, Rabelink TJ, op't Roodt J, Batman E, Cluitmans FH, Weijl NI, et al. Reversibility of capillary density after discontinuation of bevacizumab treatment. Ann Oncol. 2010;21(5):1100–5.
Beaini S, Saliba Y, Hajal J, Smayra V, Bakhos JJ, Joubran N, et al. VEGF-C attenuates renal damage in salt-sensitive hypertension. J Cell Physiol. 2019;234(6):9616–30.
Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, et al. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59(1):151–7.
Versmissen J, Mirabito Colafella KM, Koolen SLW, Danser AHJ. Vascular cardio-oncology: vascular endothelial growth factor inhibitors and hypertension. Cardiovasc Res. 2019;115(5):904–14.
Steingart RM, Bakris GL, Chen HX, Chen MH, Force T, Ivy SP, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012;163(2):156–63.
Caletti S, Paini A, Coschignano MA, De Ciuceis C, Nardin M, Zulli R, et al. Management of VEGF-targeted therapy-induced hypertension. Curr Hypertens Rep. 2018;20(8):68.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–57.
McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS, et al. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21(11):2471–9.
Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20(5):807–15.
Qi WX, Fu S, Zhang Q, Guo XM. Bevacizumab increases the risk of severe congestive heart failure in cancer patients: an up-to-date meta-analysis with a focus on different subgroups. Clin Drug Investig. 2014;34(10):681–90.
Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115(8):2108–18.
Kim S, Ding W, Zhang L, Tian W, Chen S. Clinical response to sunitinib as a multitargeted tyrosine-kinase inhibitor (TKI) in solid cancers: a review of clinical trials. Onco Targets Ther. 2014;7:719–28.
Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL, et al. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest. 2010;120(2):472–84.
Yue Z, Chen J, Lian H, Pei J, Li Y, Chen X, et al. PDGFR-β signaling regulates cardiomyocyte proliferation and myocardial regeneration. Cell Rep. 2019;28(4):966–78.e4.
Kerkela R, Woulfe KC, Durand JB, Vagnozzi R, Kramer D, Chu TF, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci. 2009;2(1):15–25.
Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44.
Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100(4):474–88.
Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52(5):918–24.
Qi WX, Shen Z, Tang LN, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92(2):71–82.
Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99(16):1232–9.
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. Jama. 2008;300(19):2277–85.
Patel JN, Jiang C, Hertz DL, Mulkey FA, Owzar K, Halabi S, et al. Bevacizumab and the risk of arterial and venous thromboembolism in patients with metastatic, castration-resistant prostate cancer treated on Cancer and Leukemia Group B (CALGB) 90401 (Alliance). Cancer. 2015;121(7):1025–31.
Ferroni P, Formica V, Roselli M, Guadagni F. Thromboembolic events in patients treated with anti-angiogenic drugs. Curr Vasc Pharmacol. 2010;8(1):102–13.
Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–95.
Arima Y, Oshima S, Noda K, Fukushima H, Taniguchi I, Nakamura S, et al. Sorafenib-induced acute myocardial infarction due to coronary artery spasm. J Cardiol. 2009;54(3):512–5.
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42.
Kattoor AJ, Kanuri SH, Mehta JL. Role of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 2019;26(9):1693–700.
Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin AV, Oparil S, et al. VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol Heart Circ Physiol. 2018;314(2):H278–h84.
Badimon L, Borrell M. Microvasculature recovery by angiogenesis after myocardial infarction. Curr Pharm Des. 2018;24(25):2967–73.
Yang Y, Shi C, Hou X, Zhao Y, Chen B, Tan B, et al. Modified VEGF targets the ischemic myocardium and promotes functional recovery after myocardial infarction. J Control Release. 2015;213:27–35.
Chen N, Ren M, Li R, Deng X, Li Y, Yan K, et al. Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer. 2015;14:140.
Adams V, Reich B, Uhlemann M, Niebauer J. Molecular effects of exercise training in patients with cardiovascular disease: focus on skeletal muscle, endothelium, and myocardium. Am J Physiol Heart Circ Physiol. 2017;313(1):H72–h88.
Beck EB, Erbs S, Möbius-Winkler S, Adams V, Woitek FJ, Walther T, et al. Exercise training restores the endothelial response to vascular growth factors in patients with stable coronary artery disease. Eur J Prev Cardiol. 2012;19(3):412–8.
Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, et al. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol. 2017;595(12):3703–19.
Mitsuhashi T, Uemoto R, Ishikawa K, Yoshida S, Ikeda Y, Yagi S, et al. Endothelial nitric oxide synthase-independent pleiotropic effects of pitavastatin against atherogenesis and limb ischemia in mice. J Atheroscler Thromb. 2018;25(1):65–80.
Chen Y, Zhang S, Peng G, Yu J, Liu T, Meng R, et al. Endothelial NO synthase and reactive oxygen species mediated effect of simvastatin on vessel structure and function: pleiotropic and dose-dependent effect on tumor vascular stabilization. Int J Oncol. 2013;42(4):1325–36.
Brown JR, Moslehi J, O'Brien S, Ghia P, Hillmen P, Cymbalista F, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796–805.
Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14(2):e0211228.
McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829–30.
Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4(12):1491–500.
Jiang L, Li L, Ruan Y, Zuo S, Wu X, Zhao Q, et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16(9):1374–82.
Yang X, An N, Zhong C, Guan M, Jiang Y, Li X, et al. Enhanced cardiomyocyte reactive oxygen species signaling promotes ibrutinib-induced atrial fibrillation. Redox Biol. 2020;30:101432.
Isaac K, Mato AR. Acalabrutinib and its therapeutic potential in the treatment of chronic lymphocytic leukemia: a short review on emerging data. Cancer Manag Res. 2020;12:2079–85.
Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–32.
Shafaattalab S, Lin E, Christidi E, Huang H, Nartiss Y, Garcia A, et al. Ibrutinib displays atrial-specific toxicity in human stem cell-derived cardiomyocytes. Stem Cell Rep. 2019;12(5):996–1006.
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e51.
Caron F, Leong DP, Hillis C, Fraser G, Siegal D. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv. 2017;1(12):772–8.
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–45.
Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15(5):835–47.
Kloth JS, Pagani A, Verboom MC, Malovini A, Napolitano C, Kruit WH, et al. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer. 2015;112(6):1011–6.
Coppola C, Rienzo A, Piscopo G, Barbieri A, Arra C, Maurea N. Management of QT prolongation induced by anti-cancer drugs: Target therapy and old agents. Different algorithms for different drugs. Cancer Treat Rev. 2018;63:135–43.
Zang J, Wu S, Tang L, Xu X, Bai J, Ding C, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS One. 2012;7(2):e30353.
Liu Y, Liu Y, Fan ZW, Li J, Xu GG. Meta-analysis of the risks of hypertension and QTc prolongation in patients with advanced non-small cell lung cancer who were receiving vandetanib. Eur J Clin Pharmacol. 2015;71(5):541–7.
Wallace E, Howard L, Liu M, O'Brien T, Ward D, Shen S, et al. Long QT syndrome: genetics and future perspective. Pediatr Cardiol. 2019;40(7):1419–30.
Foo B, Williamson B, Young JC, Lukacs G, Shrier A. hERG quality control and the long QT syndrome. J Physiol. 2016;594(9):2469–81.
El-Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin Electrophysiol. 2018;41(4):414–21.
Roden DM. A current understanding of drug-induced QT prolongation and its implications for anticancer therapy. Cardiovasc Res. 2019;115(5):895–903.
He S, Moutaoufik MT, Islam S, Persad A, Wu A, Aly KA, et al. HERG channel and cancer: a mechanistic review of carcinogenic processes and therapeutic potential. Biochim Biophys Acta Rev Cancer. 1873;2020(2):188355.
Cubeddu LX. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev. 2016;12(2):141–54.
Dennis A, Wang L, Wan X, Ficker E. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochem Soc Trans. 2007;35(Pt 5):1060–3.
Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, et al. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med. 2012;4(131):131ra50.
Yang T, Meoli DF, Moslehi J, Roden DM. Inhibition of the α-subunit of phosphoinositide 3-kinase in heart increases late sodium current and is arrhythmogenic. J Pharmacol Exp Ther. 2018;365(3):460–6.
Cohen IS, Lin RZ, Ballou LM. Acquired long QT syndrome and phosphoinositide 3-kinase. Trends Cardiovasc Med. 2017;27(7):451–9.
Lu Z, Jiang YP, Wu CY, Ballou LM, Liu S, Carpenter ES, et al. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013;62(12):4257–65.
Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 2. J Am Coll Cardiol. 2017;70(20):2552–65.
Shah SR, Park K, Alweis R. Long QT syndrome: a comprehensive review of the literature and current evidence. Curr Probl Cardiol. 2019;44(3):92–106.
Acknowledgements
We are grateful to Dr. Chenfang Dong and Mr. Zhanyu Wang of the Department of Surgical Oncology (Breast Center) of The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China, for the advice on cancer therapy and the critical reading of the manuscript.
Funding
This work was supported by The Natural Science Foundation of Shandong Province (no. ZR2020MH016), The Project of Shandong Province Higher Educational Science and Technology Program (no. J18KA285), The Cardiovascular Multidisciplinary Integrated Thinking Research Fund Scientific Research Public Welfare Project (no. Z-2016-23-2001-31), Grants from the National Natural Science Foundation of China (81871231). Youth Innovation and Science and Technology Plan of Colleges and Universities in Shandong Province (2019KJK016).
Author information
Authors and Affiliations
Contributions
Qinchao Wu collected materials, prepared the figures and tables, and wrote the paper. Baochen Bai, Chao Tian, and Daisong Li collected materials and prepared the tables. Bingxue Song and Haichu Yu collected materials and provided clinical ideas. Xianming Chu and Bing Li provided ideas and revised the paper. All authors reviewed and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Q., Bai, B., Tian, C. et al. The Molecular Mechanisms of Cardiotoxicity Induced by HER2, VEGF, and Tyrosine Kinase Inhibitors: an Updated Review. Cardiovasc Drugs Ther 36, 511–524 (2022). https://doi.org/10.1007/s10557-021-07181-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07181-3